Electrospun Nanomaterials Implementing Antibacterial Inorganic Nanophases
Abstract
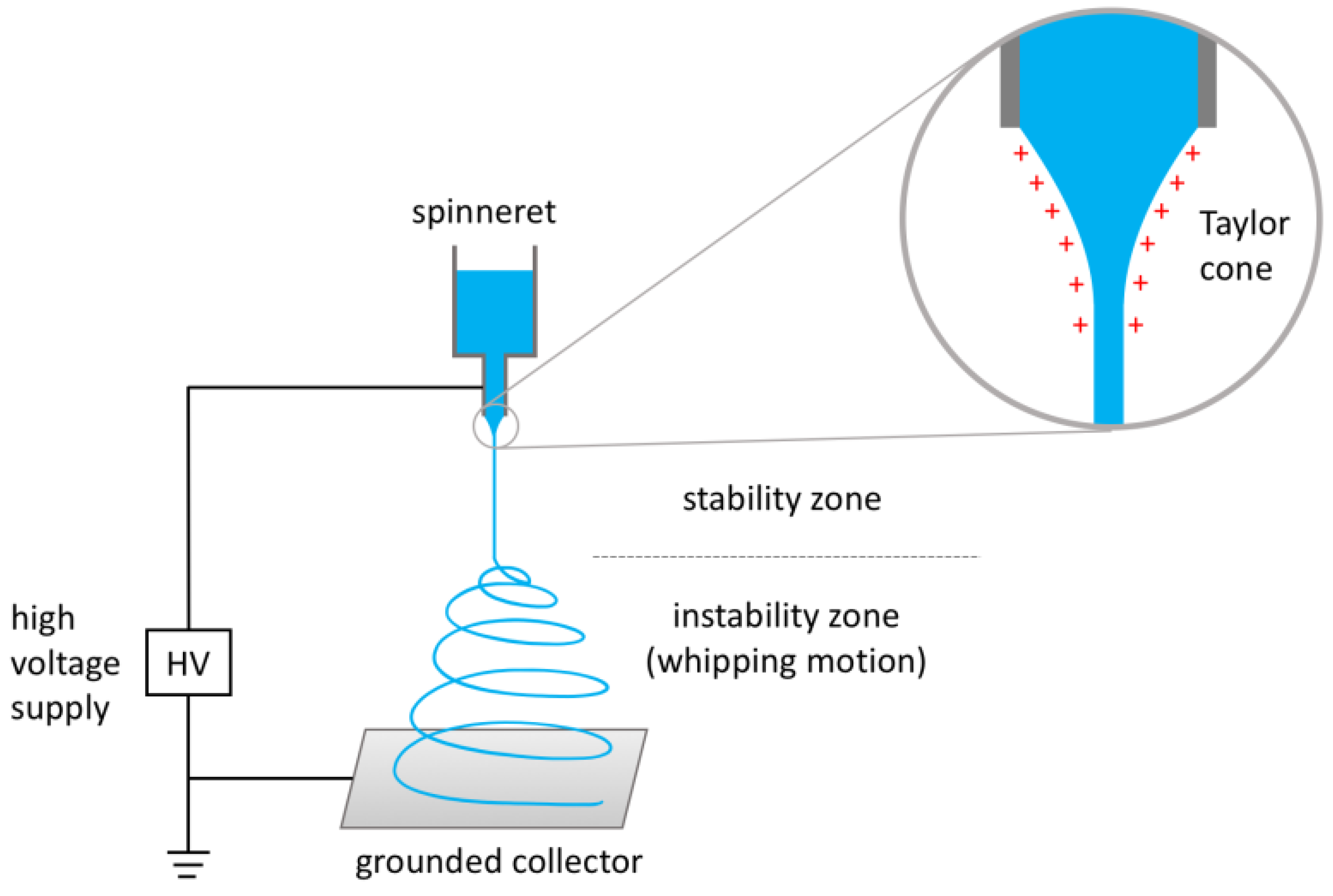
:1. Electrospinning: Generalities
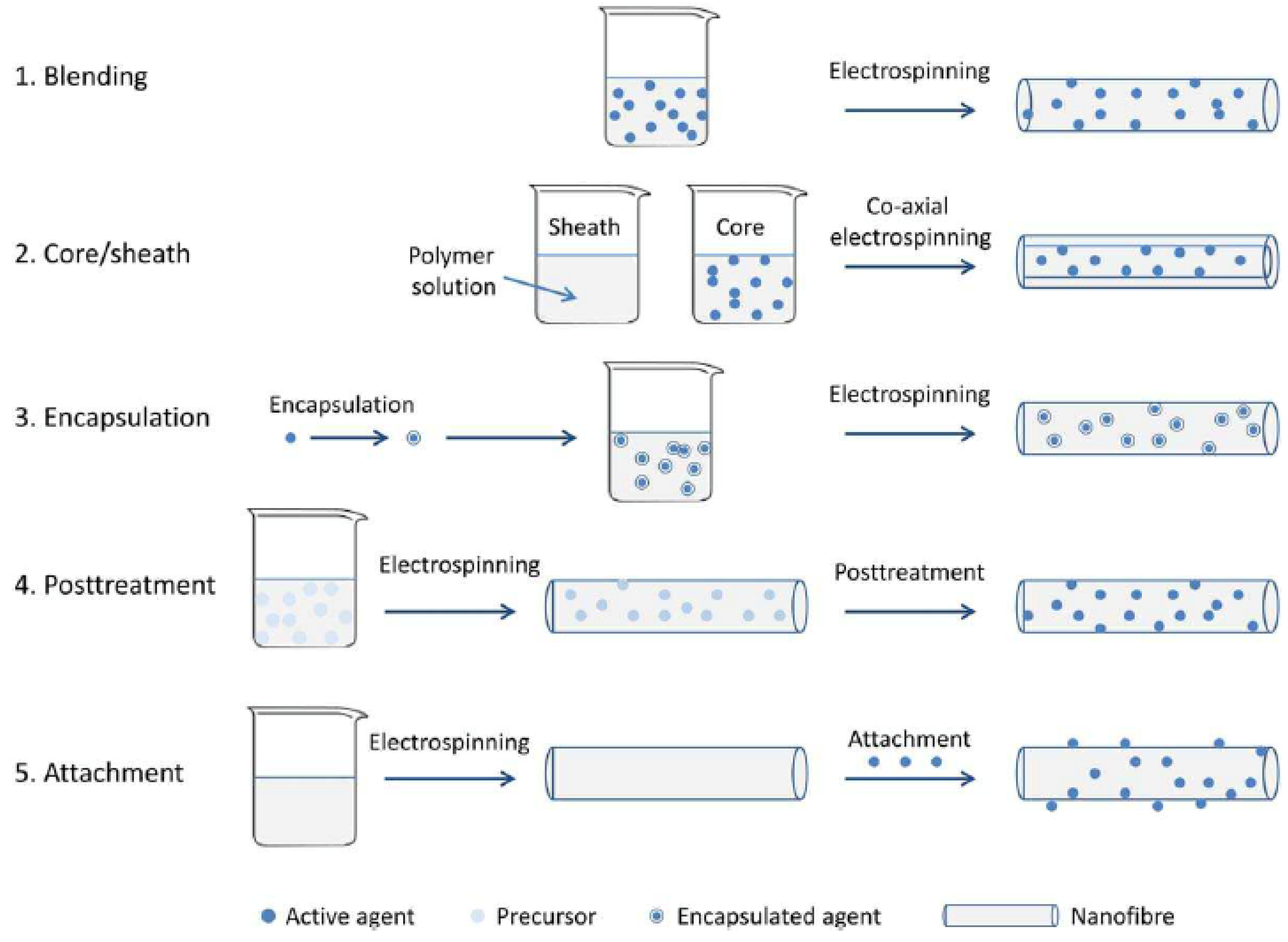
2. Nanoantimicrobials
3. Electrospun (Nano)Materials Implementing Antibacterial Properties
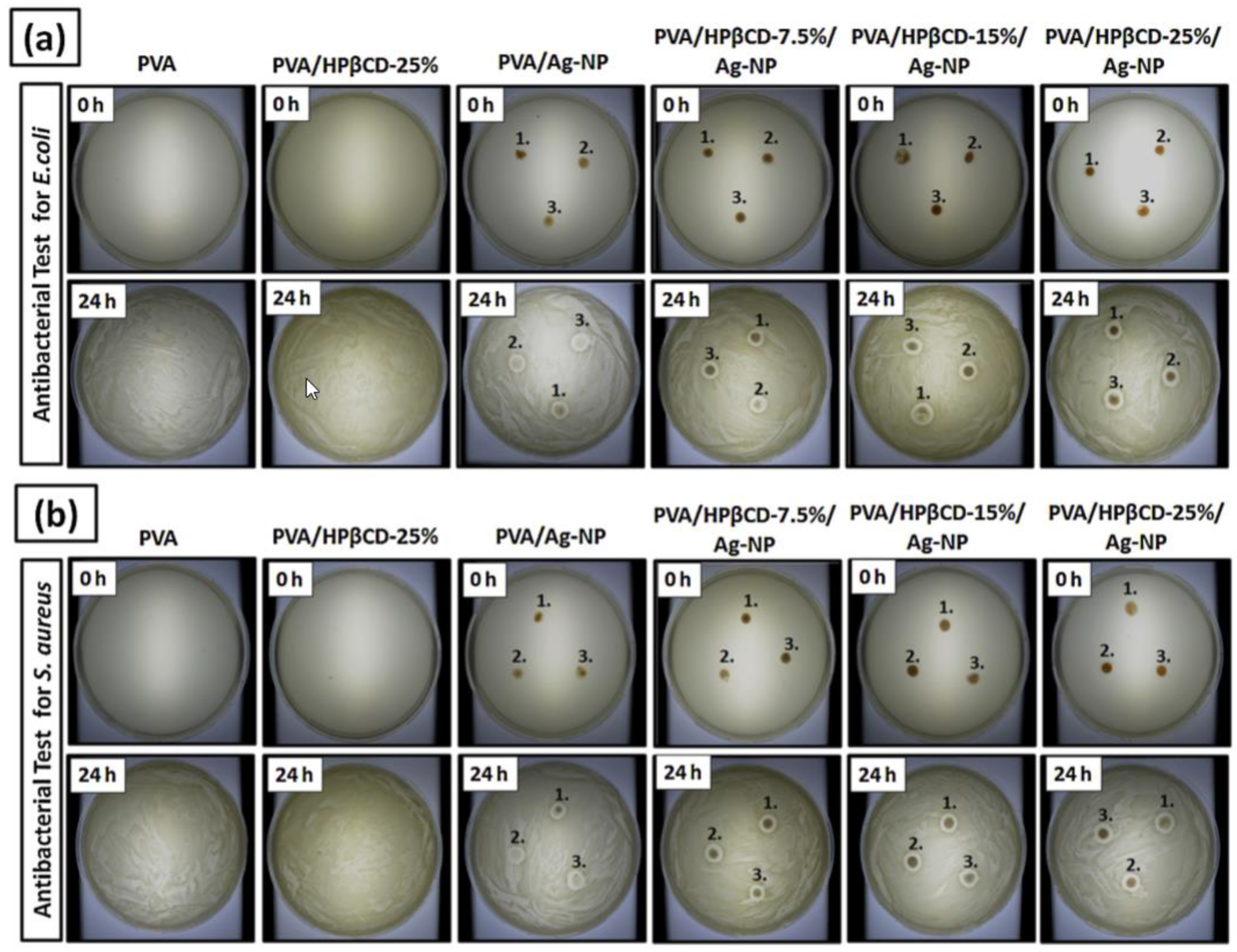
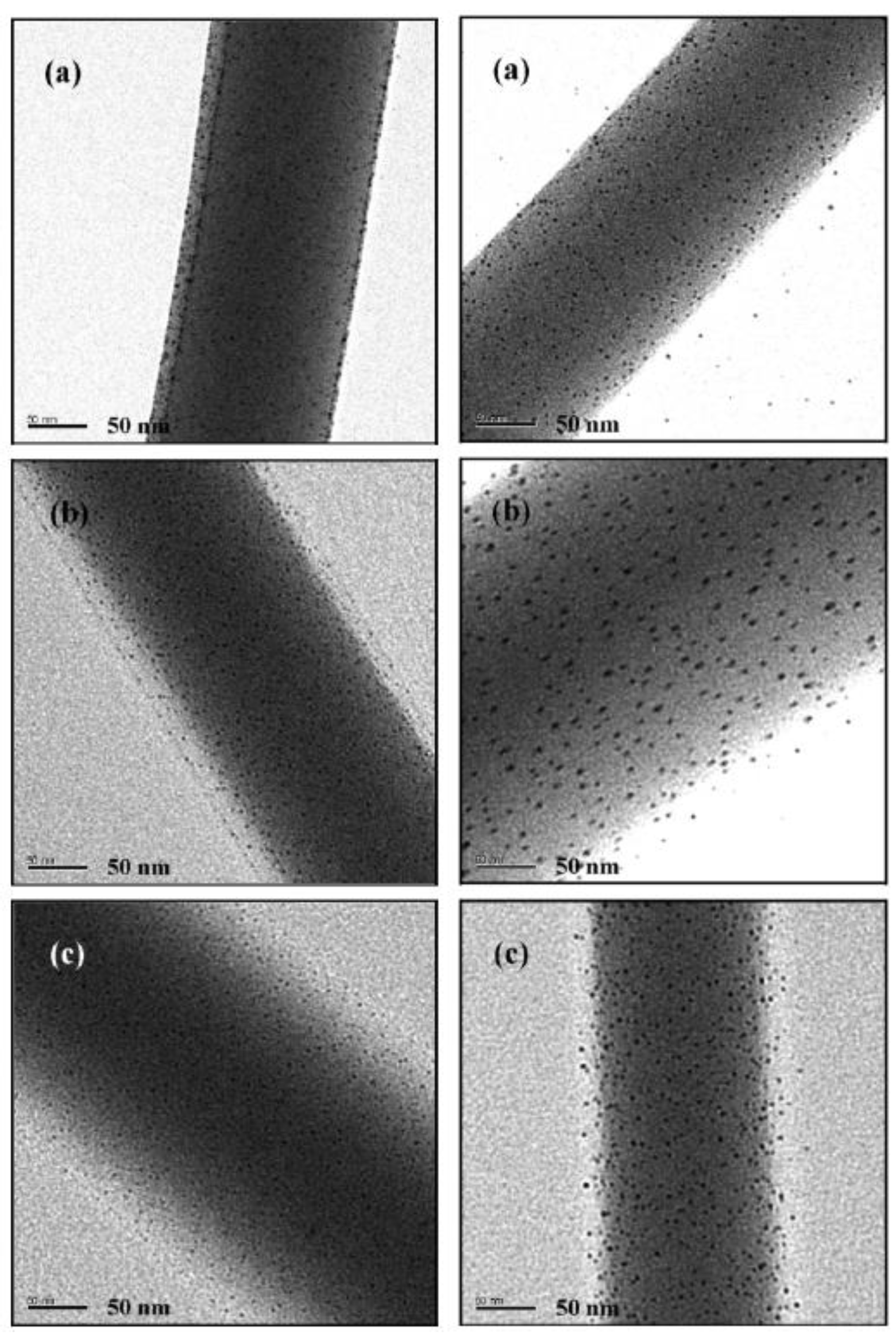
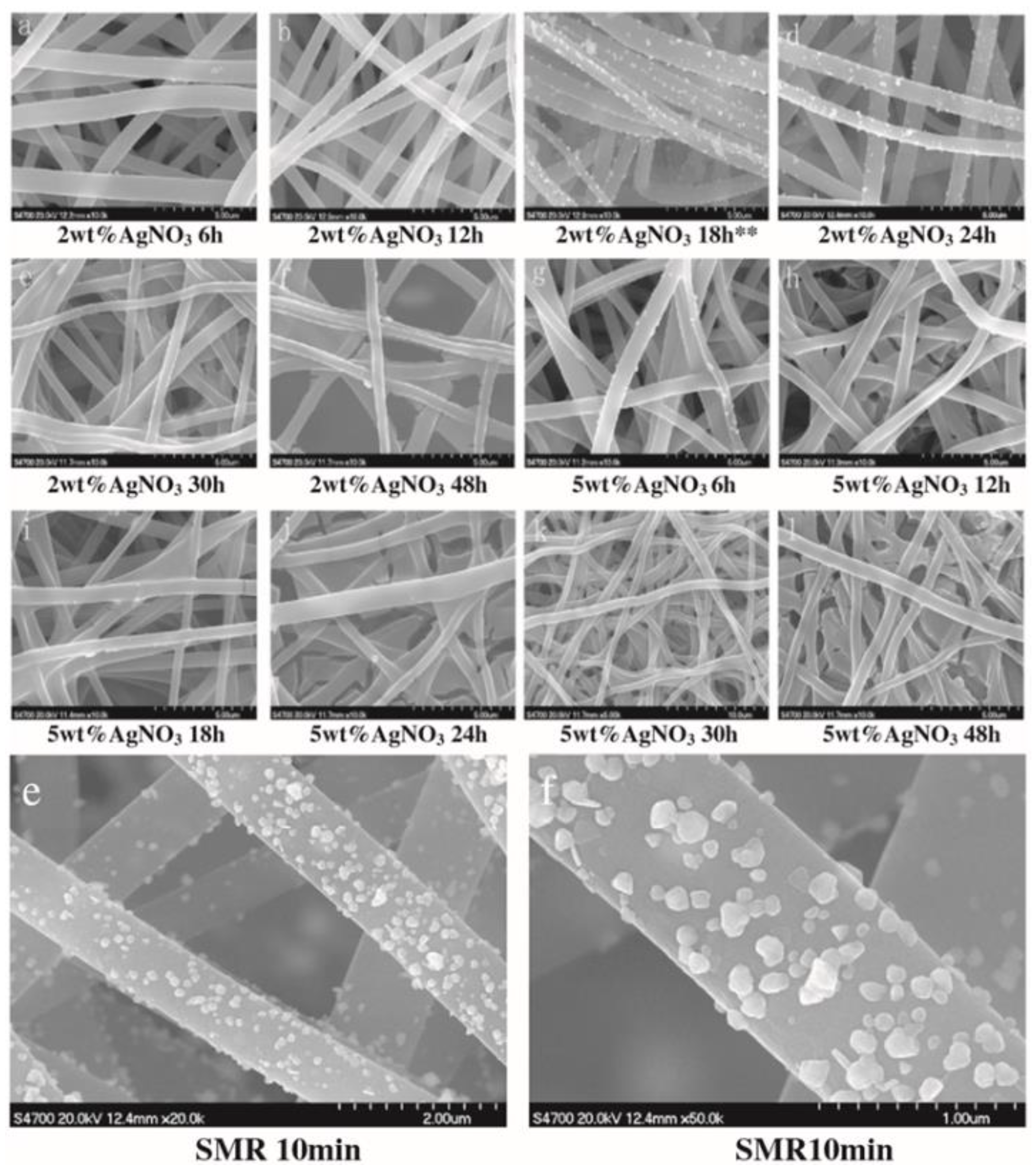
Inclusion of Metal Nanophases as Biocides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Cooley, J.F. Apparatus for Electrically Dispersing Fluids. U.S. Patent 692,631A, 6 October 1899. [Google Scholar]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Anton, F. Artificial Thread and Method of Producing Same. U.S. Patent No. 2,187,306, 28 July 1937. [Google Scholar]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. U.S. Patent 1,975,504, 2 October 1934. [Google Scholar]
- Srinivasan, G.; Reneker, D.H. Structure and morphology of small diameter electrospun aramid fibers. Polym. Int. 1995, 36, 195–201. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Taylor, G. Electrically Driven Jets. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1969, 313, 453–475. [Google Scholar] [CrossRef]
- Taylor, G. Disintegration of Water Drops in an Electric Field. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1964, 280, 383–397. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Guerrero, J.; Rivero, J.; Gundabala, V.R.; Perez-Saborid, M.; Fernandez-Nieves, A. Whipping of electrified liquid jets. Proc. Natl. Acad. Sci. USA 2014, 111, 13763–13767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing: Singapore, 2005; ISBN 981-256-415-2, 981-256-454-3. [Google Scholar]
- Seyedmahmoud, R.; Rainer, A.; Mozetic, P.; Maria Giannitelli, S.; Trombetta, M.; Traversa, E.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly (l-lactide) scaffolds for tissue engineering—PART 1: Design of experiments. J. Biomed. Mater. Res. A 2015, 103, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Seyedmahmoud, R.; Mozetic, P.; Rainer, A.; Giannitelli, S.M.; Basoli, F.; Trombetta, M.; Rinaldi, A. A primer of statistical methods for correlating parameters and properties of electrospun poly (l-lactide) scaffolds for tissue engineering—PART 2: Regression. J. Biomed. Mater. Res. A 2015, 103, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Zander, N.E. Hierarchically structured electrospun fibers. Polymers 2013, 5, 19–44. [Google Scholar] [CrossRef]
- Boland, E.D.; Wnek, G.E.; Simpson, D.G.; Pawlowski, K.J.; Bowlin, G.L. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly (glycolic acid) electrospinning. J. Macromol. Sci. A 2001, 38, 1231–1243. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Theron, A.; Zussman, E.; Yarin, A.L. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology 2001, 12, 384. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Chen, L.; Li, H.; Yin, T.; Wang, B.; Yu, Q. Electrospun nanofiber meshes with tailored architectures and patterns as potential tissue-engineering scaffolds. Biofabrication 2009, 1, 015001. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound core–shell polymer nanofibers by co-electrospinning. Adv. Mater. 2003, 15, 1929–1932. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Recent advances in multiaxial electrospinning for drug delivery. Eur. J. Pharm. Biopharm. 2017, 112, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.H.; Xu, L.; Yu, J.Y. The principle of bubble electrospinning and its experimental verification. J. Polym. Eng. 2008, 28, 55–66. [Google Scholar] [CrossRef]
- He, J.H.; Liu, Y. Control of bubble size and bubble number in bubble electrospinning. Comput. Math. Appl. 2012, 64, 1033–1035. [Google Scholar] [CrossRef]
- Son, Y.J.; Kim, W.J.; Yoo, H.S. Therapeutic applications of electrospun nanofibers for drug delivery systems. Arch. Pharm. Res. 2014, 37, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, K.; Sang, Q.; Williams, G.R.; Wu, J.; Wang, H.; Zhu, L.M. A thermosensitive drug delivery system prepared by blend electrospinning. Colloid Surf. B 2017, 159, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Suja, P.S.; Reshmi, C.R.; Sagitha, P.; Sujith, A. Electrospun nanofibrous membranes for water purification. Polym. Rev. 2017, 57, 467–504. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2017, 77, 69–94. [Google Scholar] [CrossRef]
- Cavaliere, S. (Ed.) Electrospinning for Advanced Energy and Environmental Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Li, Z.; Zhang, J.W.; Yu, L.G.; Zhang, J.W. Electrospun porous nanofibers for electrochemical energy storage. J. Mater. Sci. 2017, 52, 6173–6195. [Google Scholar] [CrossRef]
- Sapountzi, E.; Braiek, M.; Chateaux, J.F.; Jaffrezic-Renault, N.; Lagarde, F. Recent Advances in Electrospun Nanofiber Interfaces for Biosensing Devices. Sensors 2017, 17, 1887. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, M.; Yu, J.; Sun, G. Gas sensors based on electrospun nanofibers. Sensors 2009, 9, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Luzio, A.; Canesi, E.V.; Bertarelli, C.; Caironi, M. Electrospun polymer fibers for electronic applications. Materials 2014, 7, 906–947. [Google Scholar] [CrossRef] [PubMed]
- Zewe, J.W.; Steach, J.K.; Olesik, S.V. Electrospun fibers for solid-phase microextraction. Anal. Chem. 2010, 82, 5341–5348. [Google Scholar] [CrossRef] [PubMed]
- Moheman, A.; Alam, M.S.; Mohammad, A. Recent trends in electrospinning of polymer nanofibers and their applications in ultra thin layer chromatography. Adv. Colloid Interface Sci. 2016, 229, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.; Matlock-Colangelo, L.; Xiang, C.; Asiello, P.J.; Baeumner, A.J.; Frey, M.W. Electrospun nanofibers for microfluidic analytical systems. Polymer 2011, 52, 3413–3421. [Google Scholar] [CrossRef]
- Wallin, P.; Zandén, C.; Carlberg, B.; Hellström Erkenstam, N.; Liu, J.; Gold, J. A method to integrate patterned electrospun fibers with microfluidic systems to generate complex microenvironments for cell culture applications. Biomicrofluidics 2012, 6, 024131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannitelli, S.M.; Costantini, M.; Basoli, F.; Trombetta, M.; Rainer, A. Electrospinning and microfluidics: An integrated approach for tissue engineering and cancer. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices; Woodhead Publishing: Sawston, UK, 2018; pp. 139–155. [Google Scholar] [CrossRef]
- Cioffi, N.; Rai, M. Nano-Antimicrobials: Progress and Prospects, 1st ed.; Springer: Berlin, Germany, 2012; p. 504. ISBN 978-3-642-24427-8. [Google Scholar]
- World Health Organization. Available online: http://www.who.int/antimicrobial-resistance/en/ (accessed on 31 July 2018).
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Natan, M.; Banin, E. From Nano to Micro: Using nanotechnology to combat microorganisms and their multidrug resistance. FEMS Microbiol. Rev. 2017, 41, 302–322. [Google Scholar] [CrossRef] [PubMed]
- Sportelli, M.C.; Picca, R.A.; Cioffi, N. Recent advances in the synthesis and characterization of nano-antimicrobials. TRAC-Trend Anal. Chem. 2016, 84, 131–138. [Google Scholar] [CrossRef]
- PEN. The Project on Emerging Nanotechnologies. Available online: http://www.nanotechproject.org/ (accessed on 31 July 2018).
- Hochmannova, L.; Vytrasova, J. Photocatalytic and antimicrobial effects of interior paints. Prog. Org. Coat. 2010, 67, 1–5. [Google Scholar] [CrossRef]
- Eleftheriadou, M.; Pyrgiotakis, G.; Demokritou, P. Nanotechnology to the rescue: Using nano-enabled approaches in microbiological food safety and quality. Curr. Opin. Biotechnol. 2017, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuz, M. Application of Antimicrobial Nano-Materials on Leather: A Review. J. Soc. Leather Technol. Chem. 2017, 101, 173–178. [Google Scholar]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.A.S.; Weir, M.D.; Zhou, X.D.; Xu, H.H.K. Developing a new generation of antimicrobial and bioactive dental resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Windler, L.; Height, M.; Nowack, B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013, 53, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Pachuau, L. Recent developments in novel drug delivery systems for wound healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Ivask, A.; Juganson, K.; Bondarenko, O.; Mortimer, M.; Aruoja, V.; Kasemets, K.; Kahru, A. Mechanisms of toxic action of Ag, ZnO and CuO nanoparticles to selected ecotoxicological test organisms and mammalian cells in vitro: A comparative review. Nanotoxicology 2014, 8, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Sabbatini, L.; Zambonin, P.G.; Tantillo, G.; Ghibelli, L.; D’Alessio, M.; Bleve-Zacheo, T.; Traversa, E. Antifungal activity of polymer-based copper nanocomposite coatings. Appl. Phys. Lett. 2004, 85, 2417–2419. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Tütüncü, E.; Picca, R.A.; Valentini, M.; Valentini, A.; Kranz, C.; Mizaikoff, B.; Barth, H.; Cioffi, N. Inhibiting P. fluorescens biofilms with fluoropolymer-embedded silver nanoparticles: An in-situ spectroscopic study. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Picca, R.A.; Paladini, F.; Sportelli, M.C.; Pollini, M.; Giannossa, L.C.; Di Franco, C.; Mangone, A.; Valentini, A.; Cioffi, N. Combined approach for the development of efficient and safe nanoantimicrobials: The case of nanosilver-modified polyurethane foams. ACS Biomater. Sci. Eng. 2016, 3, 1417–1425. [Google Scholar] [CrossRef]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Al-Badri, Z.M.; Som, A.; Lyon, S.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Investigating the effect of increasing charge density on the hemolytic activity of synthetic antimicrobial polymers. Biomacromolecules 2008, 9, 2805–2810. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Walker, S.G.; Parker, K.A.; Sampson, N.S. Antibacterial studies of cationic polymers with alternating, random, and uniform backbones. ACS Chem. Biol. 2011, 6, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.M.; De Kwaadsteniet, M.; Dicks, L.M.T. The ability of nisin F to control Staphylococcus aureus infection in the peritoneal cavity, as studied in mice. Lett. Appl. Microbiol. 2010, 51, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Perron, G.G.; Zasloff, M.; Bell, G. Experimental evolution of resistance to an antimicrobial peptide. Proc. R. Soc. Lond. B Biol. 2006, 273, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkoun, M.; Daigle, F.; Holley, R.A.; Heuzey, M.C.; Ajji, A. Chitosan-based nanofibers as bioactive meat packaging materials. Packag. Technol. Sci. 2018, 31, 185–195. [Google Scholar] [CrossRef]
- Vineis, C.; Varesano, A. Natural polymer-based electrospun fibers for antibacterial uses. In Electrofluidodynamic Technologies (EFDTs) for Biomaterials and Medical Devices; Woodhead Publishing: Sawston, UK, 2018; pp. 275–294. [Google Scholar] [CrossRef]
- Bshena, O.; Heunis, T.D.; Dicks, L.M.; Klumperman, B. Antimicrobial fibers: Therapeutic possibilities and recent advances. Future Med. Chem. 2011, 3, 1821–1847. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Bach Truong, Y.; Zhu, Y.; Louis Kyratzis, I. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, G.; Gupta, B.S.; Tonelli, A.E. Poly (ε-caprolactone) nanowebs functionalized with α-and γ-cyclodextrins. Biomacromolecules 2014, 15, 4122–4133. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; San Keskin, N.O.; Kusku, S.I.; Durgun, E.; Uyar, T. Fast-dissolving, prolonged release, and antibacterial cyclodextrin/limonene-inclusion complex nanofibrous webs via polymer-free electrospinning. J. Agric. Food Chem. 2016, 64, 7325–7334. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Umu, O.C.; Tekinay, T.; Uyar, T. Antibacterial electrospun nanofibers from triclosan/cyclodextrin inclusion complexes. Colloid Surf. B 2014, 116, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayaci, F.; Umu, O.C.; Tekinay, T.; Uyar, T. Antibacterial electrospun poly (lactic acid) (PLA) nanofibrous webs incorporating triclosan/cyclodextrin inclusion complexes. J. Agric. Food Chem. 2013, 61, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, I.J.H.; Paladino, E.; Brozio, S.; Passarelli, M.K.; Moug, S.; Black, R.A.; Lamprou, D.A. Fabrication and characterisation of drug-loaded electrospun polymeric nanofibers for controlled release in hernia repair. Int. J. Pharm. 2017, 517, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.F.; Woodrow, K.A. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. J. Mech. Behav. Biomed. 2017, 65, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Gimeno-Alcaniz, J.V.; Ocio, M.J.; Lagaron, J.M. Comparative performance of electrospun collagen nanofibers cross-linked by means of different methods. ACS Appl. Mater. Interfaces 2008, 1, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, A.; Shaibani, P.M.; Etayash, H.; Kaur, K.; Thundat, T. Sustained drug release and antibacterial activity of ampicillin incorporated poly (methyl methacrylate)–nylon6 core/shell nanofibers. Polymer 2013, 54, 2699–2705. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Wang, H.; He, C.; Mo, X. Fabrication and characterization of biodegradable nanofibrous mats by mix and coaxial electrospinning. J. Mater. Sci. Mater. Med. 2009, 20, 2285. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; Gardella, L.; Forouharshad, M.; Pellegrino, T.; Monticelli, O. Star poly (ε-caprolactone)-based electrospun fibers as biocompatible scaffold for doxorubicin with prolonged drug release activity. Colloid Surf. B 2018, 161, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, F.; Huang, Y.; Fang, Y.; Shen, M.; Zhu, M.; Shi, X. Encapsulation of amoxicillin within laponite-doped poly (lactic-co-glycolic acid) nanofibers: Preparation, characterization, and antibacterial activity. ACS Appl. Mater. Interfaces 2012, 4, 6393–6401. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wang, S.; Wen, S.; Shen, M.; Zhu, M.; Shi, X. Characterization and antibacterial activity of amoxicillin-loaded electrospun nano-hydroxyapatite/poly (lactic-co-glycolic acid) composite nanofibers. Biomaterials 2013, 34, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. Biotechnol. 2011, 89, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Isquith, A.J.; Abbott, E.A.; Walters, P.A. Surface-bonded antimicrobial activity of an organosilicon quaternary ammonium chloride. Appl. Microbiol. 1972, 24, 859–863. [Google Scholar] [PubMed]
- Walters, P.A.; Abbott, E.A.; Isquith, A.J. Algicidal activity of a surface-bonded organosilicon quaternary ammonium chloride. Appl. Microbiol. 1973, 25, 253–256. [Google Scholar] [PubMed]
- Isquith, A.J.; McCollum, C.J. Surface kinetic test method for determining rate of kill by an antimicrobial solid. Appl. Environ. Microb. 1978, 36, 700–704. [Google Scholar]
- Nakagawa, Y.; Hayashi, H.; Tawaratani, T.; Kourai, H.; Horie, T.; Shibasaki, I. Disinfection of water with quaternary ammonium salts insolubilized on a porous glass surface. Appl. Environ. Microb. 1984, 47, 513–518. [Google Scholar]
- Jaeger, W.; Bohrisch, J.; Laschewsky, A. Synthetic polymers with quaternary nitrogen atoms—Synthesis and structure of the most used type of cationic polyelectrolytes. Prog. Polym. Sci. 2010, 35, 511–577. [Google Scholar] [CrossRef]
- Koqiso, M.; Yoshida, K.; Yase, K.; Shimizu, T. One-dimensional organization of copper nanoparticles by chemical reduction of lipid-copper hybrid nanofibers. Chem. Commun. 2002, 10, 2492. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Quirós, J.; Borges, J.P.; Boltes, K.; Rodea-Palomares, I.; Rosal, R. Antimicrobial electrospun silver-, copper-and zinc-doped polyvinylpyrrolidone nanofibers. J. Hazard. Mater. 2015, 299, 298–305. [Google Scholar] [CrossRef] [PubMed]
- SCOPUS Database. Available online: http://www.scopus.com (accessed on 31 July 2018).
- Jatoi, A.W.; Jo, Y.K.; Lee, H.; Oh, S.G.; Hwang, D.S.; Khatri, Z.; Kim, I.S. Antibacterial efficacy of poly (vinyl alcohol) composite nanofibers embedded with silver-anchored silica nanoparticles. J. Biomed. Mater. Res. B 2018, 106, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hua, D.; Pan, H.; Wang, F.; Manshian, B.; Soenen, S.J.; Huang, C. Green electrospun and crosslinked poly (vinyl alcohol)/poly (acrylic acid) composite membranes for antibacterial effective air filtration. J. Colloid Interface Sci. 2018, 511, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.J.; Jeon, H.J.; Kim, J.H.; Youk, J.H. A study on the preparation of poly (vinyl alcohol) nanofibers containing silver nanoparticles. Synth. Met. 2007, 157, 454–459. [Google Scholar] [CrossRef]
- Celebioglu, A.; Aytac, Z.; Umu, O.C.; Dana, A.; Tekinay, T.; Uyar, T. One-step synthesis of size-tunable Ag nanoparticles incorporated in electrospun PVA/cyclodextrin nanofibers. Carbohyd. Polym. 2014, 99, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, J.Y.; Kang, H.K.; Jeong, L.; Kang, Y.O.; Oh, J.E.; Yeo, I.S.; Min, B.M. Epidermal cellular response to poly (vinyl alcohol) nanofibers containing silver nanoparticles. Colloid Surf. B 2010, 78, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.H.; Park, J.L.; Sul, I.H.; Youk, J.H.; Kang, T.J. Preparation of antimicrobial poly (vinyl alcohol) nanofibers containing silver nanoparticles. J. Polym. Sci. Polym. Phys. 2006, 44, 2468–2474. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Wang, C. Electrostatic forces induce poly (vinyl alcohol)-protected copper nanoparticles to form copper/poly (vinyl alcohol) nanocables via electrospinning. Macromol. Rapid Commun. 2006, 27, 152–155. [Google Scholar] [CrossRef]
- Khalil, A.; Jouiad, M.; Khraisheh, M.; Hashaikeh, R. Facile synthesis of copper oxide nanoparticles via electrospinning. J. Nanomater. 2014, 2014, 80. [Google Scholar] [CrossRef]
- Malwal, D.; Gopinath, P. Efficient adsorption and antibacterial properties of electrospun CuO-ZnO composite nanofibers for water remediation. J. Hazard. Mater. 2017, 321, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Cao, Y.; Xin, B.; Liu, Y.; Chen, Z.; Lin, L.; Sun, Y. Fabrication and characterization of electrospun cellulose/polyacrylonitrile nanofibers with Cu (II) ions. Cellulose 2018, 25, 2955–2963. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Bai, J.; Wang, J.; Li, H. Synthesis, characterization, and antibacterial activity of Cu NPs embedded electrospun composite nanofibers. Colloid Polym. Sci. 2015, 293, 2525–2530. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Preparation of antimicrobial ultrafine cellulose acetate fibers with silver nanoparticles. Macromol. Rapid Commun. 2004, 25, 1632–1637. [Google Scholar] [CrossRef]
- Shalaby, T.; Hamad, H.; Ibrahim, E.; Mahmoud, O.; Al-Oufy, A. Electrospun nanofibers hybrid composites membranes for highly efficient antibacterial activity. Ecotoxicol. Environ. Saf. 2018, 162, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.; Aljaafari, A.; Al-Omair, M.A. Functional electrospun cellulosic nanofiber mats for antibacterial bandages. Fibers Polym. 2017, 18, 2379–2386. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Zhang, J.; Yu, Z.; Yang, D. Electrospun polyacrylonitrile nanofibers loaded with silver nanoparticles by silver mirror reaction. Mater. Sci. Eng. C 2015, 51, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Castro Mayorga, J.L.; Fabra Rovira, M.J.; Cabedo Mas, L.; Sánchez Moragas, G.; Lagarón Cabello, J.M. Antimicrobial nanocomposites and electrospun coatings based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and copper oxide nanoparticles for active packaging and coating applications. J. Appl. Polym. Sci. 2018, 135, 45673. [Google Scholar] [CrossRef]
- Quirós, J.; Gonzalo, S.; Jalvo, B.; Boltes, K.; Perdigón-Melón, J.A.; Rosal, R. Electrospun cellulose acetate composites containing supported metal nanoparticles for antifungal membranes. Sci. Total Environ. 2016, 563, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, B.; Joh, H.I.; Jo, S.M.; Park, J.H.; Yi, K.B.; Lee, S. Enhanced reactive H2S adsorption using carbon nanofibers supported with Cu/CuxO nanoparticles. Appl. Surf. Sci. 2018, 429, 253–257. [Google Scholar] [CrossRef]
- Santos, J.P.F.; da Silva, A.B.; Arjmand, M.; Sundararaj, U.; Bretas, R.E.S. Nanofibers of poly (vinylidene fluoride)/copper nanowire: Microstructural analysis and dielectric behavior. Eur. Polym. J. 2018, 101, 46–55. [Google Scholar] [CrossRef]
- Sheikh, F.A.; Kanjwal, M.A.; Saran, S.; Chung, W.J.; Kim, H. Polyurethane nanofibers containing copper nanoparticles as future materials. Appl. Surf. Sci. 2011, 257, 3020–3026. [Google Scholar] [CrossRef]
- Ahmad, Z.; Vargas-Reus, M.A.; Bakhshi, R.; Ryan, F.; Ren, G.G.; Oktar, F.; Allaker, R.P. Antimicrobial properties of electrically formed elastomeric polyurethane–copper oxide nanocomposites for medical and dental applications. In Methods in Enzymology; Academic Press: Cambridge, CA, USA, 2012; Volume 509, pp. 87–99. [Google Scholar]
- Pareek, V.; Gupta, R.; Panwar, J. Do physico-chemical properties of silver nanoparticles decide their interaction with biological media and bactericidal action? A review. Mater. Sci. Eng. C 2018, 90, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver nanoparticle-mediated cellular responses in various cell lines: An in vitro model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Altern. Med. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, E.D.; de Figueiredo, L.F.P.; Otoch, J.P.; Seckler, M.M.; de Oliveira, R.A.; Franco, F.F.; Costa, S.F. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankier, C.; Cheong, Y.; Mahalingam, S.; Edirisinghe, M.; Ren, G.; Cloutman-Green, E.; Ciric, L. A comparison of methods to assess the antimicrobial activity of nanoparticle combinations on bacterial cells. PLoS ONE 2018, 13, e0192093. [Google Scholar] [CrossRef] [PubMed]


| Antibacterial Agent | Electrospun Polymer Composite | References |
|---|---|---|
| CuNPs, CuO NPs | Copper salt + PVP | [92] |
| Commercial CuO + PHBV | [109] | |
| Supported copper salt + CA | [110] | |
| Cu/CuOx + PAN | [103,104,111] | |
| Copper salt + PVA | [100,101] | |
| Copper salt + CMC | [107] | |
| Copper salt + PVDF | [112] | |
| Copper salt + PU | [113,114] | |
| AgNPs | Silver salt + PVP | [92,93] |
| Supported silver salt + CA | [105,110] | |
| Silver salt + PAN | [108] | |
| Silver salt + PVA | [94,95,96,97,98,99] | |
| Other Me/Me oxides NPs | Copper salt + zinc salt + PVA | [102] |
| Zinc salt + CMC | [107] | |
| Iron salt + CMC | [107] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditaranto, N.; Basoli, F.; Trombetta, M.; Cioffi, N.; Rainer, A. Electrospun Nanomaterials Implementing Antibacterial Inorganic Nanophases. Appl. Sci. 2018, 8, 1643. https://doi.org/10.3390/app8091643
Ditaranto N, Basoli F, Trombetta M, Cioffi N, Rainer A. Electrospun Nanomaterials Implementing Antibacterial Inorganic Nanophases. Applied Sciences. 2018; 8(9):1643. https://doi.org/10.3390/app8091643
Chicago/Turabian StyleDitaranto, Nicoletta, Francesco Basoli, Marcella Trombetta, Nicola Cioffi, and Alberto Rainer. 2018. "Electrospun Nanomaterials Implementing Antibacterial Inorganic Nanophases" Applied Sciences 8, no. 9: 1643. https://doi.org/10.3390/app8091643









