Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Properties
2.2. Membrane Distillation and Crystallization Setup
2.3. Fouling/Scaling Analysis
2.4. Footprint, Weight, and Waste Reduction Analysis
2.5. Crystal Characterization
3. Results and Discussion
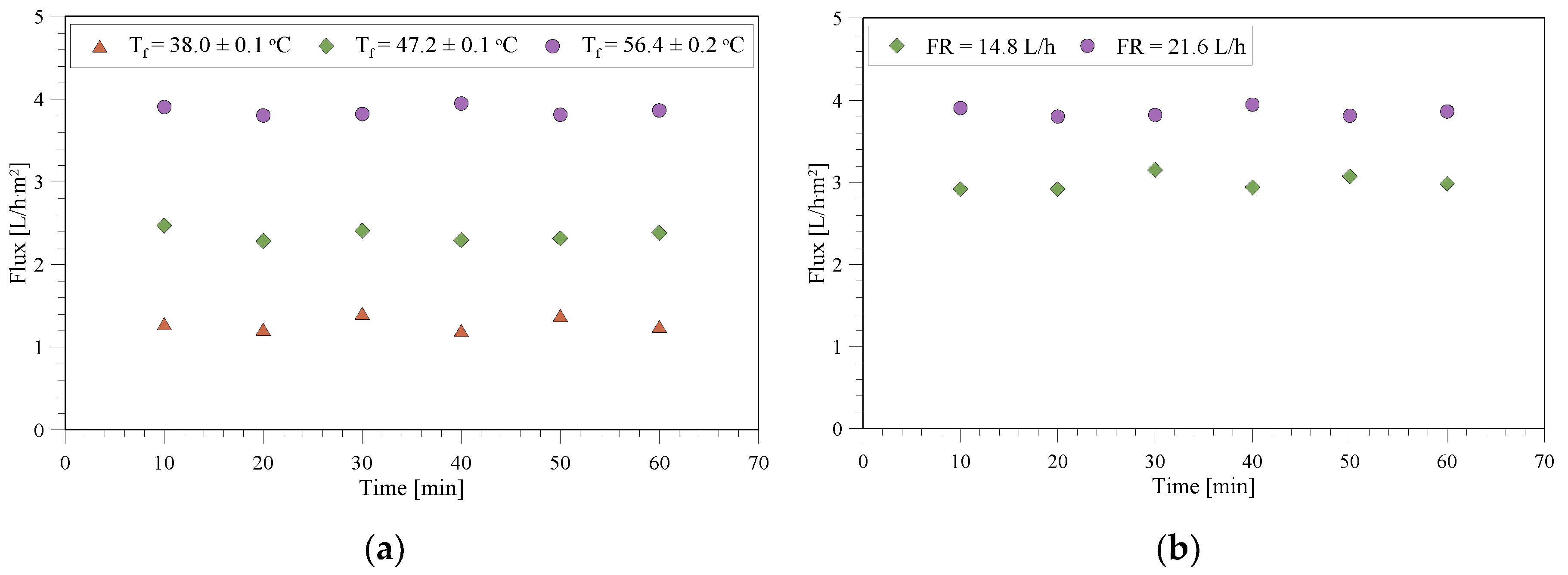
3.1. Membrane Characterization with Pure Water
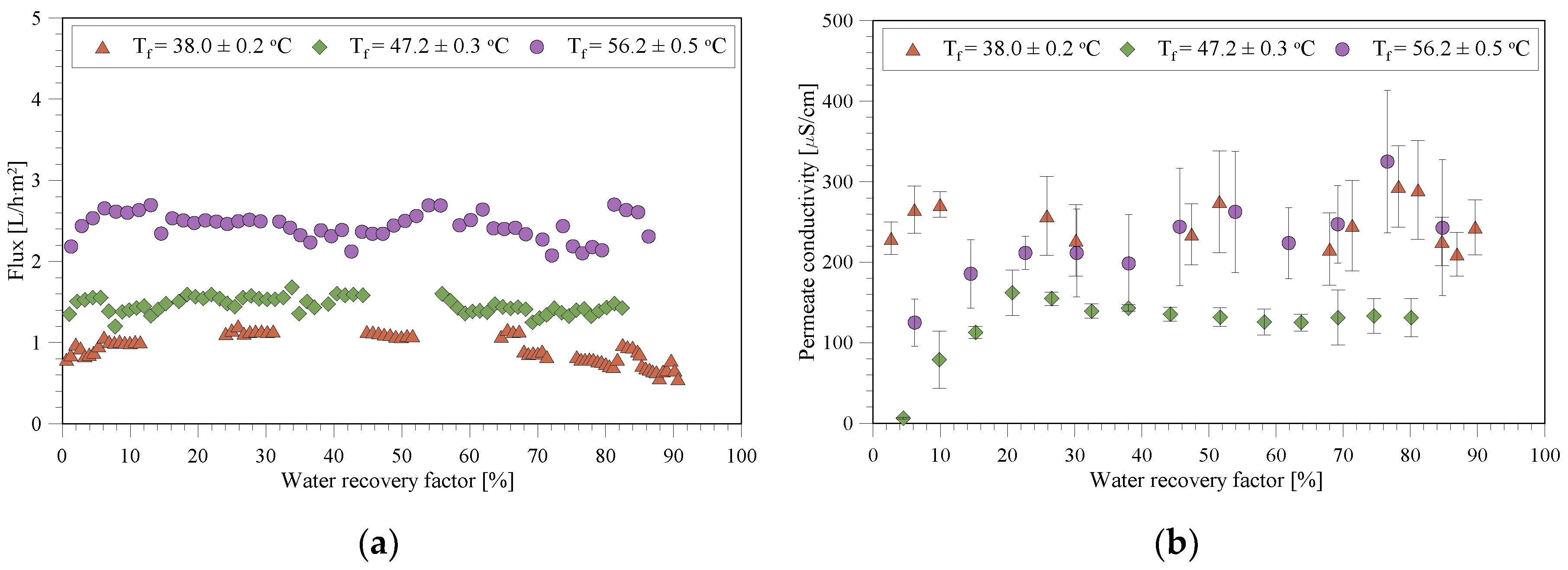
3.2. MD/MCr Tests with the Wastewater
3.3. Crystallization Study
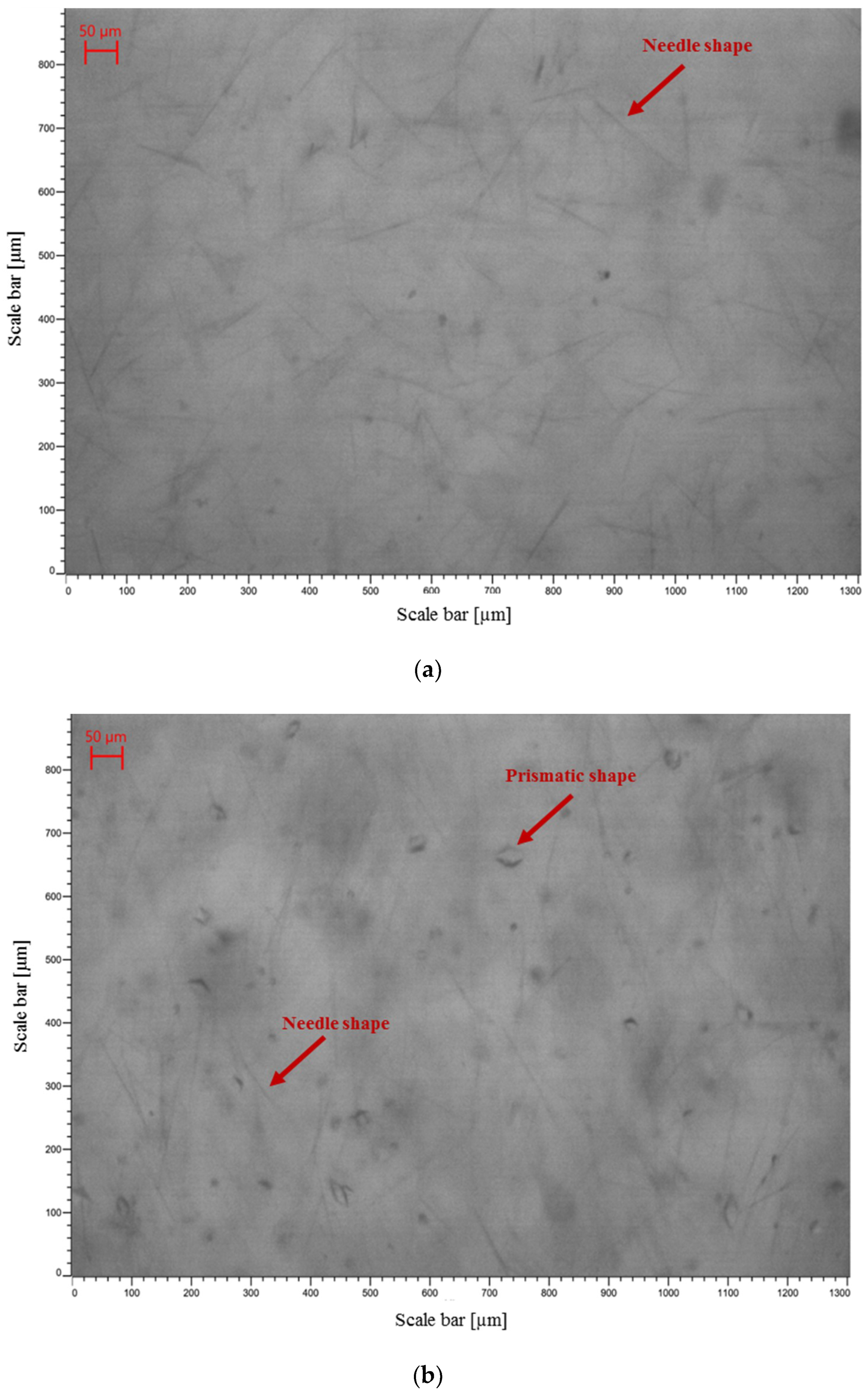
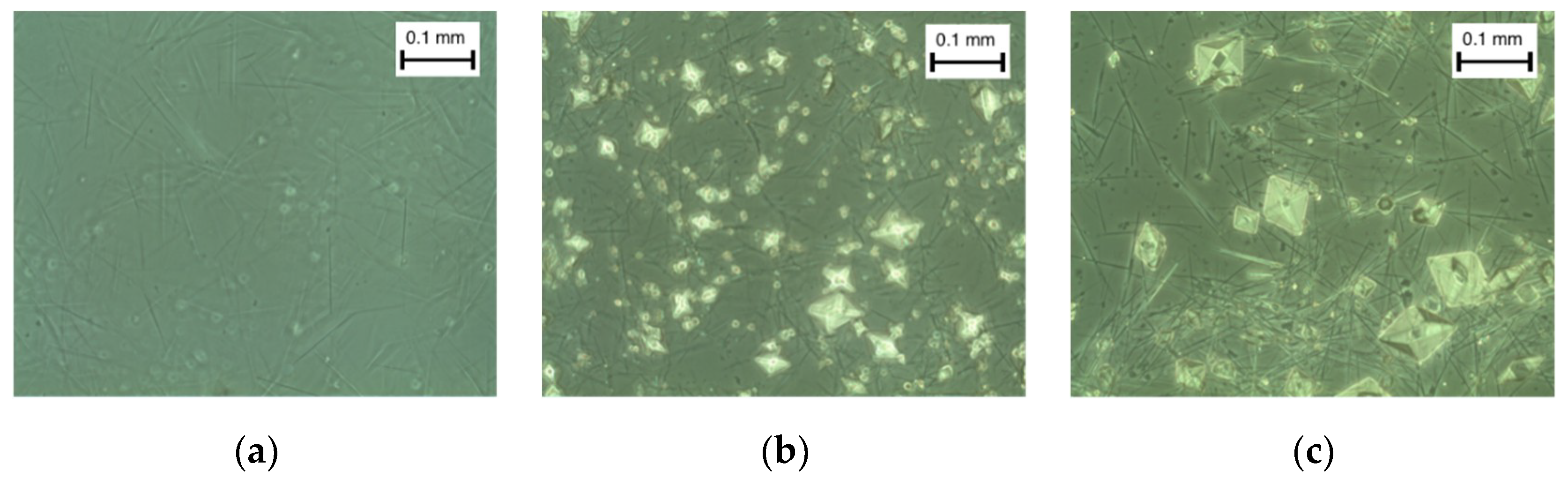
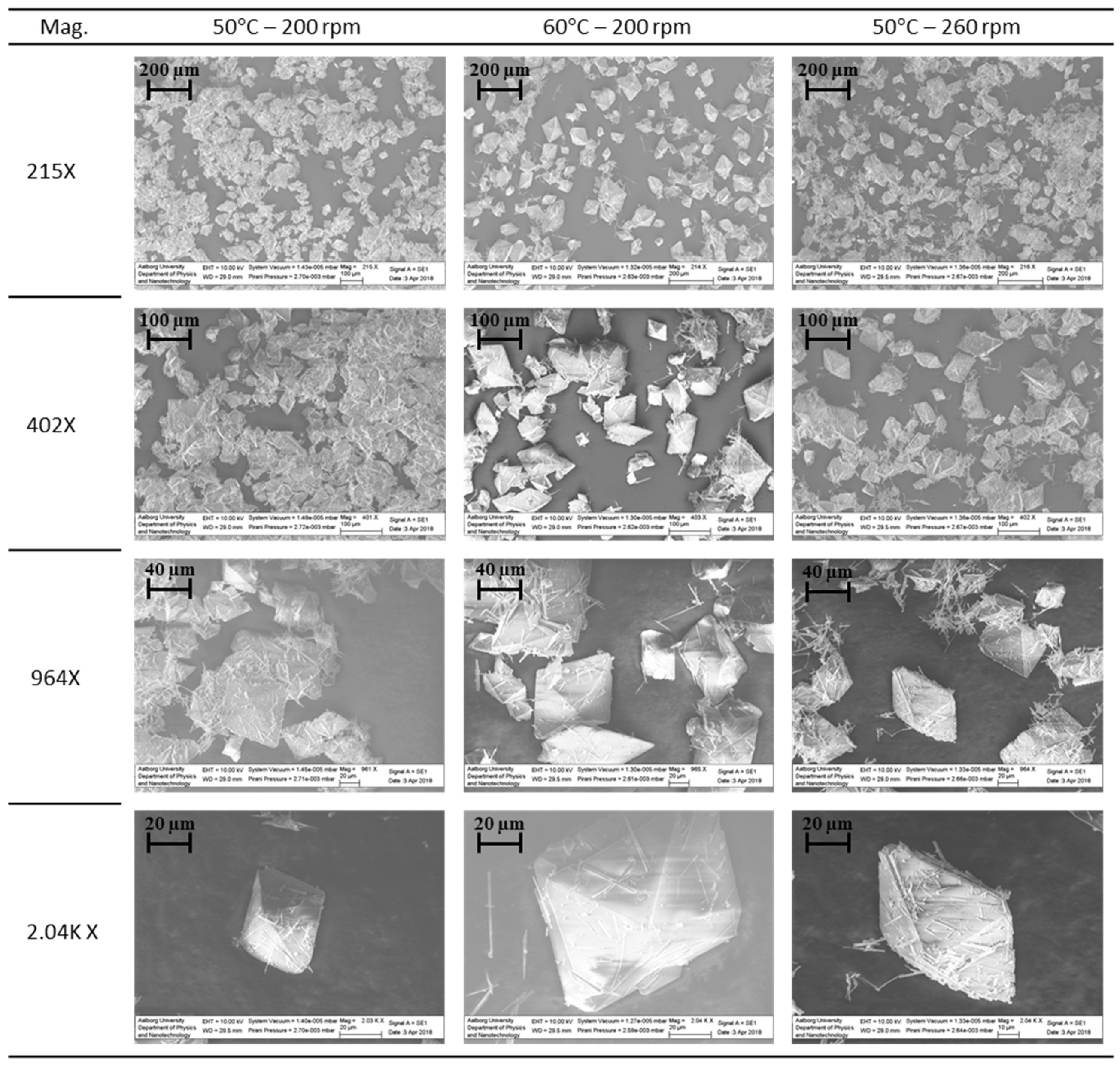
3.3.1. Crystal Morphology
3.3.2. Identification and Purity of the Crystals
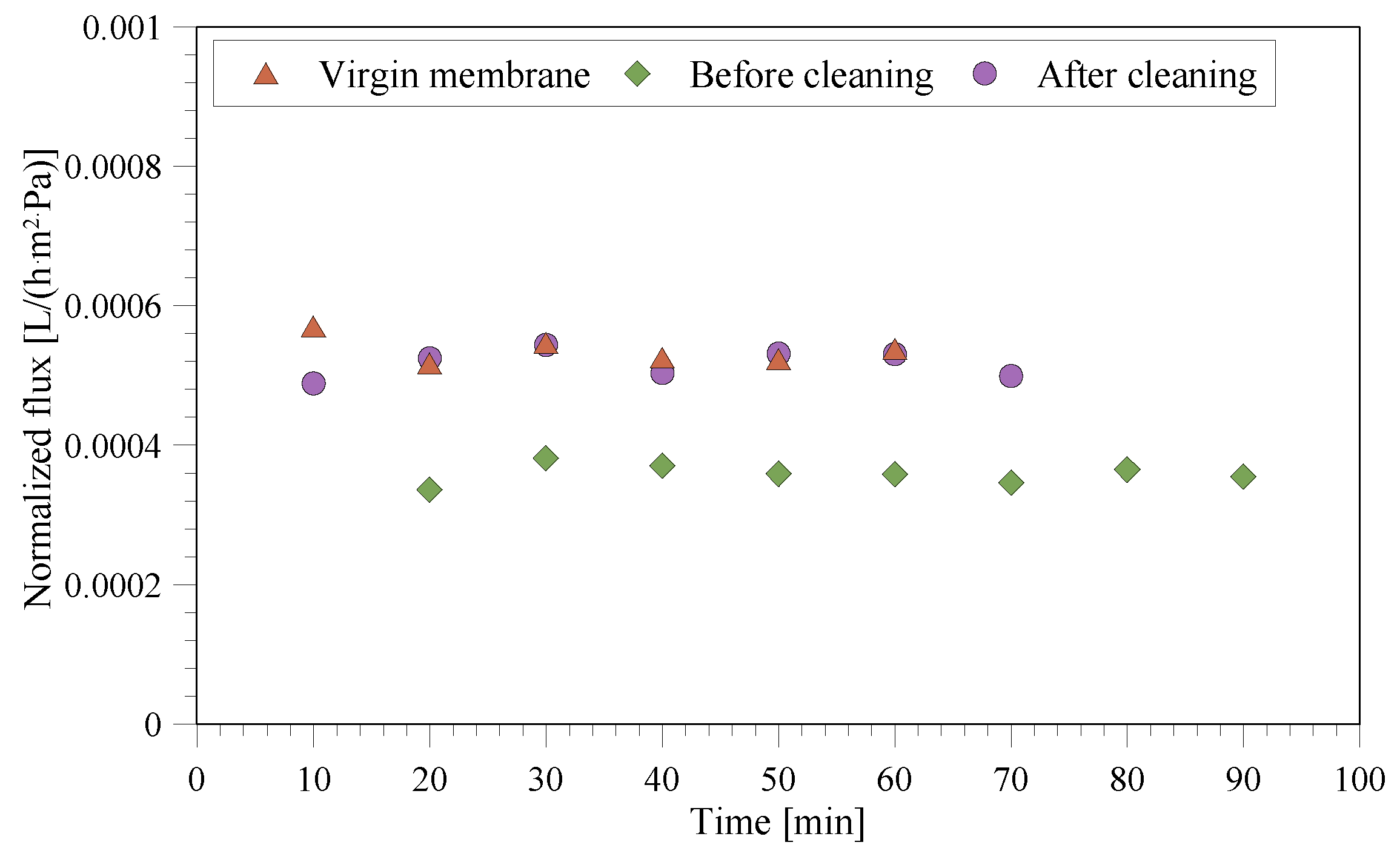
3.4. Fouling/Scaling Analysis
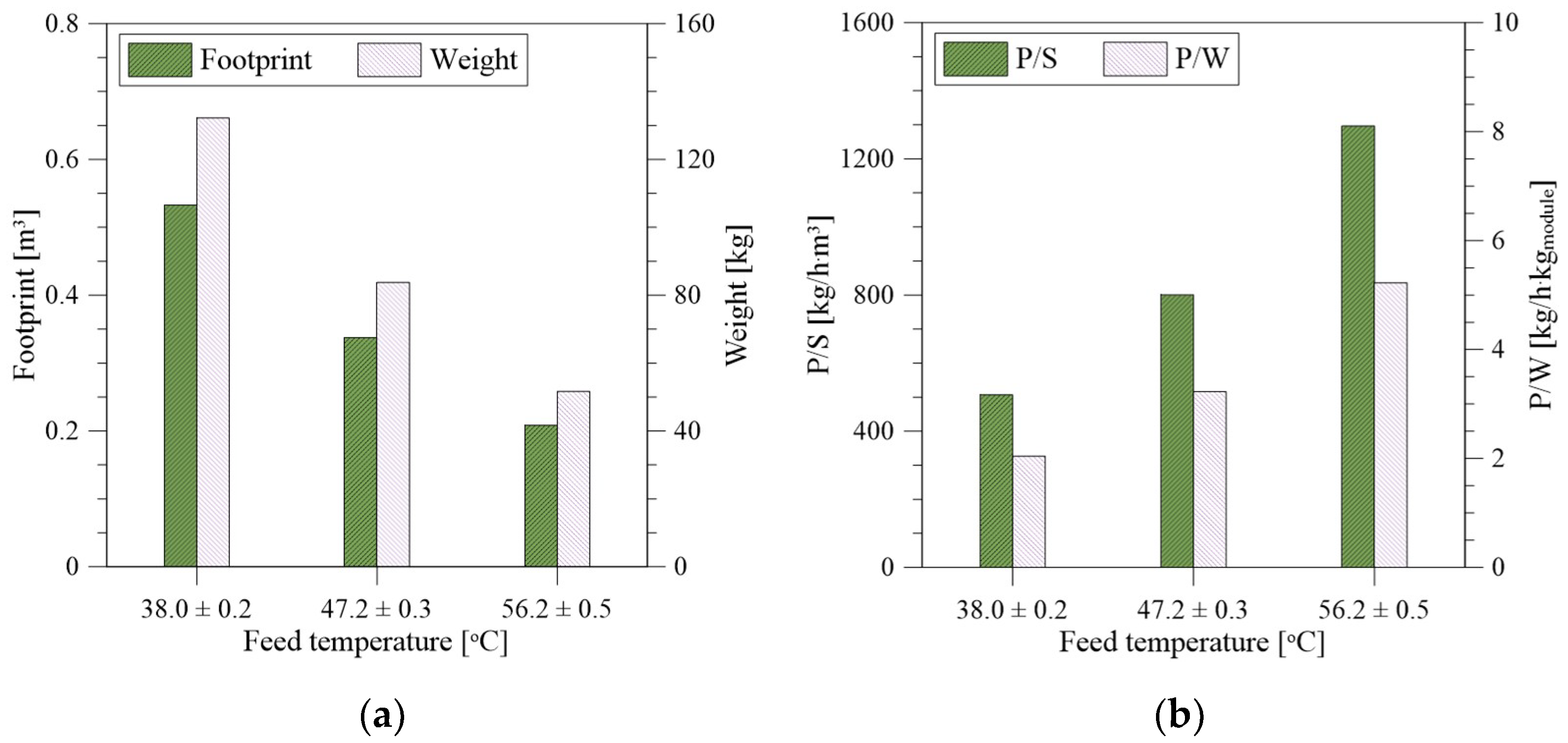
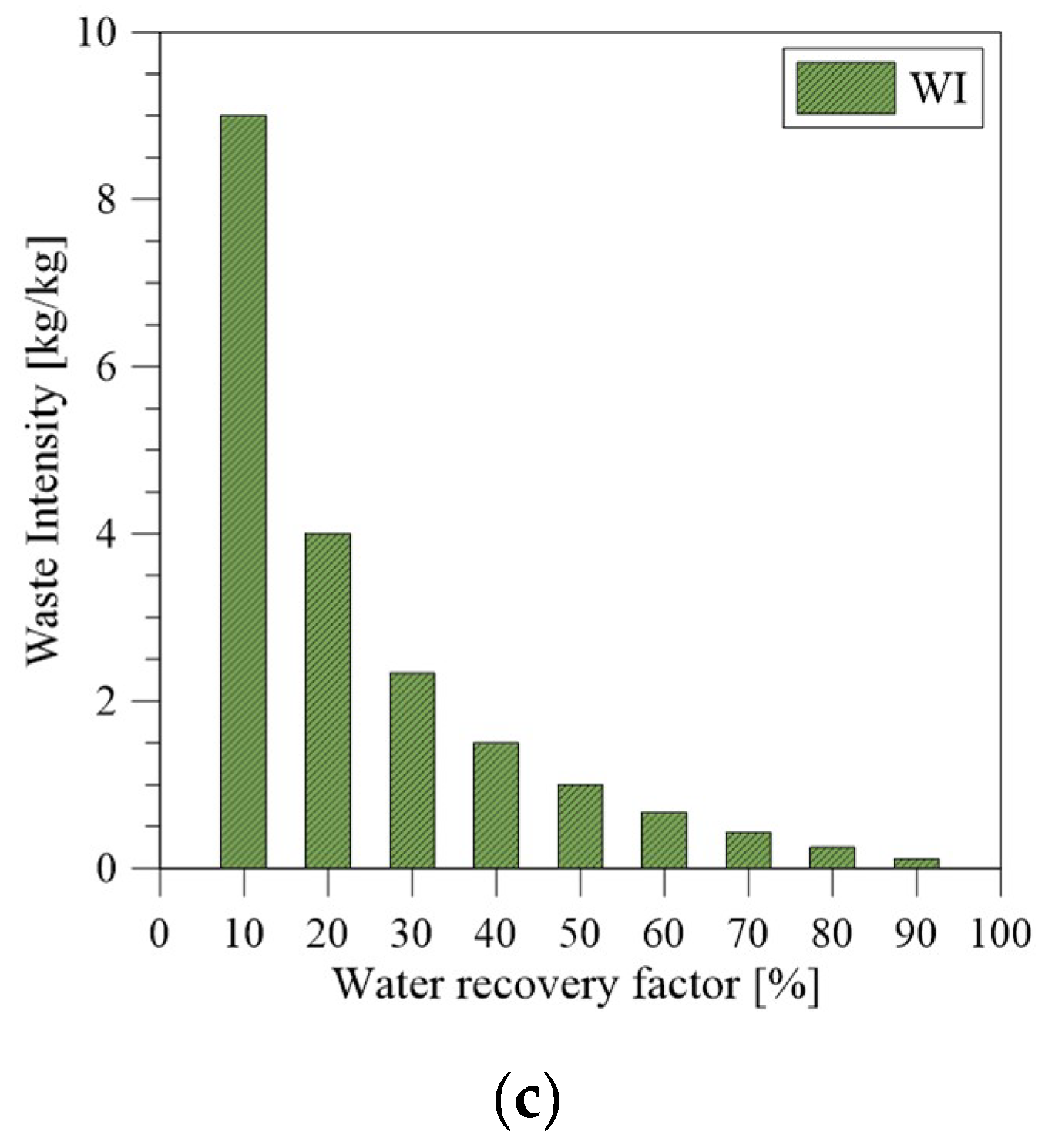
3.5. Weight, Size, and Waste Reduction Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevenson, M.F. Anodizing. In ASM Handbook; ASM Press: Washington, DC, USA, 1994; Volume 5, pp. 482–493. [Google Scholar]
- Patrick, G.; McCune, L. Aluminium Anodizer Implements Zero Liquid Discharge. Products Finishing. 2016. Available online: http://www.pfonline.com/articles/aluminum-anodizer-implements-zero-liquid-discharge (accessed on 14 October 2018).
- Amer, S. Treating Metal Finishing Wastewater; AQUACHEM INC.: Denver, CO, USA, 1998; pp. 1–7. [Google Scholar]
- CIE Waste Water Treatment & Zero Liquid Discharge Plants. Available online: http://www.cieeng.com/site/new/industries/aluminum/extrusion/ (accessed on 14 October 2018).
- Pramanik, B.K.; Thangavadivel, K.; Shu, L.; Jegatheesan, V. A critical review of membrane crystallization for the purification of water and recovery of minerals. Rev. Environ. Sci. Biotechnol. 2016, 15, 411–439. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Drioli, E.; Macedonio, F. Evaluation of integrated microfiltration and membrane distillation/crystallization processes for produced water treatment. Desalination 2018, 434, 161–168. [Google Scholar] [CrossRef]
- Criscuoli, A.; Drioli, E. New Metrics for Evaluating the Performance of Membrane Operations in the Logic of Process Intensification. Ind. Eng. Chem. Res. 2007, 46, 2268–2271. [Google Scholar] [CrossRef]
- Ali, A.; Quist-jensen, C.A.; Macedonio, F.; Drioli, E. Application of Membrane Crystallization for Minerals’ Recovery from Produced Water. Membranes (Basel) 2015, 5, 772–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quist-Jensen, C.A.; Macedonio, F.; Conidi, C.; Cassano, A.; Aljlil, S.; Alharbi, O.A.; Drioli, E. Direct contact membrane distillation for the concentration of clarified orange juice. J. Food Eng. 2016, 187, 37–43. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Kargari, A. A Review on Applications of Membrane Distillation (MD) Process for Wastewater Treatment. J. Membr. Sci. Res. 2015, 1, 101–112. [Google Scholar]
- Khayet, M. Membranes and theoretical modeling of membrane distillation: A review. Adv. Colloid Interface Sci. 2011, 164, 56–88. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Sørensen, J.M.; Svenstrup, A.; Scarpa, L.; Carlsen, T.S.; Jensen, H.C.; Wybrandt, L.; Christensen, M.L. Membrane crystallization for phosphorus recovery and ammonia stripping from reject water from sludge dewatering process. Desalination 2018, 440, 156–160. [Google Scholar] [CrossRef]
- Lu, D.; Li, P.; Xiao, W.; He, G.; Jiang, X. Simultaneous Recovery and Crystallization Control of Saline Organic Wastewater by Membrane Distillation Crystallization. AIChE J. 2017, 63, 2187–2197. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Integrated membrane desalination systems with membrane crystallization units for resource recovery: A new approach for mining from the sea. Crystals 2016, 6, 36. [Google Scholar] [CrossRef]
- Ji, X.; Curcio, E.; Al Obaidani, S.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane distillation-crystallization of seawater reverse osmosis brines. Sep. Purif. Technol. 2010, 71, 76–82. [Google Scholar] [CrossRef]
- Li, W.; Van der Bruggen, B.; Luis, P. Integration of reverse osmosis and membrane crystallization for sodium sulphate recovery. Chem. Eng. Process. Process Intensif. 2014, 85, 57–68. [Google Scholar] [CrossRef]
- Curcio, E.; Ji, X.; Matin, A.; Barghi, S.; Di, G.; Fontananova, E.; Macleod, T.; Drioli, E. Hybrid nanofiltration-membrane crystallization system for the treatment of sulfate wastes. J. Membr. Sci. 2010, 360, 493–498. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Macedonio, F.; Horbez, D.; Drioli, E. Reclamation of sodium sulfate from industrial wastewater by using membrane distillation and membrane crystallization. Desalination 2017, 401, 112–119. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. Optimization of module length for continuous direct contact membrane distillation process. Chem. Eng. Process. Process Intensif. 2016, 110, 188–200. [Google Scholar] [CrossRef]
- Gill, W.N.; Wiley, D.E.; Fell, C.J.D.; Fane, A.G. Effect of viscosity on concentration polarization in ultrafiltration. AIChE J. 1988, 34, 1563–1567. [Google Scholar] [CrossRef]
- Ali, A.; Tsai, J.-H.; Tung, K.-L.; Drioli, E. On designing and optimization of continuous direct contact membrane distillation process. Desalination 2018, 426, 97–107. [Google Scholar] [CrossRef]
- Ali, A.; Quist-Jensen, C.A.; Macedonio, F.; Drioli, E. On designing of membrane thickness and thermal conductivity for large scale membrane distillation modules. J. Membr. Sci. Res. 2016, 2, 179–185. [Google Scholar]
- Rodriguez-Navarro, C.; Doehne, E.; Sebastian, E. How does sodium sulfate crystallize? Implications for the decay and testing of building materials. Cem. Concr. Res. 2000, 30, 1527–1534. [Google Scholar] [CrossRef] [Green Version]
- Naruse, H.; Tanaka, K.; Morikawa, H.; Marumo, F.; Mehrotra, B.N. Structure of Na2SO4 (I) at 693 K. Acta Crystallogr. Sect. B Struct. Sci. 1987, 43, 143–146. [Google Scholar] [CrossRef]
- Eyser, W.; Components, T. Grystal Ghemistry of the System NarSOn-KrSOn-KrCrOn-Na,CrOn and of the Glaserite Phase. Am. Mineral. 1973, 58, 736–747. [Google Scholar]
- Rasmussen, S.E.; Jørgensen, J.E.; Lundtoft, B. Structures and Phase Transitions of Na2SO4. J. Appl. Crystallogr. 1996, 29, 42–47. [Google Scholar] [CrossRef]
- Bouchrit, R.; Boubakri, A.; Mosbahi, T.; Hafiane, A.; Bouguecha, S.A.T. Membrane crystallization for mineral recovery from saline solution: Study case Na2SO4 crystals. Desalination 2017, 412, 1–12. [Google Scholar] [CrossRef]




| pH | 7.36 |
| Redox potential [mV] | 102 |
| Conductivity [mS/cm] | 37.61 |
| Dry matter [g/L] | 64.4 ± 0.4 |
| Inorganic matter [%] | 88.7 |
| Organic matter [%] | 11.3 |
| Supernatant [mg/L] | |
|---|---|
| Dry matter [g/L] | 43.3 |
| Inorganic matter [%] | 97.9 |
| Organic matter [%] | 2.1 |
| Conductivity [ms/cm] | 39 |
| Mn2+ | 3.648 |
| Fe2+ | 6 |
| Mg2+ | 7.813 |
| P3− | 90 |
| Na+ | 2302 |
| SO42− | 18990 |
| Material | Polypropylene |
| Type | Hollow fiber |
| No. of fibers | 19 |
| Length of fibers (cm) | 42 |
| Inner fiber diameter (mm) | 1.8 |
| Outer fiber diameter (mm) | 2.7 |
| Membrane thickness (mm) | 0.45 |
| Average pore size (μm) | 0.2 |
| Porosity (%) | 73 |
| Surface area (cm2) | 45.1 |
| Concentration [mg/L] | |
|---|---|
| Mn2+ | 0.0043 |
| Mg2+ | 0.076 |
| P3− | 0.0367 |
| Na+ | 16.6 |
| Experiment | Al | Fe | Mg | Mn | Na | P |
|---|---|---|---|---|---|---|
| 47 °C-21.6 L/h | nd | nd | 0.00042 | 0.0021 | 28.47 | nd |
| 56 °C-21.6 L/h | 0.065 | nd | 0.0233 | 0.0032 | 32.84 | 0.0053 |
| 38 °C-21.6 L/h | 0.012 | 0.00005 | 0.016 | 0.0023 | 26.21 | 0.0048 |
| 47 °C-30.7 L/h | 0.0032 | 0.0008 | 0.015 | 0.0026 | 30.53 | 0.0028 |
| 47 °C-14.8 L/h | 0.146 | 0.0027 | 0.045 | 0.0059 | 23.32 | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Hvid Jacobsen, J.; Casper Jensen, H.; Lykkegaard Christensen, M.; Quist-Jensen, C.A. Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Appl. Sci. 2019, 9, 287. https://doi.org/10.3390/app9020287
Ali A, Hvid Jacobsen J, Casper Jensen H, Lykkegaard Christensen M, Quist-Jensen CA. Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Applied Sciences. 2019; 9(2):287. https://doi.org/10.3390/app9020287
Chicago/Turabian StyleAli, Aamer, Josephine Hvid Jacobsen, Henriette Casper Jensen, Morten Lykkegaard Christensen, and Cejna Anna Quist-Jensen. 2019. "Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization" Applied Sciences 9, no. 2: 287. https://doi.org/10.3390/app9020287





