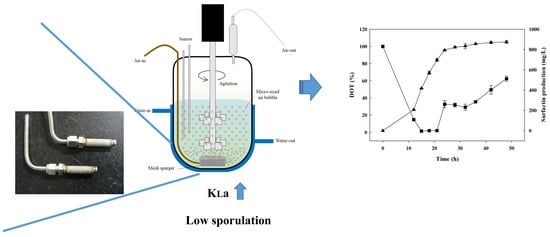
Effect of Oxygen Supply on Surfactin Production and Sporulation in Submerged Culture of Bacillus subtilis Y9
Abstract
:1. Introduction
2. Materials and Methods
2.1. Micro-Organisms and Media
2.2. Cultivation
2.3. Measurement of Surfactin
2.4. Determination of Dry Cell Weight
2.5. Determination of Reducing Sugars
2.6. Endospore Staining
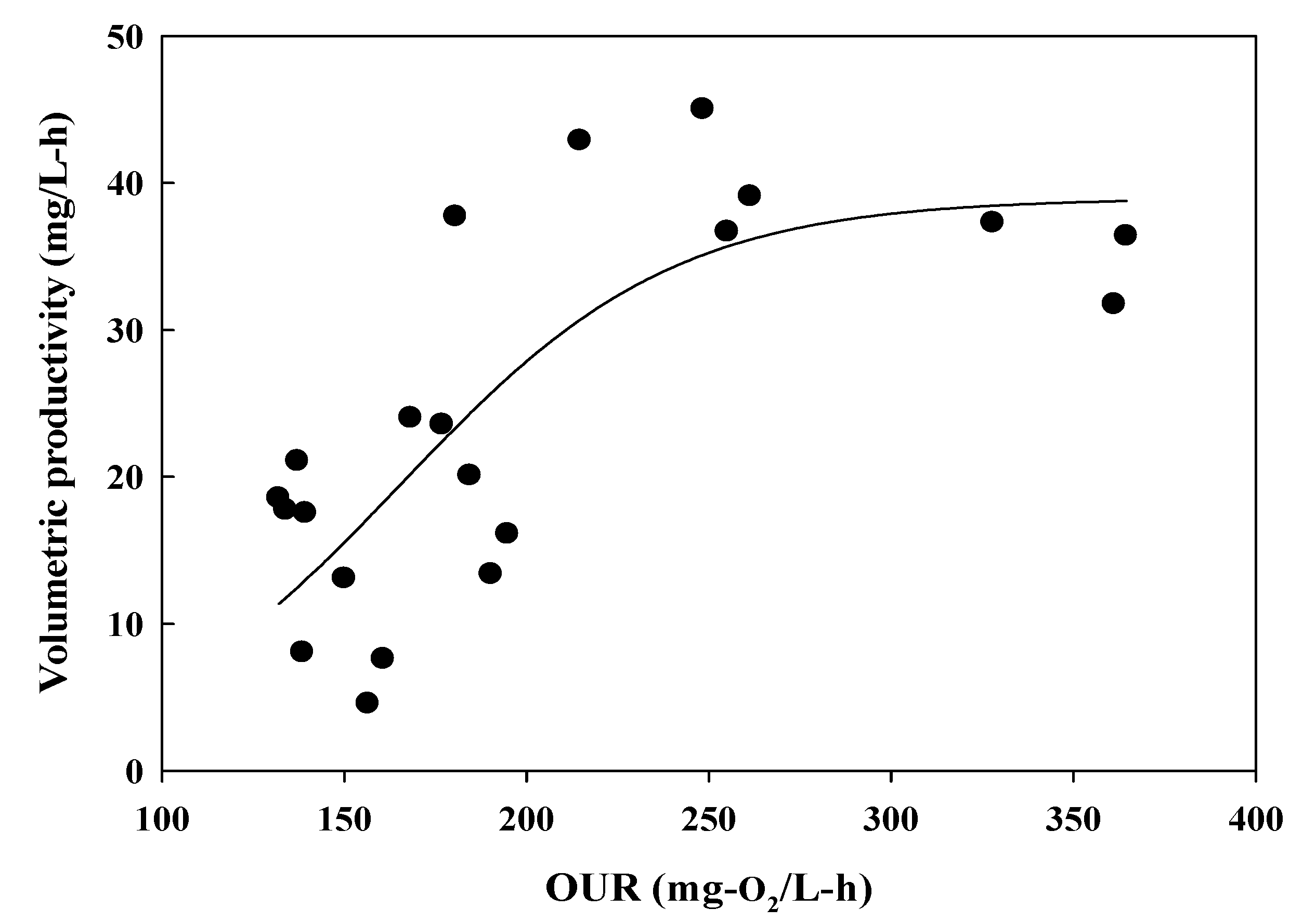
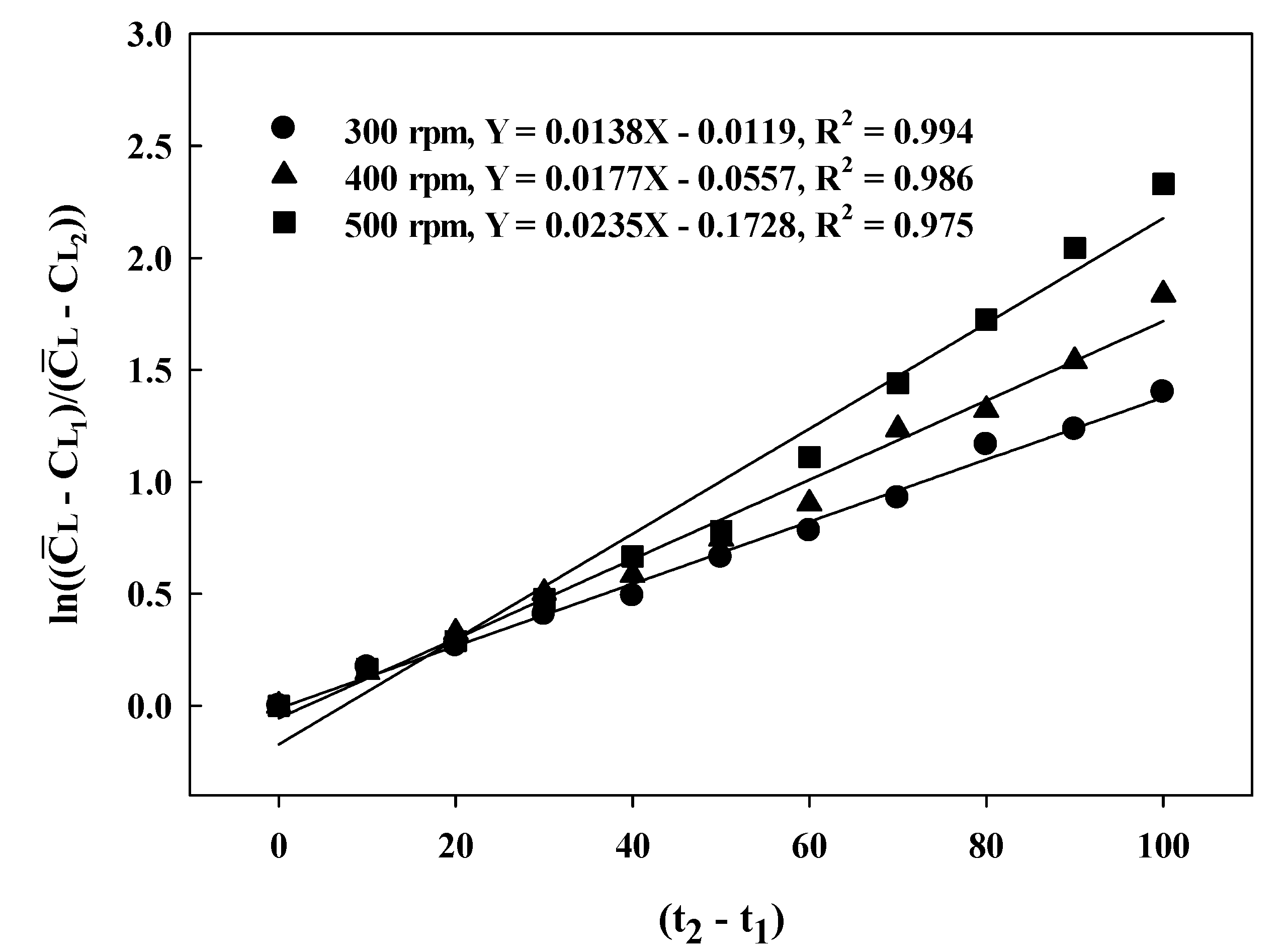
2.7. Determination of the Volumetric Mass Transfer Rate (KLa) and Oxygen-Uptake Rate (OUR)
2.8. Statistical Analysis
3. Results
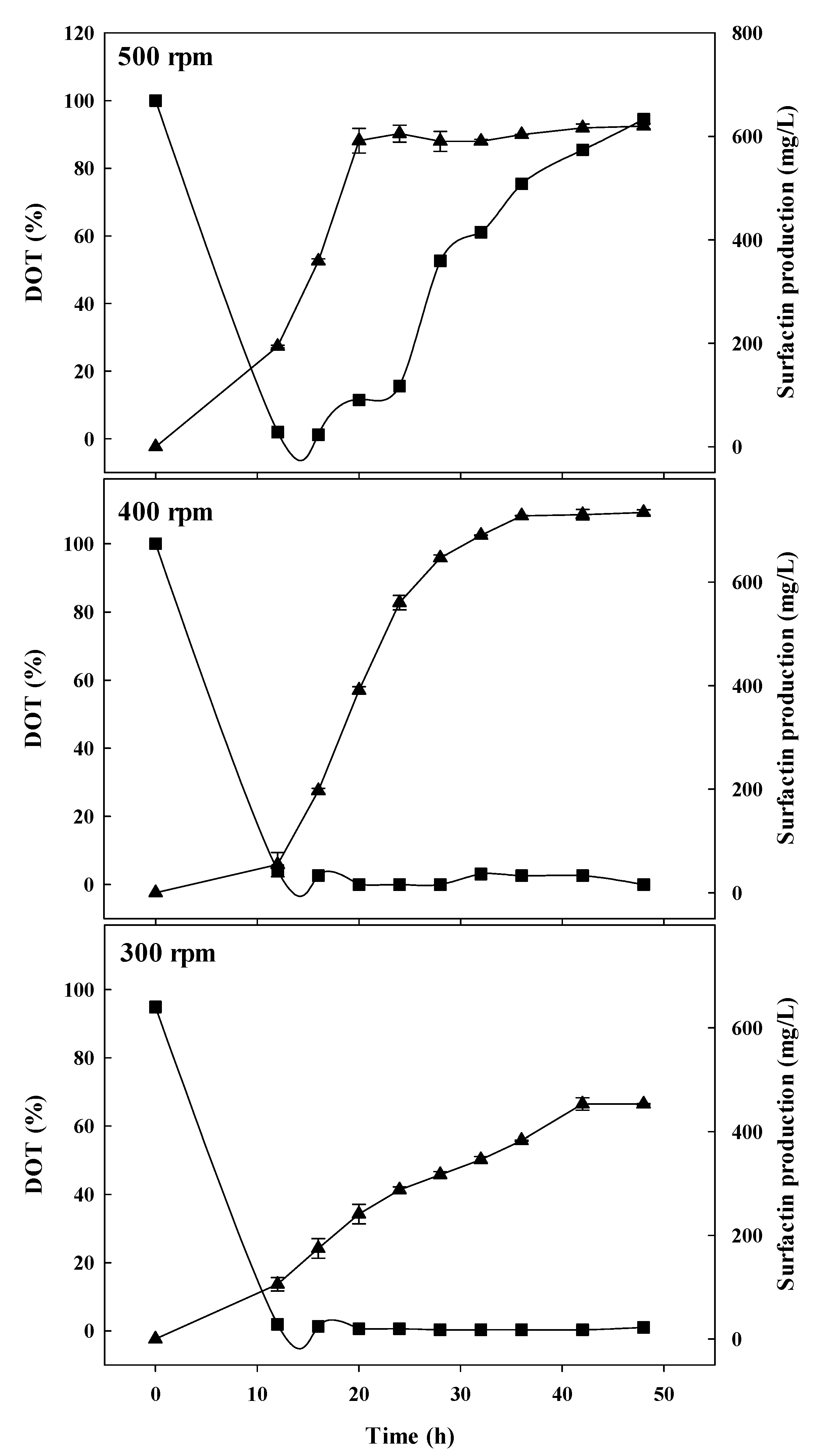
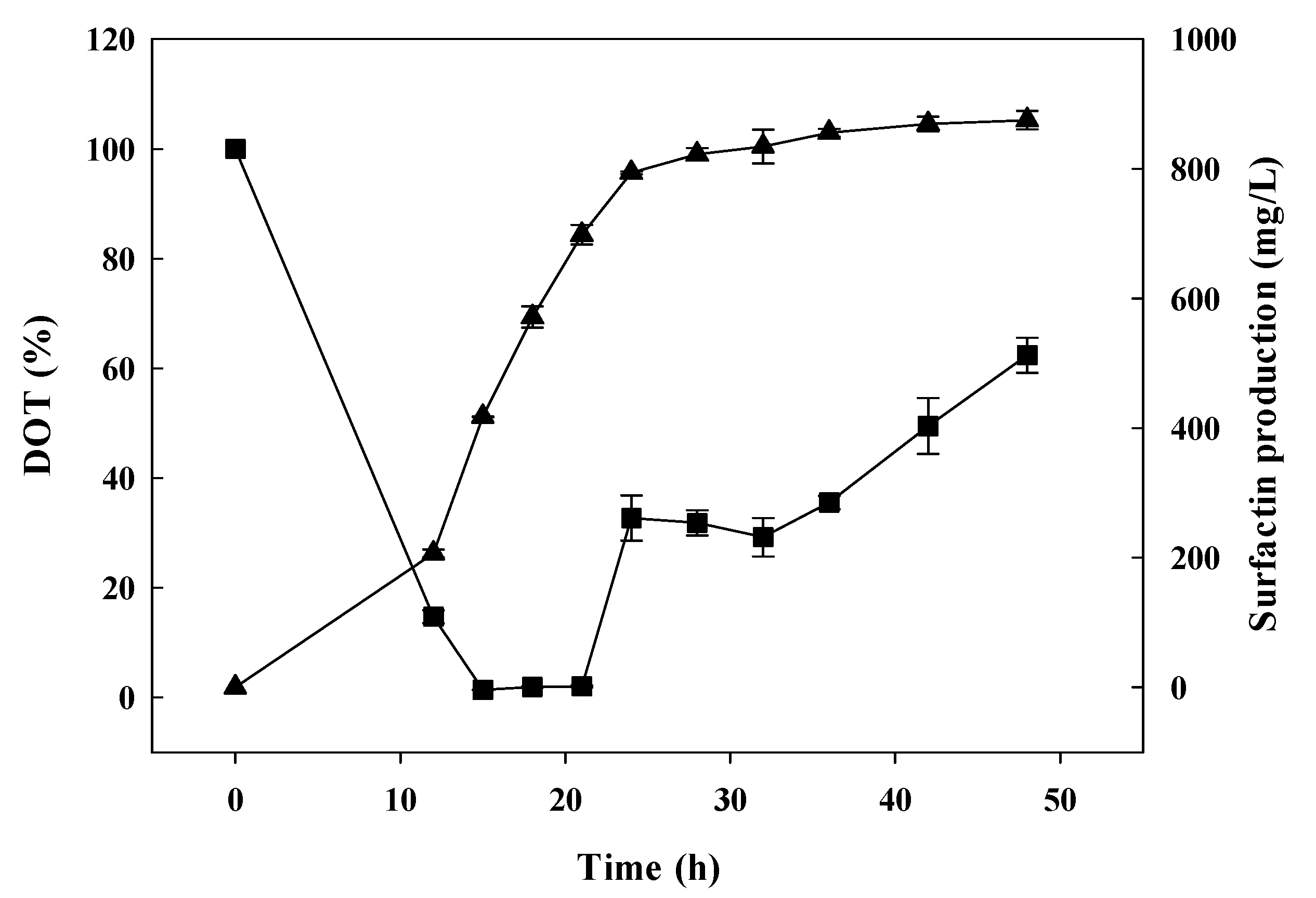
3.1. Batch-Fermentation Characteristics at Different Agitation Speeds
3.2. Spore Ratio at Different Agitation Speeds
3.3. Batch-Fermentation Characteristics with a Mesh-Type Sparger
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Banat, I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: A review. Bioresour. Technol. 1995, 51, 1–12. [Google Scholar] [CrossRef]
- Velioglu, Z.; Urek, R.O. Biosurfactant production by Pleurotus ostreatus in submerged and solid-state fermentation systems. Turkish J. Biol. 2015, 39, 160–166. [Google Scholar] [CrossRef]
- Raza, Z.A.; Rehman, A.; Hussain, M.T.; Masood, R.; ul Haq, A.; Saddique, M.T.; Javid, A.; Ahmad, N. Production of rhamnolipid surfactant and its application in bioscouring of cotton fabric. Carbohydr. Res. 2014, 391, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Seydlova, G.; Svobodova, J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. 2008, 3, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.Y.; Lim, D.J.; Noh, M.Y.; Kim, J.-C.; Kim, Y.C.; Kim, I.S. Characterization of biosurfactants as insecticidal metabolites produced by Bacillus subtilis Y9. Entomol. Res. 2017, 47, 55–59. [Google Scholar] [CrossRef]
- Das, P.; Mukherjee, S.; Sen, R. Genetic regulations of the biosynthesis of microbial surfactants: An overview. Biotechnol. Genet. Eng. Rev. 2008, 25, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, N.S.; Singhal, R.S. Surfactin—A review on biosynthesis, fermentation, purification and applications. Food Technol. Biotechnol. 2010, 48, 119–134. [Google Scholar]
- Fonseca, R.R.; Silva, A.J.R.; De Franc, F.P.; Cardoso, V.L.; Sérvulo, E.F. Optimizing carbon/nitrogen ratio for biosurfactant production by a Bacillus subtilis strain. Appl. Biochem. Biotechnol. 2007, 137, 471–486. [Google Scholar] [PubMed]
- Ghribi, D.; Ellouze-Chaabouni, S. Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol. Res. Int. 2011, 2011, 653654. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.S.; Wei, Y.H.; Chang, J.S. Enhanced production of surfactin from Bacillus subtilis by addition of solid carriers. Biotechnol. Prog. 2005, 21, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Hussey, M.A.; Zayaitz, A. Endospore Stain Protocol; American Society for Microbiology: Wasinghton, DC, USA, 2012. [Google Scholar]
- Bandyopadhyay, B.; Humphrey, A.E. Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Biotechnol. Bioeng. 1967, 9, 533–544. [Google Scholar] [CrossRef]
- Avignone-Rossa, C.; Arcas, J.; Mignone, C. Bacillus thuringiensis growth, sporulation and δ-endotoxin production in oxygen limited and non-limited cultures. World J. Microbiol. Biotechnol. 1992, 8, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Kraemer-Schafhalter, A.; Moser, A. Kinetic study of Bacillus thuringiensis var. israelensis in lab-scale batch process. Bioprocess Eng. 1996, 14, 139–144. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.H.; Roh, Y.H.; Seong, B.L.; Shin, C.S. Optimization of enterokinase fermentation using a recombinant Saccharomyces cerevisiae. Process Biochem. 2005, 40, 717–722. [Google Scholar] [CrossRef]
- Ardestani, F.; Fatemi, S.S.; Yakhchali, B.; Hosseyni, S.M.; Najafpour, G. Evaluation of mycophenolic acid production by Penicillium brevicompactum MUCL 19011 in batch and continuous submerged cultures. Biochem. Eng. J. 2010, 50, 99–103. [Google Scholar] [CrossRef]
- Yeh, M.S.; Wei, Y.H.; Chang, J.S. Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Process Biochem. 2006, 41, 1799–1905. [Google Scholar] [CrossRef]
- Boniolo, F.S.; Rodrigues, R.C.; Prata, A.M.R.; López, M.L.; Jacinto, T.; da Silveira, M.M.; Berbert-Molina, M.A. Oxygen supply in Bacillus thuringiensis fermentations: Bringing new insights on their impact on sporulation and δ-endotoxin production. Appl. Microbiol. Biotechnol. 2012, 94, 625–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghribi, D.; Zouari, N.; Trigui, W.; Jaoua, S. Use of sea water as salts source in starch- and soya bean-based media, for the production of Bacillus thuringiensis bioinsecticides. Process Biochem. 2007, 42, 374–378. [Google Scholar] [CrossRef]
- Yezza, A.; Tyagi, R.D.; Valéro, J.R.; Surampalli, R.Y. Production of Bacillus thuringiensis-based biopesticides in batch and fed batch cultures using wastewater sludge as a raw material. J. Chem. Technol. Biotechnol. 2005, 80, 502–510. [Google Scholar] [CrossRef]
- Flores, E.R.; Pérez, F.; de la Torre, M. Scale-up of Bacillus thuringiensis fermentation based on oxygen transfer. J. Ferment. Bioeng. 1997, 83, 561–564. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.; Kim, H.M.; Chun, H.H.; Hwang, I.M.; Lee, J.-H.; Kim, J.-C.; Kim, I.S.; Park, H.W. Effect of Oxygen Supply on Surfactin Production and Sporulation in Submerged Culture of Bacillus subtilis Y9. Appl. Sci. 2018, 8, 1660. https://doi.org/10.3390/app8091660
Ha S, Kim HM, Chun HH, Hwang IM, Lee J-H, Kim J-C, Kim IS, Park HW. Effect of Oxygen Supply on Surfactin Production and Sporulation in Submerged Culture of Bacillus subtilis Y9. Applied Sciences. 2018; 8(9):1660. https://doi.org/10.3390/app8091660
Chicago/Turabian StyleHa, Sanghyun, Ho Myeong Kim, Ho Hyun Chun, In Min Hwang, Jong-Hee Lee, Jin-Cheol Kim, In Seon Kim, and Hae Woong Park. 2018. "Effect of Oxygen Supply on Surfactin Production and Sporulation in Submerged Culture of Bacillus subtilis Y9" Applied Sciences 8, no. 9: 1660. https://doi.org/10.3390/app8091660