Microstructures of Three In-Situ Reinforcements and the Effect on the Tensile Strengths of an Al-Si-Cu-Mg-Ni Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Intermediate Alloy Preparation
2.2. Experimental Process
2.2.1. The Process of Matrix Alloy
2.2.2. The Process of Composite Alloys
2.2.3. The Microstructure Analysis
2.2.4. The Mechanical Tensile Tests
3. Results and Discussion
3.1. Metallurgical Analysis of Al-3Ti-0.25C Intermediate Alloy
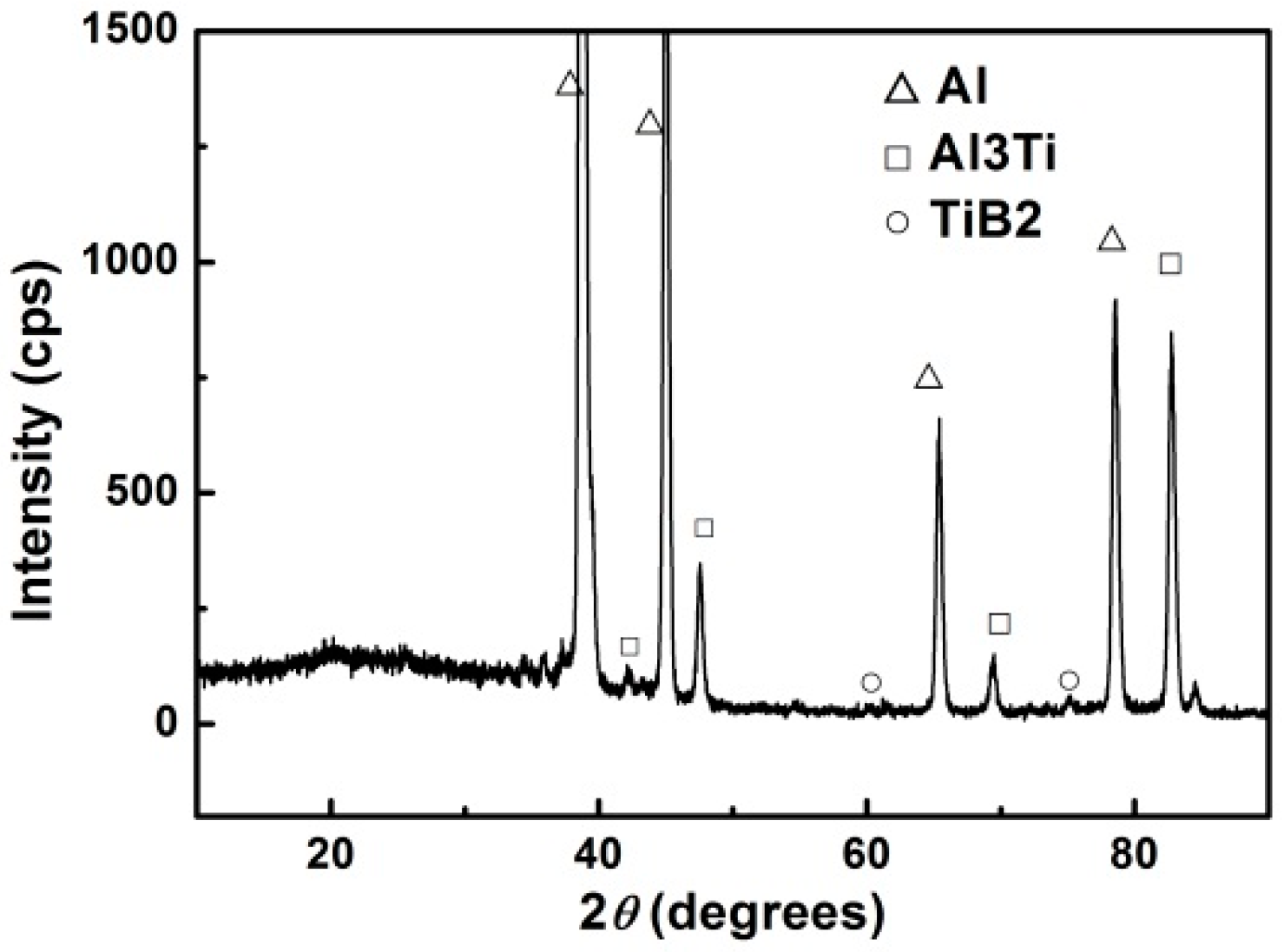
3.1.1. X-ray Diffraction Analysis
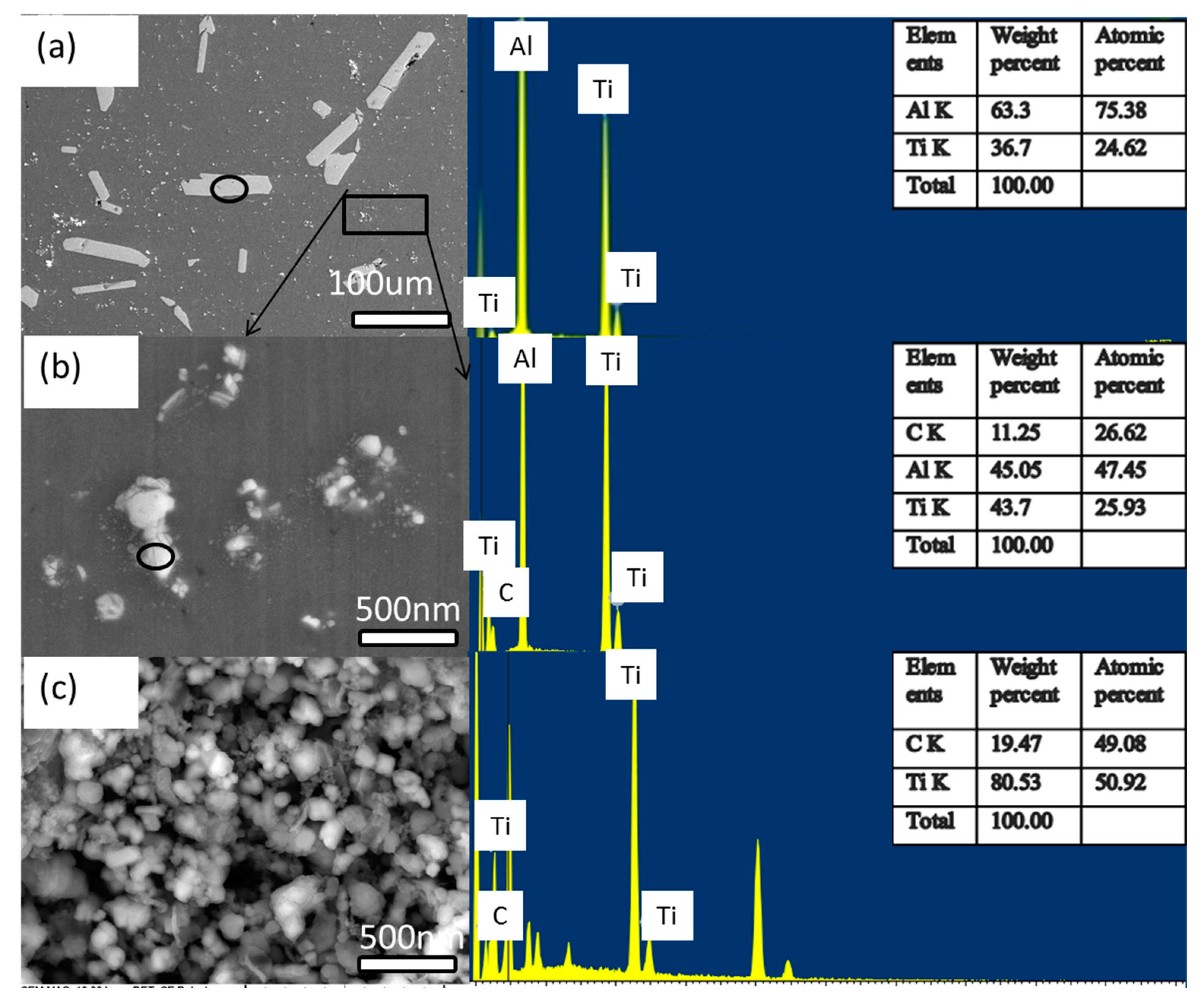
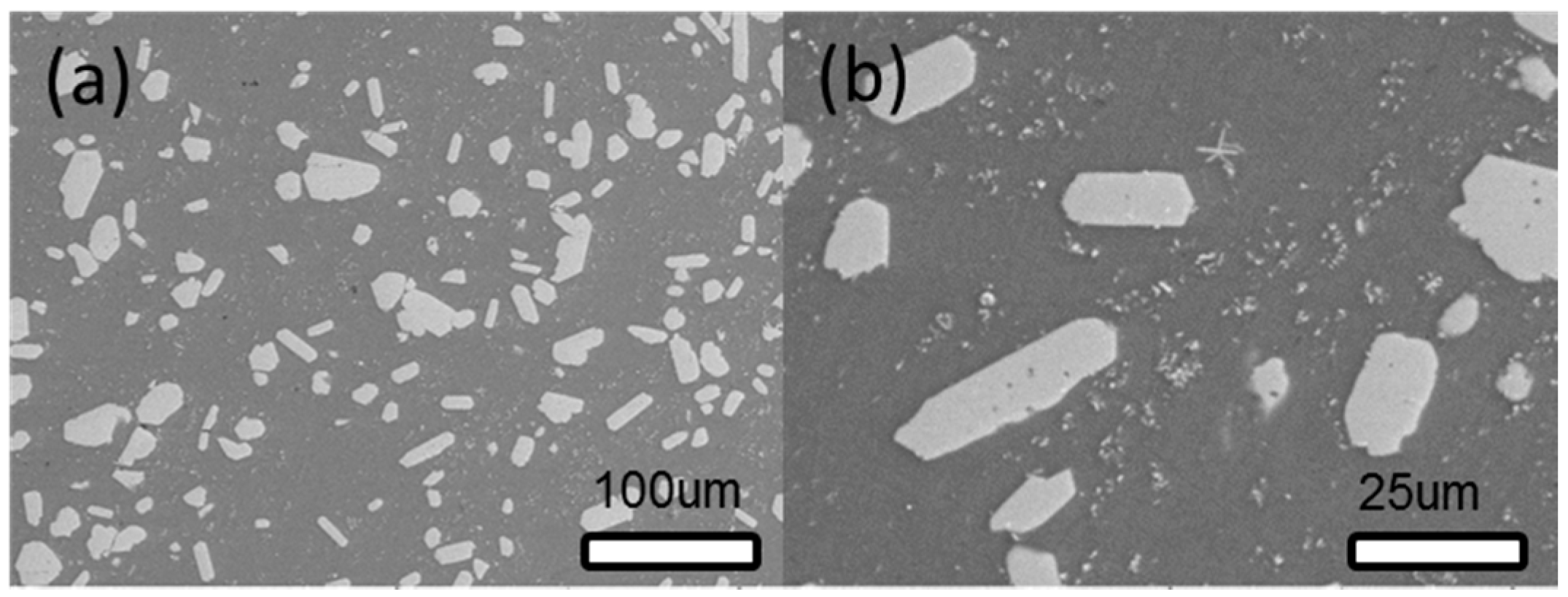
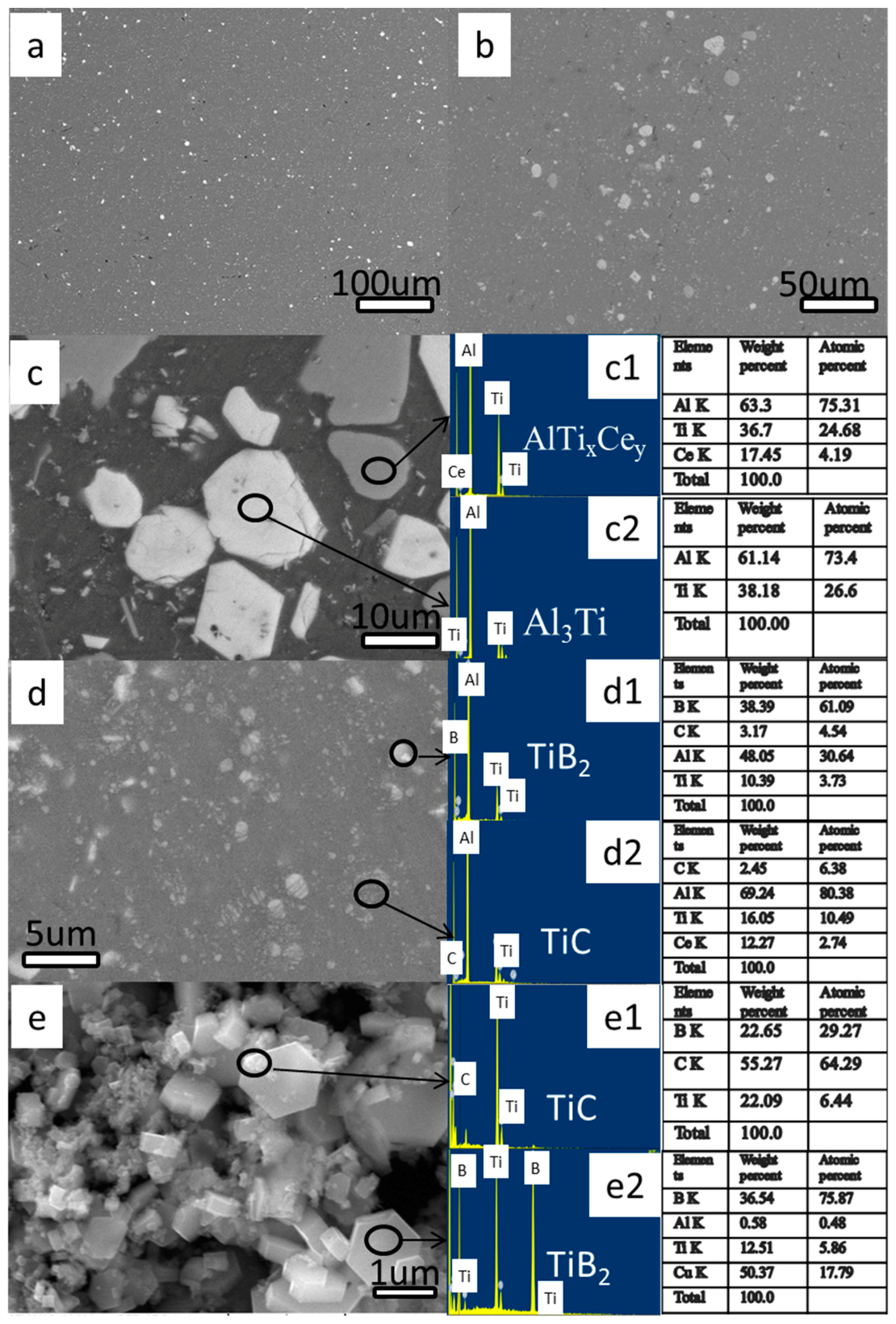
3.1.2. SEM Analysis
3.1.3. TEM Analysis
3.2. Al-5Ti-B Intermediate Alloy Phase Analysis
3.2.1. X-ray Diffraction Analysis
3.2.2. SEM Analysis
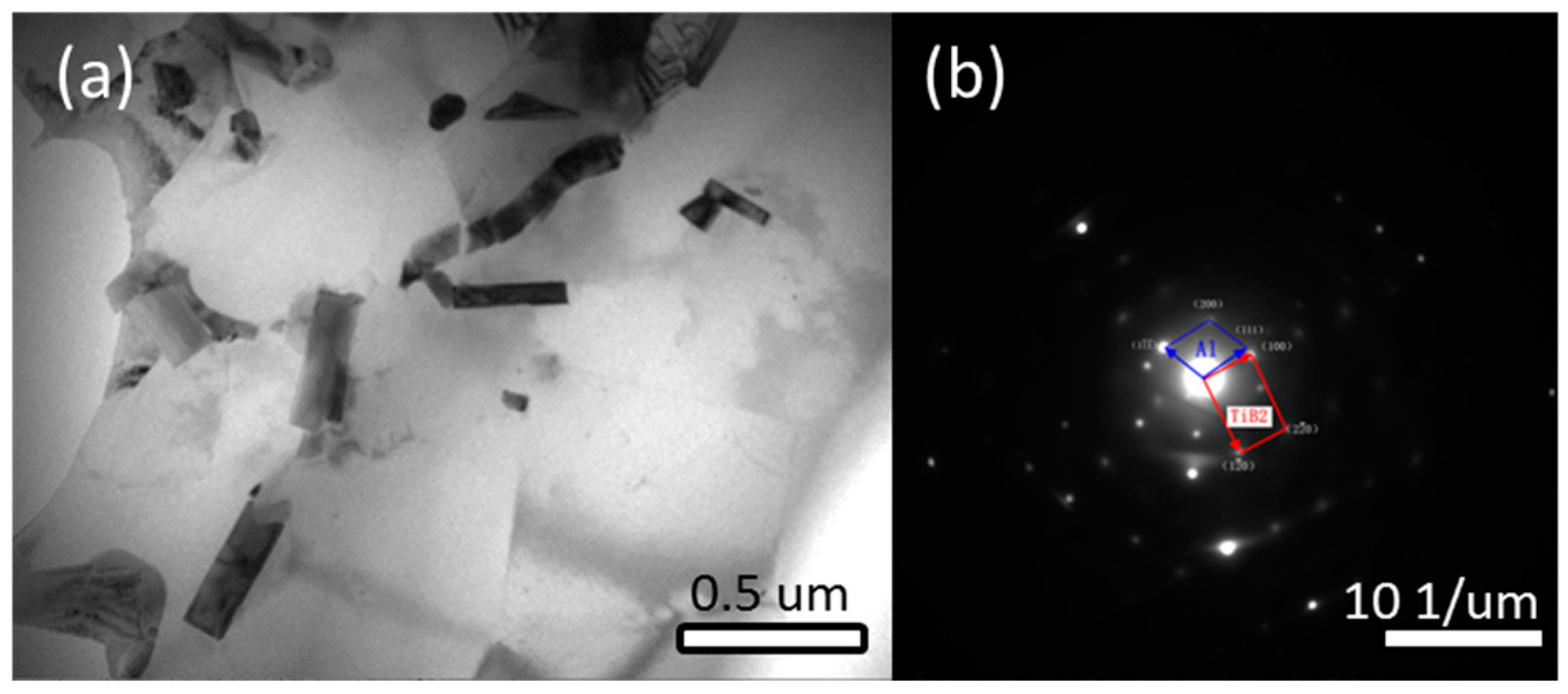
3.2.3. TEM Analysis
3.3. Al-5Ti-B-C-Ce Phase Analysis
3.3.1. XRD Analysis
3.3.2. SEM Analysis
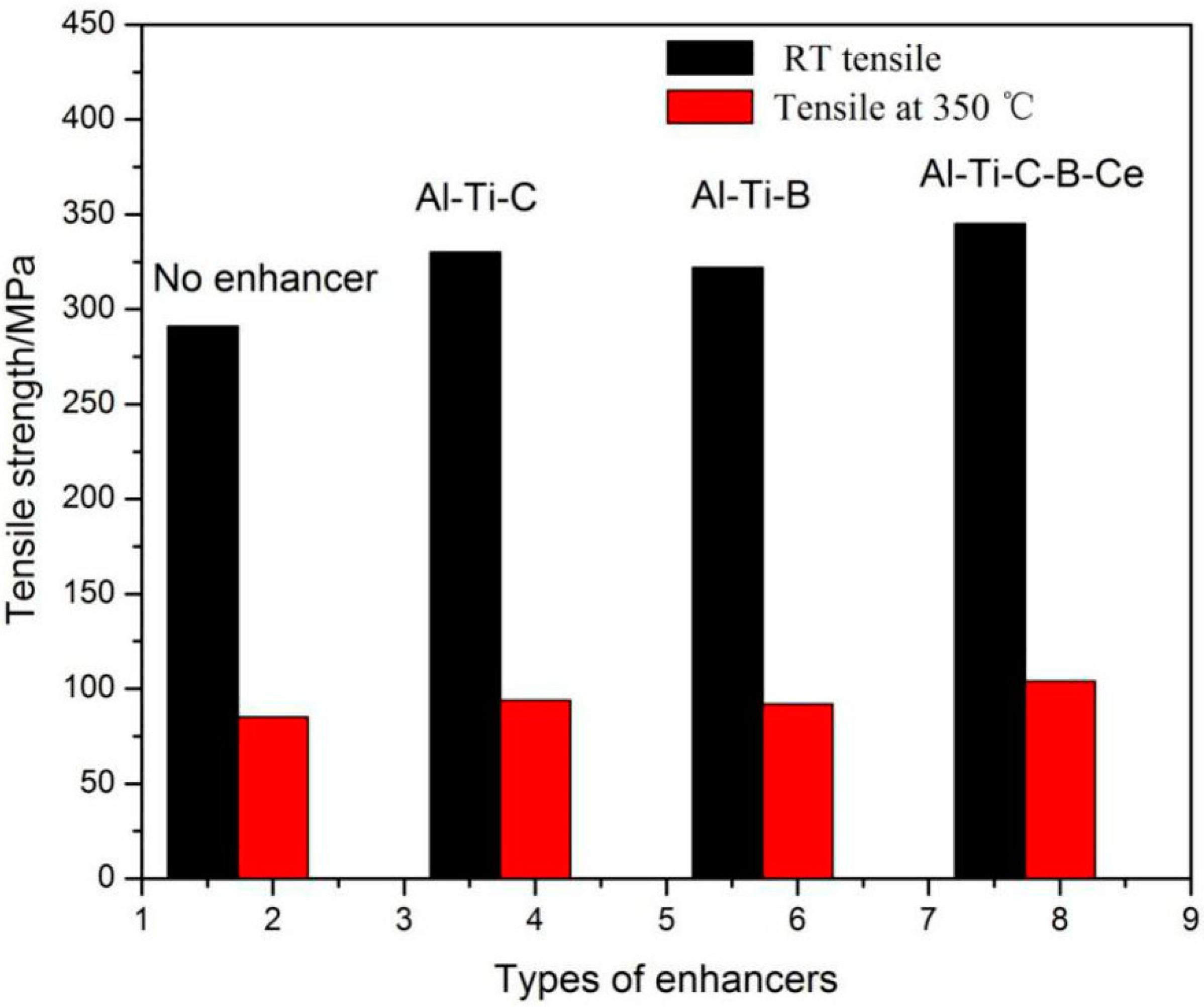
3.4. Effects of Three Different Reinforcements on the Tensile Properties of the Al Alloy
3.5. Strengthening Mechanism
4. Conclusions
- After doping with trace amounts of elements B and Ce, the size of reinforcement Al3Ti phase changed from 80 µm (un-reinforced) to about 10 µm, with the simultaneous formation of Ti2Al20Ce phase.
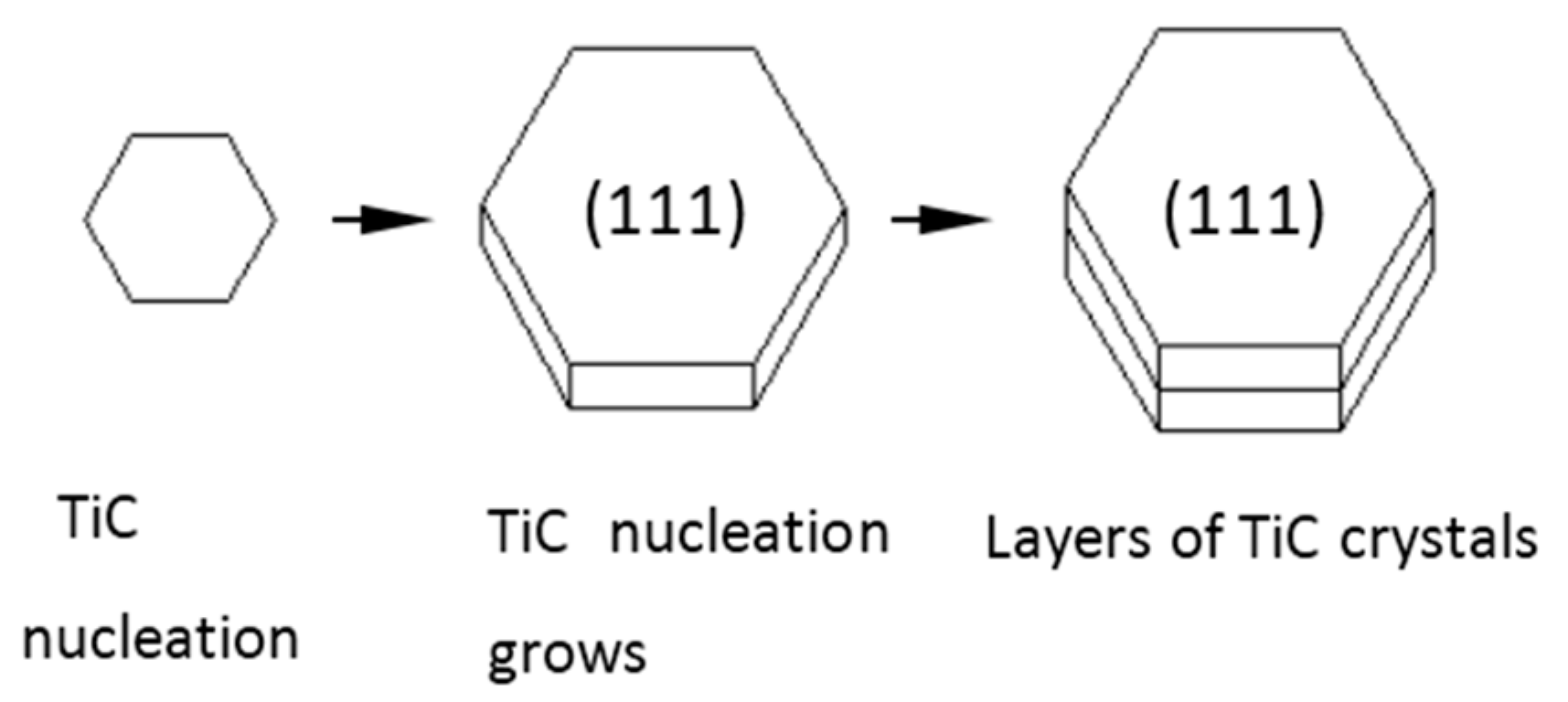
- Since doping with element B destroys the equilibrium growth conditions for TiC phase, small nano phases of TiC attached to the surface and vicinity of hexagonal TiB2. Compared with alloy without element B doping, the hexagonal TiB2 phase turns out to be a growth carrier for TiC nano-phase, and the distribution of TiC nano-phase is more uniform.
- All three in situ reinforcements were effective to the Al-13Si-4Cu-2Ni-1Mg alloy, and the addition of Al-5Ti-B-C-Ce reinforcement was more effective than the other two reinforcements in increasing the room temperature and high-temperature tensile strength. The RT strength increased by 19.0%, and the 350 °C-strength increased by 18.4%.
Author Contributions
Funding
Conflicts of Interest
References
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites: Applications; Cambridge University Press: Cambridge, UK, 1993; p. 454. [Google Scholar]
- Moore, J.J.; Feng, H.J. Combustion synthesis of advanced materials: Part I. Reaction parameters. J. Prog. Mater Sci. 1995, 39, 243–273. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Self-propagating high temperature synthesis and powder metallurgy: Unity of goals and competition of principles. Adv. Powder Metall. Part Mater. 1992, 9, 341–368. [Google Scholar]
- Merzhanov, A.G. Academician. Processes of self-propagating high-temperature synthesis at new frontiers Academician. Ind. Ceram. 2009, 29, 196–199. [Google Scholar]
- Nampoothiri, J.; Harini, R.S.; Nayak, S.K.; Raj, B.; Ravi, K.R. Post in-situ reaction ultrasonic treatment for generation of Al-4.4Cu/TiB2 nanocomposite: A route to enhance the strength of metal matrix nanocomposite. J. Alloys Compd. 2016, 683, 370–378. [Google Scholar] [CrossRef]
- Tjong, S.C.; Mai, Y.W. Processing-structure-property aspects of particulate and whisker-reinforced titanium matrix composites. Compos. Sci. Technol. 2008, 68, 583–601. [Google Scholar] [CrossRef]
- Ramesh, C.S.; Keshavamurthy, R.; Channabasappa, B.H.; Pramod, S. Influence of heat treatment on slurry erosive wear resistance of Al6061 alloy. Mater. Des. 2009, 30, 3713–3722. [Google Scholar] [CrossRef]
- Macke, A.; Schultz, B.F.; Rohatgi, P. Metal matrix composites offer the auto-motive industry an opportunity to reduce vehicle weight, improve performance. Adv. Mater. Processes 2012, 170, 19–23. [Google Scholar]
- Yang, J.; Pan, L.; Gu, W.; Qiu, T.; Zhang, Y. Microstructure and mechanical properties of in situ synthesized (TiB2 + TiC)/Ti3SiC2 composites. Ceram. Int. 2012, 38, 649–655. [Google Scholar] [CrossRef]
- Choi, Y.; Rhee, S.W. Effect of carbon sources on the combustion synthesis of TiC. J. Mater. Sci. 1993, 28, 6669–6675. [Google Scholar] [CrossRef]
- Kaftelen, H.; Unlu, N.; Goller, G. Comparative processing-structure–property studies of Al-Cu matrix composites reinforced with TiC particulates. Compos. Part A Appl. Sci. Manuf. 2011, 42, 812–824. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Q.; Li, J. Fabrication of in situ Al3Ti/Al composites by using ultrasound assisted direct reaction between solid Ti powders and liquid Al. Powder Technol. 2013, 247, 55–59. [Google Scholar] [CrossRef]
- Tang, P.; Li, W.F.; Wang, K.; Du, J.; Chen, X.Y.; Zhao, Y.J.; Li, W.Z. Effect of Al-Ti-C master alloy addition on microstructures and mechanical properties of cast eutectic Al-Si-Fe-Cu alloy. Mater. Des. 2017, 115, 147–157. [Google Scholar] [CrossRef]
- Sadeghi, E.; Karimzadeh, F.; Abbasi, M.H. Thermodynamic analysis of Ti-Al-C intermetallics formation by mechanical alloying. J. Alloys Compd. 2013, 576, 317–323. [Google Scholar] [CrossRef]
- Rai, R.N.; Datta, G.L.; Chakraborty, M.; Chattopadhyay, A.B. A study on the machinability behaviour of Al-TiC composite prepared by in situ technique. Mater. Sci. Eng. A 2006, 428, 34–40. [Google Scholar] [CrossRef]
- Li, P.T.; Li, Y.G.; Wu, Y.Y.; Ma, G.L.; Liu, X.F. Distribution of TiB2 particles and its effect on the mechanical properties of A390 alloy. Mater. Sci. Eng. A 2012, 546, 146–152. [Google Scholar] [CrossRef]
- Liu, Z.D.; Tian, J.; Li, B.; Zhao, L.P. Microstrcuture and mechanical behaviors of in situ TiC particulates reinforced Ni matrix composites. Mater. Sci. Eng. A 2010, 527, 3898–3903. [Google Scholar] [CrossRef]
- Wangjara, P. Characterization of Ti-6% Al-4%V/TiC Particulate Reinforced Metal Matrix Composites Consolidated by Sintering and Thermomechanical Processing. Ph.D. Thesis, Mcgill University, Montreal, QC, Canada, 1999. [Google Scholar]
- Pearson, W.B. A Handbook of Lattice Spacings and Structures of Metals and Alloys; Pergamon Press: Oxford, UK, 1958. [Google Scholar]
- Ramesh, C.S.; Pramod, S.; Keshavamurthy, R. A study on microstructure and mechanical properties of Al 6061—TiB2 in-situ composites. Mater. Sci. Eng. A 2011, 528, 4125–4132. [Google Scholar] [CrossRef]
- Wang, E.Z.; Gao, T.; Nie, J.F.; Liu, X.F. Grain refinement limit and mechanical properties of 6063 alloy inoculated by Al-Ti-C (B) master alloys. J. Alloys Compd. 2014, 594, 7–11. [Google Scholar] [CrossRef]
- Zhao, H.L.; Song, Y.; Li, M.; Guan, S.K. Grain refining efficiency and microstructure of Al-Ti-C-RE master alloy. J. Alloys Compd. 2010, 508, 206–211. [Google Scholar] [CrossRef]
- Tabrizi, S.G.; Sajjadi, S.A.; Babakhani, A.; Lu, W. Influence of spark plasma sintering and subsequent hot rolling on microstructure and flexural behavior of in-situ TiB and TiC reinforced Ti6Al4V composite. Mater. Sci. Eng. A 2015, 624, 271–278. [Google Scholar] [CrossRef]
- El-Mahallawy, N.; Taha, M.A.; Jarfors, A.E.W.; Fredriksson, H. On the reaction between aluminium K2TiF6 and KBF4. J. Alloys Compd. 1999, 292, 221–229. [Google Scholar] [CrossRef]
- Xuan, Q.Q.; Shu, S.L.; Qiu, F.; Jin, S.B.; Jiang, Q.C. Different strain-rate dependent compressive properties and work-hardening capacities of 50 vol% TiCx/Al and TiB2/Al composites. Mater. Sci. Eng. A. 2012, 538, 335–339. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, S.S.; Lu, S.L.; Duan, X.C.; Zhong, Z.Y. Preparation of in-situ TiB2 and Mg2Si hybrid particulates reinforced Al-matrix composites. J. Alloys Compd. 2015, 651, 521–527. [Google Scholar] [CrossRef]
- Nie, J.F.; Liu, X.F.; Ma, X.G. Influence of trace boron on the morphology of titanium carbide in an Al-Ti-C-B master alloy. J. Alloys Compd. 2010, 491, 113–117. [Google Scholar] [CrossRef]
- Wu, Z.S. Preparation and Properties of TiC-Based Composites. Ph.D. Thesis, Jiangsu University, Nanjing, China, 2004. [Google Scholar]
- Ma, M.Z.; Cai, D.Y.; Wei, T.H. Microstructure and mechanical properties of TiCp/Al-4.5Cu-0.8Mg compsites by direct reaction synthesis. J. Mater. Sci. Technol. 2003, 19, 447–449. [Google Scholar]
- Le, Y.K.; Zhang, Y.Y. Research status of particle reinforced aluminum matrix composites. Dev. Appl. 1997, 5, 33–37. [Google Scholar]
- Tee, K.L.; Lu, L.; Lai, M.O. Synthesis of in situ Al-TiB2 composites using stir cast route. Compos. Struct. 1999, 47, 589–593. [Google Scholar] [CrossRef]
- Mandal, A.; Chakraborty, M.; Murty, B.S. Ageing behaviour of A356 alloy reinforced with in-situ formed TiB2 particles. Mater. Sci. Eng. A 2008, 489, 220–226. [Google Scholar] [CrossRef]
- Zhao, D.G.; Liu, X.F.; Bian, X.F. Preparation and mechanical properties of TiB2 + SiC/ZL109. Casting 2004, 53, 97–100. [Google Scholar]
- Song, W.; Wang, L.; Chen, X.; Chen, Y.; Li, Q. Effects of Al-Ti-C-Ce master alloy on As-cast microstructure and mechanical properties of 7010 aluminum alloy. Spec. Cast. Nonferrous Alloy 2011, 4, 39. [Google Scholar]
- Cheng, L.H.; Sun, Y.; Sun, G.X. Effect of Al-5Ti-1B on the microstructure of near-eutectic Al-13%Si alloys modified with Sr. J. Mater. Sci. 2002, 37, 3489–3495. [Google Scholar]
- Wang, K.; Cui, C.; Wang, Q.; Liu, S.; Gu, C. The microstructure and formation mechanism of core–shell-like TiAl3/Ti2Al20Ce in melt-spun Al-Ti-B-Re grain refiner. Mater. Lett. 2012, 85, 153–156. [Google Scholar] [CrossRef]
- Yin, J.B.; Li, Y.T.; Bi, J.M.; Pang, X.Z.; Li, L.J. Research on grain refinement of Al-2Ti-B-Ce and Al-5Ti-B-Ce master alloys on industrial pure aluminum. Light Alloy Fabr. Technol. 2015, 1, 5. [Google Scholar]





| Particle | Density/(g·cm−3) | Melting Point/°C | Coefficient of Thermal Expansion/ (10−6 °C−1) | Thermal Conductivity | Elasticity Modulus/(GPa) |
|---|---|---|---|---|---|
| TiC | 4.93 | 3160 | 7.74 | 24.28 | 450 |
| TiB2 | 4.25 | 2980 | 4.6–8.01 | 24.4–26 | 530 |
| TiB | 4.5 | 2473 | 8.6 | - | 550 |
| SiC | 3.19 | 2970 | 4.3 | - | 430 |
| VC | 5.36 | 2810 | 4.2 | 24.7 | 430 |
| WC | 15.55 | 2720 | 3.84 | 29.31 | 810 |
| Al2O3 | 3.9 | 2050 | 8.6 | 28.89 | 420 |
| Al3Ti | 3.3 | 1623 | - | - | 220 |
| Reinforcement Types | Advantages | Disadvantages |
|---|---|---|
| Al-Ti-B |
|
|
| Al-Ti-C |
|
|
| Al-Ti-B-C-Ce |
|
|
| Materials | Enhanced Phase Content/vol. % | Tensile Strength/MPa | Elongationδ/% | Reference |
|---|---|---|---|---|
| TiC/Al | 15 | 610 | 4.9 | [28] |
| TiC/Al-4.5Cu | 20 | 540 | 18 | [29] |
| TiB2/Al | 16 | 349 | 3.8 | [30] |
| TiB2/Al | 5 | 124 | 9.2 | [31] |
| TiB2/A356 | 2.5 | 290 | 10 | [32] |
| TiB2/A356 | 5 | 302 | 9 | [32] |
| TiB2/A356 | 7.5 | 317 | 8 | [32] |
| TiB2/A356 | 10 | 328 | 6 | [32] |
| TiB2/ZL109 | - | 258.7 | 7.5 | [33] |
| Powders | Particle Size | Purity | Manufacturer |
|---|---|---|---|
| Al | 200 mesh | ≥99.0% | Shanghai shanpu chemical Co., Ltd. (Shanghai, China) |
| Ti | 200–300 mesh | ≥99.0% | Sinopharm chemical reagent Co., Ltd. (Shanghai, China) |
| C | 2000 mesh | ≥99.9% | Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China) |
| K2TiF6 | / | ≥99.0% | Shanghai SSS Reagent Co., Ltd. (Shanghai, China) |
| KBF4 | / | ≥99.0% | Shanghai SSS Reagent Co., Ltd. (Shanghai, China) |
| B4C | 100–300 mesh | ≥99.0% | Nangong ruiteng alloy material Co., Ltd. (Nangong, China) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Guo, Y.; Li, J.; Xia, F.; Liang, M.; Duan, H.; Wang, P.; Wang, J. Microstructures of Three In-Situ Reinforcements and the Effect on the Tensile Strengths of an Al-Si-Cu-Mg-Ni Alloy. Appl. Sci. 2018, 8, 1523. https://doi.org/10.3390/app8091523
Tian L, Guo Y, Li J, Xia F, Liang M, Duan H, Wang P, Wang J. Microstructures of Three In-Situ Reinforcements and the Effect on the Tensile Strengths of an Al-Si-Cu-Mg-Ni Alloy. Applied Sciences. 2018; 8(9):1523. https://doi.org/10.3390/app8091523
Chicago/Turabian StyleTian, Lusha, Yongchun Guo, Jianping Li, Feng Xia, Minxian Liang, Hongbo Duan, Ping Wang, and Jianli Wang. 2018. "Microstructures of Three In-Situ Reinforcements and the Effect on the Tensile Strengths of an Al-Si-Cu-Mg-Ni Alloy" Applied Sciences 8, no. 9: 1523. https://doi.org/10.3390/app8091523




