Carotenoids Overproduction in Dunaliella Sp.: Transcriptional Changes and New Insights through Lycopene β Cyclase Regulation
Abstract
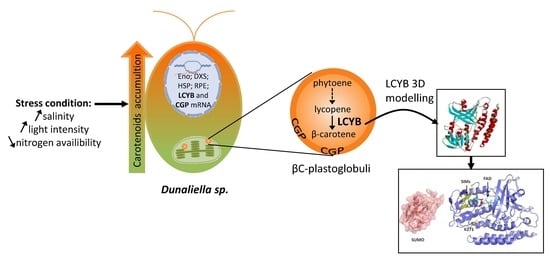
:1. Introduction
2. Materials and Methods
2.1. Microalga and Growth Conditions
2.2. Pigments Extraction
2.3. Flow Cytometry Analysis
2.4. RNA Extraction
2.5. qRT-PCR
2.5.1. Primers Design and Validation, Reference Gene Selection
2.5.2. qPCR Conditions
2.6. Retrieval of Protein Sequences and In Silico Analysis
2.7. Three-Dimensional Protein Modeling
2.8. Flavin Adenine Dinucleotide (FAD)-Docking
3. Results
3.1. Characterization of Dunaliella in the Two Cultural Conditions
3.1.1. Influence of Stressed Condition on Cell Growth
3.1.2. Pigments Accumulation
3.1.3. Flow Cytometry Analysis
3.2. Transcriptional Analysis
3.2.1. Evaluation of Reference Gene Stability
3.2.2. Gene Expression Profile under Stressed Conditions
3.3. In Silico Analysis
3.3.1. Enzyme Modeling
3.3.2. Alignment Analysis
3.3.3. Overall 3D Model of D. salina LCYB
3.3.4. Overall 3D Model of D. salina SUMO Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ye, Z.-W.; Jiang, J.-G.; Wu, G.-H. Biosynthesis and regulation of carotenoids in Dunaliella: Progresses and prospects. Biotechnol. Adv. 2008, 26, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Polle, J.; Tran, D.; Cushman, J.C.; Jin, E.; Varela, J.C. The unicellular green alga Dunaliella salina Teod. as a model for abiotic stress tolerance: Genetic advances and future perspectives. Algae 2011, 26, 3–20. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.-G. Osmotic responses of Dunaliella to the changes of salinity. J. Cell. Physiol. 2009, 219, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Merhan, O. The biochemistry and antioxidant properties of carotenoids. Carotenoids 2017, 5, 51. [Google Scholar]
- García-González, M.; Moreno, J.; Manzano, J.C.; Florencio, F.J.; Guerrero, M.G. Production of Dunaliella salina biomass rich in 9-cis-β-carotene and lutein in a closed tubular photobioreactor. J. Biotechnol. 2005, 115, 81–90. [Google Scholar] [CrossRef]
- Domonkos, I.; Kis, M.; Gombos, Z.; Ughy, B. Carotenoids, versatile components of oxygenic photosynthesis. Prog. Lipid Res. 2013, 52, 539–561. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Shaish, A.; Avron, M. Mode of action of the massively accumulated beta-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol. 1989, 91, 1040–1043. [Google Scholar] [CrossRef] [Green Version]
- Raja, R.; Hemaiswarya, S.; Rengasamy, R. Exploitation of Dunaliella for β-carotene production. Appl. Microbiol. Biotechnol. 2007, 74, 517–523. [Google Scholar] [CrossRef]
- Nishino, H.; Tokuda, H.; Murakoshi, M.; Satomi, Y.; Masuda, M.; Onozuka, M.; Yamaguchi, S.; Takayasu, J.; Tsuruta, J.; Okuda, M.; et al. Cancer prevention by natural carotenoids. BioFactors Oxf. Engl. 2000, 13, 89–94. [Google Scholar] [CrossRef]
- Pasquet, V. Recherche Bioguidée de Molécules Anticancéreuses Issues de Microalgues Marines. Ph.D. Thesis, Université de La Rochelle, La Rochelle, France, 2011. [Google Scholar]
- Jayappriyan, K.R.; Rajkumar, R.; Venkatakrishnan, V.; Nagaraj, S.; Rengasamy, R. In Vitro Anticancer Activity of Natural β-Carotene from Dunaliella Salina EU5891199 in PC-3 Cells. Biomed. Prev. Nutr. 2013, 3, 99–105. [Google Scholar]
- Yang, D.-J.; Lin, J.-T.; Chen, Y.-C.; Liu, S.-C.; Lu, F.-J.; Chang, T.-J.; Wang, M.; Lin, H.-W.; Chang, Y.-Y. Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS-stimulated pro-inflammatory mediators in RAW264.7 cells via NF-κB and JNK inactivation. J. Funct. Foods 2013, 5, 607–615. [Google Scholar] [CrossRef]
- Jaswir, I. Carotenoids: Sources, medicinal properties and their application in food and nutraceutical industry. J. Med. Plants Res. 2011, 5, 7119–7131. [Google Scholar]
- Ben-Amotz, A.; Katz, A.; Avron, M. Accumulation of β-carotene in halotolerant algae: Purification and characterization of β-carotene-rich globules from Dunaliella bardawil (Chlorophyceae). J. Phycol. 1982, 18, 529–537. [Google Scholar] [CrossRef]
- Chen, X.; Han, H.; Jiang, P.; Nie, L.; Bao, H.; Fan, P.; Lv, S.; Feng, J.; Li, Y. Transformation of β-lycopene cyclase genes from Salicornia europaea and Arabidopsis conferred salt tolerance in Arabidopsis and tobacco. Plant Cell Physiol. 2011, 52, 909–921. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Wang, Y.; Qin, S. Molecular evolution of lycopene cyclases involved in the formation of carotenoids in eukaryotic algae. Plant Mol. Biol. Report. 2011, 29, 1013–1020. [Google Scholar] [CrossRef]
- Ramos, A.; Coesel, S.; Marques, A.; Rodrigues, M.; Baumgartner, A.; Noronha, J.; Rauter, A.; Brenig, B.; Varela, J. Isolation and characterization of a stress-inducible Dunaliella salina Lcy-beta gene encoding a functional lycopene beta-cyclase. Appl. Microbiol. Biotechnol. 2008, 79, 819–828. [Google Scholar] [CrossRef]
- Fazeli, M.R.; Tofighi, H.; Madadkar-Sobhani, A.; Shahverdi, A.R.; Nejad-Sattari, T.; Mirzaie, S.; Jamalifar, H. Nicotine inhibition of lycopene cyclase enhances accumulation of carotenoid intermediates by Dunaliella salina CCAP 19/18. Eur. J. Phycol. 2009, 44, 215–220. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Jiang, J.-G.; Chen, Q. Characterization of cDNA of lycopene beta-cyclase responsible for a high level of beta-carotene accumulation in Dunaliella salina. Biochem. Cell Biol. Biochim. Biol. Cell. 2008, 86, 285–292. [Google Scholar] [CrossRef]
- Lv, H.; Cui, X.; Wahid, F.; Xia, F.; Zhong, C.; Jia, S. Analysis of the Physiological and Molecular Responses of Dunaliella salina to Macronutrient Deprivation. PLoS ONE 2016, 11, e0152226. [Google Scholar] [CrossRef]
- Mendoza, H.; Río, M.J.D.; Reina, G.G.; Ramazanov, Z. Low-temperature-induced β-carotene and fatty acid synthesis, and ultrastructural reorganization of the chloroplast in Dunaliella salina (Chlorophyta). Eur. J. Phycol. 1996, 31, 329–331. [Google Scholar] [CrossRef]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliella salina, a unicellular green microalga. J. Biotechnol. 2012, 162, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yilancioglu, K.; Cokol, M.; Pastirmaci, I.; Erman, B.; Cetiner, S. Oxidative stress is a mediator for increased lipid accumulation in a newly isolated Dunaliella salina strain. PLoS ONE 2014, 9, e91957. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S. Effects of sulfur, iron and manganese starvation on growth, β-carotene production and lipid profile of Dunaliella salina. J. Young Pharm. 2017, 9, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Wongsnansilp, T.; Juntawong, N.; Wu, Z. Effects of phosphorus on the growth and chlorophyll fluorescence of a Dunaliella salina strain isolated from saline soil under nitrate limitation. J. Biol. Res. Boll. Della Soc. Ital. Biol. Sper. 2016, 89, 5866. [Google Scholar] [CrossRef] [Green Version]
- Gomez, P.I.; Barriga, A.; Cifuentes, A.S.; Gonzalez, M.A. Effect of salinity on the quantity and quality of carotenoids accumulated by Dunaliella salina (strain CONC-007) and Dunaliella bardawil (strain ATCC 30861) Chlorophyta. Biol. Res. 2003, 36, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rad, F.A.; Aksoz, N.; Hejazi, M.A. Effect of salinity on cell growth and β-carotene production in Dunaliella sp. isolates from Urmia Lake in northwest of Iran. Afr. J. Biotechnol. 2011, 10, 2282–2289. [Google Scholar]
- Farhat, N.; Rabhi, M.; Falleh, H.; Jouini, J.; Abdelly, C.; Smaoui, A. Optimization of Salt Concentrations for a Higher Carotenoid Production in Dunaliella salina (chlorophyceae). J. Phycol. 2011, 47, 1072–1077. [Google Scholar] [CrossRef]
- Lamers, P.P.; Janssen, M.; De Vos, R.C.H.; Bino, R.J.; Wijffels, R.H. Exploring and exploiting carotenoid accumulation in Dunaliella salina for cell-factory applications. Trends Biotechnol. 2008, 26, 631–638. [Google Scholar] [CrossRef]
- Davidi, L.; Levin, Y.; Ben-Dor, S.; Pick, U. Proteome Analysis of Cytoplasmatic and Plastidic β-Carotene Lipid Droplets in Dunaliella bardawil. Plant Physiol. 2015, 167, 60–79. [Google Scholar] [CrossRef] [Green Version]
- Davidi, L.; Shimoni, E.; Khozin-Goldberg, I.; Zamir, A.; Pick, U. Origin of β-carotene-rich plastoglobuli in Dunaliella bardawil. Plant Physiol. 2014, 164, 2139–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, A.; Jimenez, C.; Pick, U. Isolation and characterization of a protein associated with carotene globules in the alga Dunaliella bardawil. Plant Physiol. 1995, 108, 1657–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Amor, F.; Elleuch, F.; Ben Hlima, H.; Garnier, M.; Saint-Jean, B.; Barkallah, M.; Pichon, C.; Abdelkafi, S.; Fendri, I. Proteomic analysis of the Chlorophyta Dunaliella new strain AL-1 revealed global changes of metabolism during high carotenoid production. Mar. Drugs 2017, 15, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chtourou, H.; Dahmen, I.; Jebali, A.; Karray, F.; Hassairi, I.; Abdelkafi, S.; Ayadi, H.; Sayadi, S.; Dhouib, A. Characterization of Amphora sp., a newly isolated diatom wild strain, potentially usable for biodiesel production. Bioprocess Biosys. Eng. 2015, 38, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls A and B of Leaf Extracts in Different Solvents. In Advances in Photosynthesis Research; Advances in Agricultural Biotechnology; Springer: Dordrecht, The Netherlands, 1984; pp. 9–12. ISBN 978-90-247-2943-2. [Google Scholar]
- Mendoza, H.; De la Jara, A.; Freijanes, K.; Carmona, L.; Ramos, A.A.; de Sousa Duarte, V.; Varela, S.; Carlos, J. Characterization of Dunaliella salina strains by flow cytometry: A new approach to select carotenoid hyperproducing strains. Electron. J. Biotechnol. 2008, 11, 5–6. [Google Scholar] [CrossRef]
- Dammak, M.; Haase, S.M.; Miladi, R.; Ben Amor, F.; Barkallah, M.; Gosset, D.; Pichon, C.; Huchzermeyer, B.; Fendri, I.; Denis, M.; et al. Enhanced lipid and biomass production by a newly isolated and identified marine microalga. Lipids Health Dis. 2016, 15, 209. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Lao, Y.-M.; Jiang, J.-G. Effects of salinities on the gene expression of a (NAD+)-dependent glycerol-3-phosphate dehydrogenase in Dunaliella salina. Sci. Total Environ. 2011, 409, 1291–1297. [Google Scholar] [CrossRef]
- Chen, X.-J.; Zhang, X.-H.; Hu, L.-D.; Zhang, J.-Q.; Jiang, Y.; Yang, Y.; Yan, Y.-B. DsCaf1 is involved in environmental stress response of Dunaliella salina. Int. J. Biol. Macromol. 2016, 82, 369–374. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Li, H.; Gao, B.; Yang, H.; Zhang, Y.; Wood, A.J. Characterization of reference genes for RT-qPCR in the desert moss Syntrichia caninervis in response to abiotic stress and desiccation/rehydration. Front. Plant Sci. 2015, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Ben Amor, F.; Ben Hlima, H.; Abdelkafi, S.; Fendri, I. Insight into Arthrospira platensis Δ9 desaturase: A key enzyme in poly-unsaturated fatty acid synthesis. Mol. Biol. Rep. 2018, 45, 1873–1879. [Google Scholar] [CrossRef]
- Xia, J.-X.; Ikeda, M.; Shimizu, T. ConPred_elite: A highly reliable approach to transmembrane topology predication. Comput. Biol. Chem. 2004, 28, 51–60. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Thornton, J.M. PROCHECK: Validation of Protein-Structure Coordinates. Available online: http://xrpp.iucr.org/Fb/ch21o4v0001/ (accessed on 13 February 2018).
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Shin, W.-H.; Kim, J.-K.; Kim, D.-S.; Seok, C. GalaxyDock2: Protein-ligand docking using beta-complex and global optimization. J. Comput. Chem. 2013, 34, 2647–2656. [Google Scholar] [CrossRef]
- Pedrós, R.; Moya, I.; Goulas, Y.; Jacquemoud, S. Chlorophyll fluorescence emission spectrum inside a leaf. Photochem. Photobiol. Sci. 2008, 7, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Nonomura, A.M.; Coder, D.M. Improved Phycocatalysis of Carotene Production by Flow Cytometry and Cell Sorting. Biocatalysis 1988, 1, 333–338. [Google Scholar] [CrossRef]
- Ukibe, K.; Katsuragi, T.; Tani, Y.; Takagi, H. Efficient screening for astaxanthin-overproducing mutants of the yeast Xanthophyllomyces dendrorhous by flow cytometry. FEMS Microbiol. Lett. 2008, 286, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.-W.; Kim, K.-J. Crystal structure of 1′-OH-carotenoid 3,4-desaturase from Nonlabens dokdonensis DSW-6. Enzym. Microb. Technol. 2015, 77, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Xu, C.; Zhang, J.; Zhang, X.; Tu, X. Solution structure of SUMO from Trypanosoma brucei and its interaction with Ubc9. Proteins Struct. Funct. Bioinform. 2009, 76, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Estudillo, L.; Freile-Pelegrin, Y.; Rivera-Madrid, R.; Robledo, D.; Narváez-Zapata, J.A. Regulation of two photosynthetic pigment-related genes during stress-induced pigment formation in the green alga. Dunaliella salina. Biotechnol. Lett. 2006, 28, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Jha, B. Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour. Technol. 2009, 100, 3382–3386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gong, W.; Chen, X.; Chen, D. Characterization of genes and enzymes in Dunaliella salina involved in glycerol metabolism in response to salt changes. Phycol. Res. 2013, 61, 37–45. [Google Scholar] [CrossRef]
- Xia, B.-B.; Wang, S.-H.; Duan, J.-B.; Bai, L.-H. The relationship of glycerol and glycolysis metabolism patway under hyperosmotic stress in Dunaliella salina. Cent. Eur. J. Biol. 2014, 9, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Fachet, M.; Hermsdorf, D.; Rihko-Struckmann, L.; Sundmacher, K. Flow cytometry enables dynamic tracking of algal stress response: A case study using carotenogenesis in Dunaliella salina. Algal Res. 2016, 13, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Polle, J.E.W.; Melis, A.; Lee, T.K.; Jin, E. Up-regulation of photoprotection and PSII-repair gene expression by irradiance in the unicellular green alga Dunaliella salina. Mar. Biotechnol. 2006, 8, 120–128. [Google Scholar] [CrossRef]
- Ruan, K.; Duan, J.; Bai, F.; Lemaire, M.; Ma, X.; Bai, L. Function of Dunaliella salina (Dunaliellaceae) enolase and its expression during stress. Eur. J. Phycol. 2009, 44, 207–214. [Google Scholar] [CrossRef]
- Polle, J.E.W.; Neofotis, P.; Huang, A.; Chang, W.; Sury, K.; Wiech, E.M. Carbon Partitioning in Green Algae (Chlorophyta) and the Enolase Enzyme. Metabolites 2014, 4, 612–628. [Google Scholar] [CrossRef] [Green Version]
- Peng, G.; Wang, C.; Song, S.; Fu, X.; Azam, M.; Grierson, D.; Xu, C. The role of 1-deoxy-d-xylulose-5-phosphate synthase and phytoene synthase gene family in citrus carotenoid accumulation. Plant Physiol. Biochem. 2013, 71, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Kinohira, S.; Ando, A.; Shimoyama, M.; Kato, M.; Fukuzawa, H. Accumulation of squalene in a microalga Chlamydomonas reinhardtii by genetic modification of squalene synthase and squalene epoxidase genes. PLoS ONE 2015, 10, e0120446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsushima, D.; Jenke-Kodama, H.; Sato, Y.; Fukunaga, Y.; Sumimoto, K.; Kuzuyama, T.; Matsunaga, S.; Okada, S. The single cellular green microalga Botryococcus braunii, race B possesses three distinct 1-deoxy-d-xylulose 5-phosphate synthases. Plant Sci. 2012, 185–186, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Ghirardo, A.; Wright, L.P.; Bi, Z.; Rosenkranz, M.; Pulido, P.; Rodríguez-Concepción, M.; Niinemets, Ü.; Brüggemann, N.; Gershenzon, J.; Schnitzler, J.-P. Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol. 2014, 165, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Guevara-García, A.; San Román, C.; Arroyo, A.; Cortés, M.E.; de la Luz Gutiérrez-Nava, M.; León, P. Characterization of the Arabidopsis clb6 Mutant Illustrates the Importance of Posttranscriptional Regulation of the Methyl-d-Erythritol 4-Phosphate Pathway. Plant Cell 2005, 17, 628–643. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Wu, Y.; Banerjee, R.; Li, Y.; Yan, H.; Sharkey, T.D. Feedback Inhibition of Deoxy-d-xylulose-5-phosphate Synthase Regulates the Methylerythritol 4-Phosphate Pathway. J. Biol. Chem. 2013, 288, 16926–16936. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.-H.; Lu, Y.; Chen, H.-H.; Jiang, J.-G. The salt-regulated element in the promoter of lycopene β-cyclase gene confers a salt regulatory pattern in carotenogenesis of Dunaliella bardawil. Environ. Microbiol. 2017, 19, 982–989. [Google Scholar] [CrossRef]
- Liang, M.-H.; Jiang, J.-G. Analysis of carotenogenic genes promoters and WRKY transcription factors in response to salt stress in Dunaliella bardawil. Sci. Rep. 2017, 7, 37025. [Google Scholar] [CrossRef]
- Rabbani, S.; Beyer, P.; Lintig, J.V.; Hugueney, P.; Kleinig, H. Induced β-Carotene Synthesis Driven by Triacylglycerol Deposition in the Unicellular Alga Dunaliella bardawil. Plant Physiol. 1998, 116, 1239–1248. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-C.; Chen, L.-J.; Su, P.-H.; Li, H. Evolution of chloroplast J proteins. PLoS ONE 2013, 8, e70384. [Google Scholar] [CrossRef] [Green Version]
- Yokthongwattana, K.; Chrost, B.; Behrman, S.; Casper-Lindley, C.; Melis, A. Photosystem II Damage and Repair Cycle in the Green Alga Dunaliella salina: Involvement of a Chloroplast-Localized HSP70. Plant Cell Physiol. 2001, 42, 1389–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trösch, R.; Mühlhaus, T.; Schroda, M.; Willmund, F. ATP-dependent molecular chaperones in plastids—More complex than expected. Biochim. Biophys. Acta-Bioenerg. 2015, 1847, 872–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.-P.; Williams, E.; Wang, D.; Xie, Z.-X.; Hsia, R.; Jenck, A.; Halden, R.; Li, J.; Chen, F.; Place, A.R. Responses of Nannochloropsis oceanica IMET1 to Long-Term Nitrogen Starvation and Recovery1. Plant Physiol. 2013, 162, 1110–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enserink, J.M. Sumo and the cellular stress response. Cell Div. 2015, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Knobbe, A.R.; Horken, K.M.; Plucinak, T.M.; Balassa, E.; Cerutti, H.; Weeks, D.P. SUMOylation by a stress-specific small ubiquitin-like modifier E2 conjugase is essential for survival of Chlamydomonas reinhardtii under stress conditions. Plant Physiol. 2015, 167, 753–765. [Google Scholar] [CrossRef] [Green Version]
- Trujillo-Hdz, J.A.; Cárdenas-Conejo, Y.; Turriza, P.E.; Aguilar-Espinosa, M.; Carballo-Uicab, V.; Garza-Caligaris, L.E.; Comai, L.; Rivera-Madrid, R. Functional polymorphism in lycopene beta-cyclase gene as a molecular marker to predict bixin production in Bixa orellana L. (achiote). Mol. Breed. 2016, 36, 135. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Schaub, P.; Ghisla, S.; Al-Babili, S.; Krieger-Liszkay, A.; Beyer, P. The lycopene cyclase CrtY from Pantoea ananatis (formerly Erwinia uredovora) catalyzes an FADred-dependent non-redox reaction. J. Biol. Chem. 2010, 285, 12109–12120. [Google Scholar] [CrossRef] [Green Version]
- Moise, A.R.; Al-Babili, S.; Wurtzel, E.T. Mechanistic aspects of carotenoid biosynthesis. Chem. Rev. 2014, 114, 164–193. [Google Scholar] [CrossRef] [Green Version]


 Model,
Model,  confidence interval (Mean 95%),
confidence interval (Mean 95%),  confidence interval (obs. 95%).
confidence interval (obs. 95%).
 Model,
Model,  confidence interval (Mean 95%),
confidence interval (Mean 95%),  confidence interval (obs. 95%).
confidence interval (obs. 95%).




| Gene Name | Abbreviation | Primer Sequence (5′->3′) | Amplicon Size (bp) | Tm (°C) | E (%) | Reference/Access Number | |
|---|---|---|---|---|---|---|---|
| Actin | Act | F | ACCACACCTTCTTCAACGAG | 150 | 86.76 | 111.35 | [39] |
| R | GGATGGCTACATACATGGCA | ||||||
| 18S RNA | 18S | F | TTGGGTAGTCGGGCTGGTC | - | 83.2 | 92.6 | [40] |
| R | CGCTGCGTTCTTCATCGTT | ||||||
| Alpha tubulin | α-TUB | F | GAGATCACCAACGCTGCCTTTGA | 105 | 86.03 | 106.2 | JF346103.1 |
| R | CACCACATCACCGCGGTACAT | ||||||
| Beta tubulin | β-TUB | F | AGGAGGGCGAGTTTGAGGGT | 137 | 83.12 | 92.4 | DQ080916.1 |
| R | AGCGCGGTGGACTTGAACAG | ||||||
| Enolase | ENO | F | TGAAGGGCATGGACCCCAGG | 123 | 85.66 | 97.23 | KM008612.1 |
| R | TGGCAGTTGCCAGGGAGACA | ||||||
| 1-deoxy-D-xylulose 5-phosphate synthase | DXS | F | AGGACTTACGCCTGGCCACAT | 102 | 85.67 | 94.86 | FJ469276.1 |
| R | GCAGCAACCAGGCCAGTGTT | ||||||
| Lycopene β-cyclase | LCYB | F | CCTGCAATCACCACAGGCGG | 130 | 87.91 | 97.1 | EU327876.1 |
| R | GGGGGTTTCTGGCGTCCTCT | ||||||
| Carotene globule protein | CGP | F | GGCAACCGAGGCAGTGACTACCA | 104 | 85.18 | 111 | JX646677.1 |
| R | GGAACTCCGGTGGACATCTGGTT | ||||||
| Chloroplast-localized heat shock protein | HSP70 | F | TGCAGGCTGGTGTGCTGTCT | 124 | 87.88 | 99.03 | AJ271605.2 |
| R | AGGGTGGTGTTGCGGGTGAT | ||||||
| Chloroplast ribulose phosphate-3-epimerase | RPE | F | TGATCAAGGACCTGGGCTGCAA | 120 | 85.96 | 97.33 | JF346108.1 |
| R | AGCCAGGGTTCACGGACATGA |
| Enzyme | Position | Group | Score |
|---|---|---|---|
| CGP | K308 | WLLGM AKPE DEAKK | 0.79 |
| K262 | AVSEA FKEG ETEIS | 0.68 | |
| LCYB | K271 | HARKL VKYD QEFNP | 0.93 |
| 124–128 | QRQPAADLLVVGSGPSGLA | 0.70 | |
| 149–153 | AGGFSVCVIDLDPYAPMIP | 0.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elleuch, F.; Hlima, H.B.; Barkallah, M.; Baril, P.; Abdelkafi, S.; Pichon, C.; Fendri, I. Carotenoids Overproduction in Dunaliella Sp.: Transcriptional Changes and New Insights through Lycopene β Cyclase Regulation. Appl. Sci. 2019, 9, 5389. https://doi.org/10.3390/app9245389
Elleuch F, Hlima HB, Barkallah M, Baril P, Abdelkafi S, Pichon C, Fendri I. Carotenoids Overproduction in Dunaliella Sp.: Transcriptional Changes and New Insights through Lycopene β Cyclase Regulation. Applied Sciences. 2019; 9(24):5389. https://doi.org/10.3390/app9245389
Chicago/Turabian StyleElleuch, Fatma, Hajer Ben Hlima, Mohamed Barkallah, Patrick Baril, Slim Abdelkafi, Chantal Pichon, and Imen Fendri. 2019. "Carotenoids Overproduction in Dunaliella Sp.: Transcriptional Changes and New Insights through Lycopene β Cyclase Regulation" Applied Sciences 9, no. 24: 5389. https://doi.org/10.3390/app9245389