Optimization of the Antioxidant Potentials of Red Pitaya Peels and Its In Vitro Skin Whitening Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of Red Pitaya Peels
2.3. Experimental Design
2.4. Analytical Procedure for Optimization Process
2.4.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.3. Beta-Carotene Bleaching (BCB) Assay
2.5. Statistical Analysis
2.6. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the Optimized Red Pitaya Peel Extract
2.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of the Optimized Red Pitaya Peel Extract
2.8. Evaluation of Vitamin C Content and Anti-Tyrosinase Activity of the Optimized Red Pitaya Peel Extract
2.8.1. Vitamin C Content Assay
2.8.2. Anti-Tyrosinase Assay
3. Result and Discussion
3.1. Fitting the Models
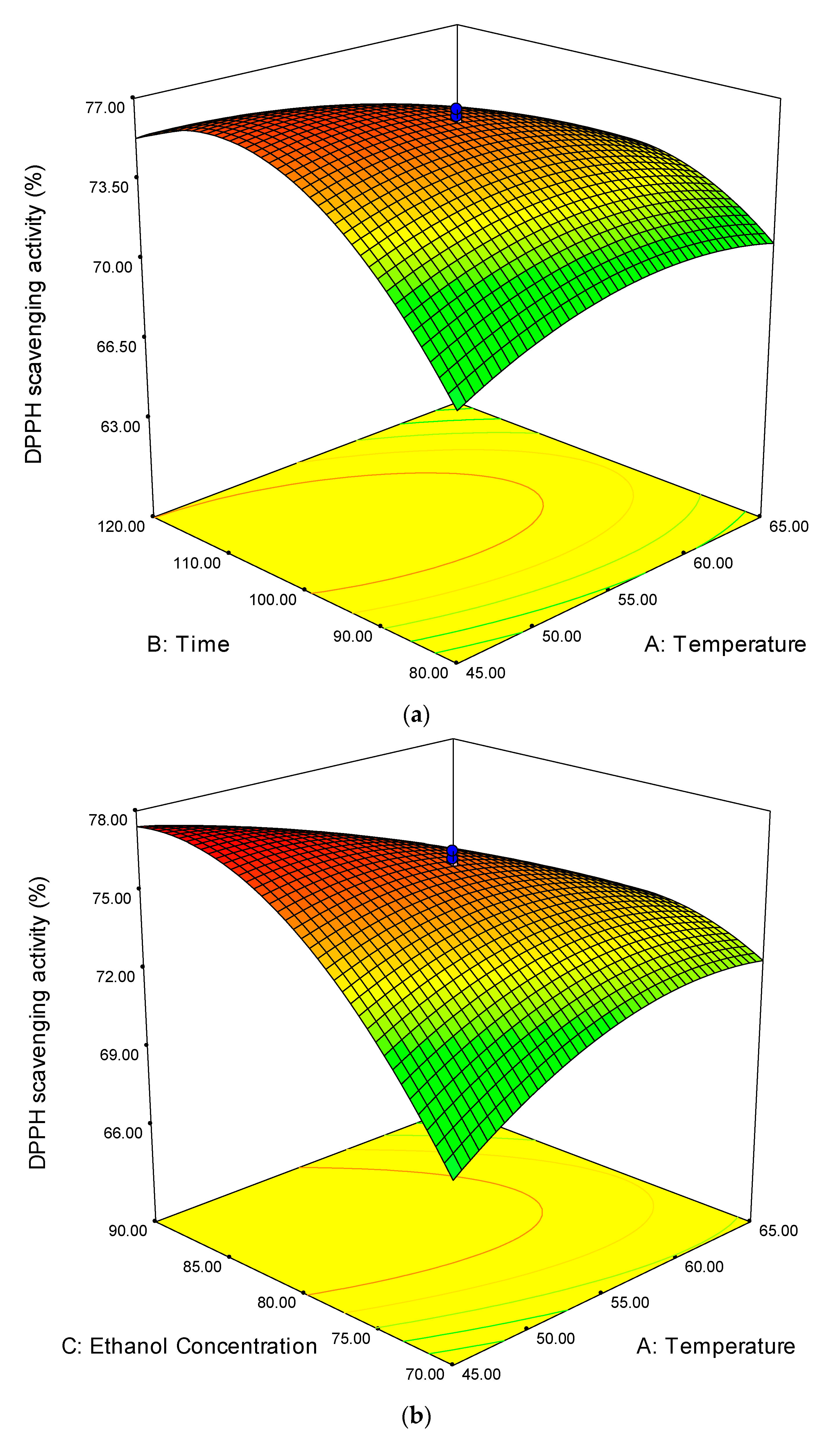
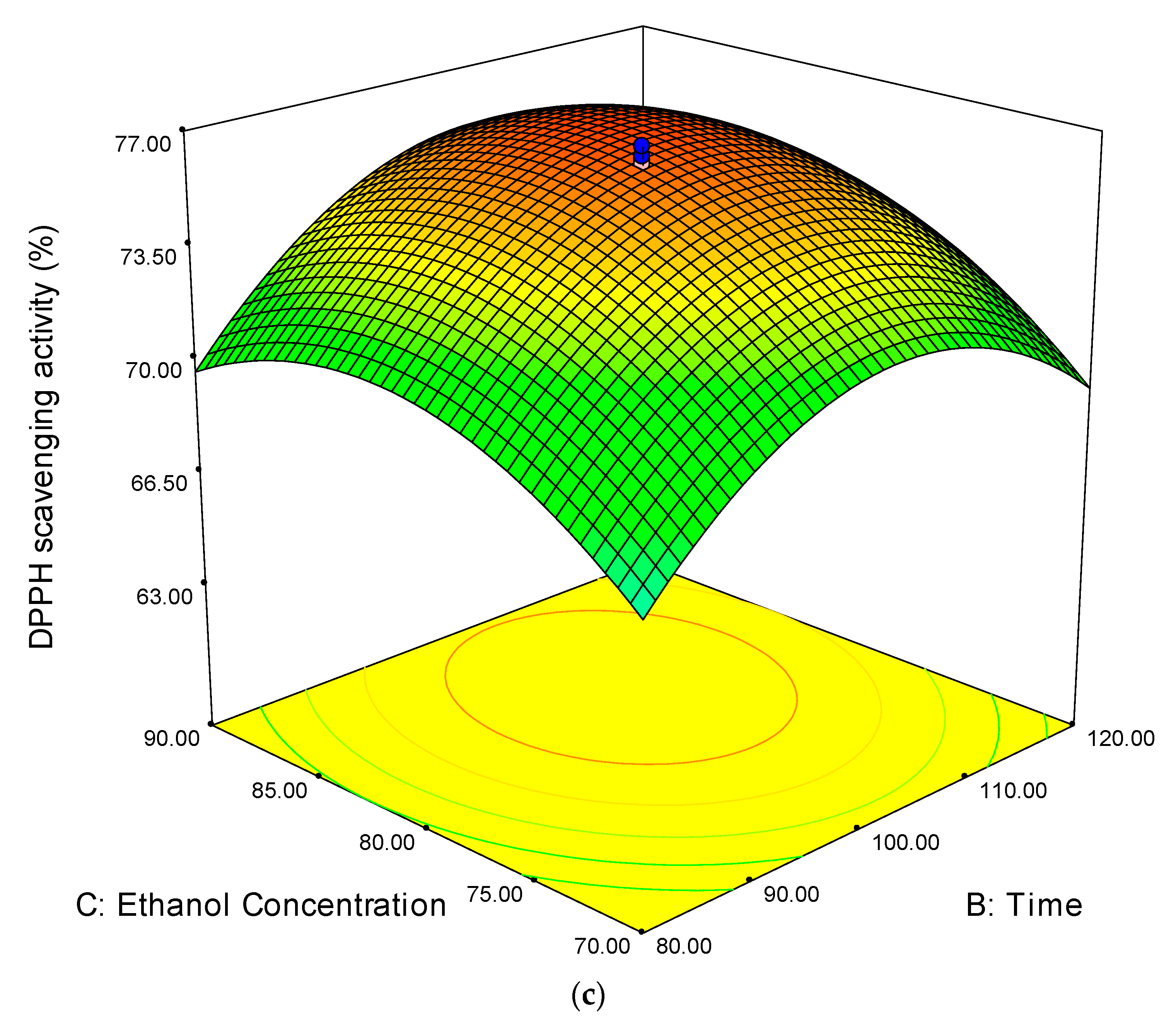
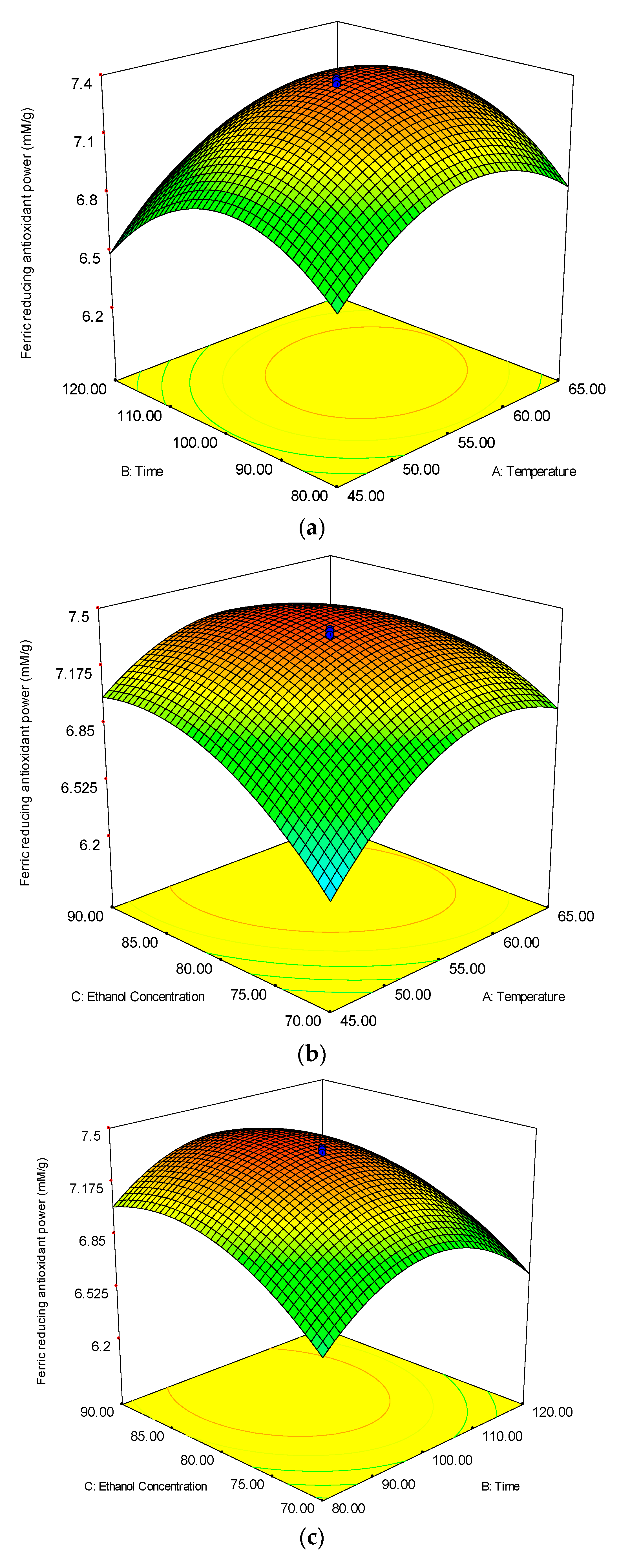
3.2. Analysis of Response Surface
3.2.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Scavenging Activity
3.2.2. Ferric Ion Antioxidant Reducing Power (FRAP)
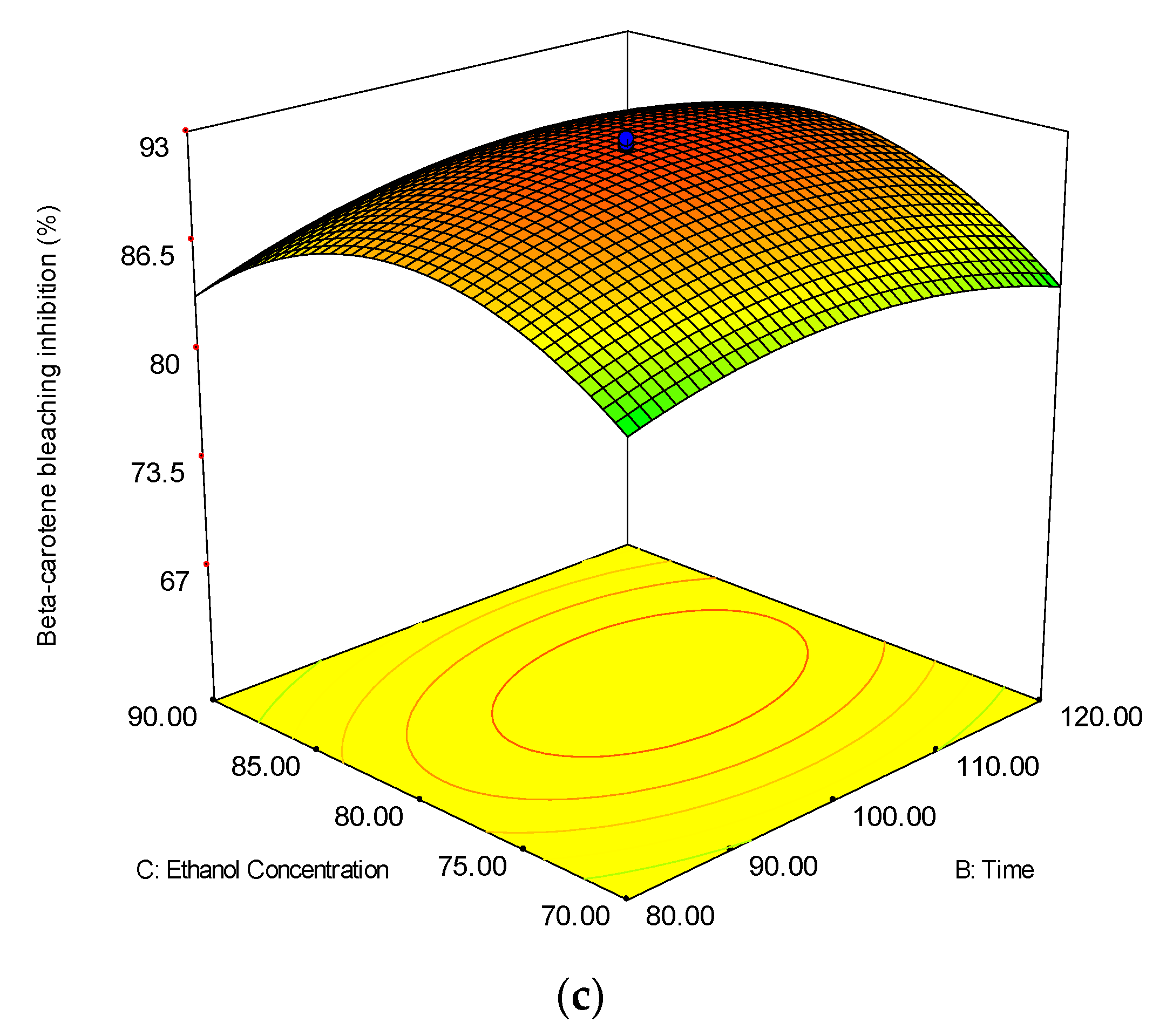
3.2.3. Beta-Carotene Bleaching (BCB) Inhibition
3.3. Optimization of Extraction Parameters
3.4. Verification of Predictive Model
3.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the Optimized Red Pitaya Peel Extract
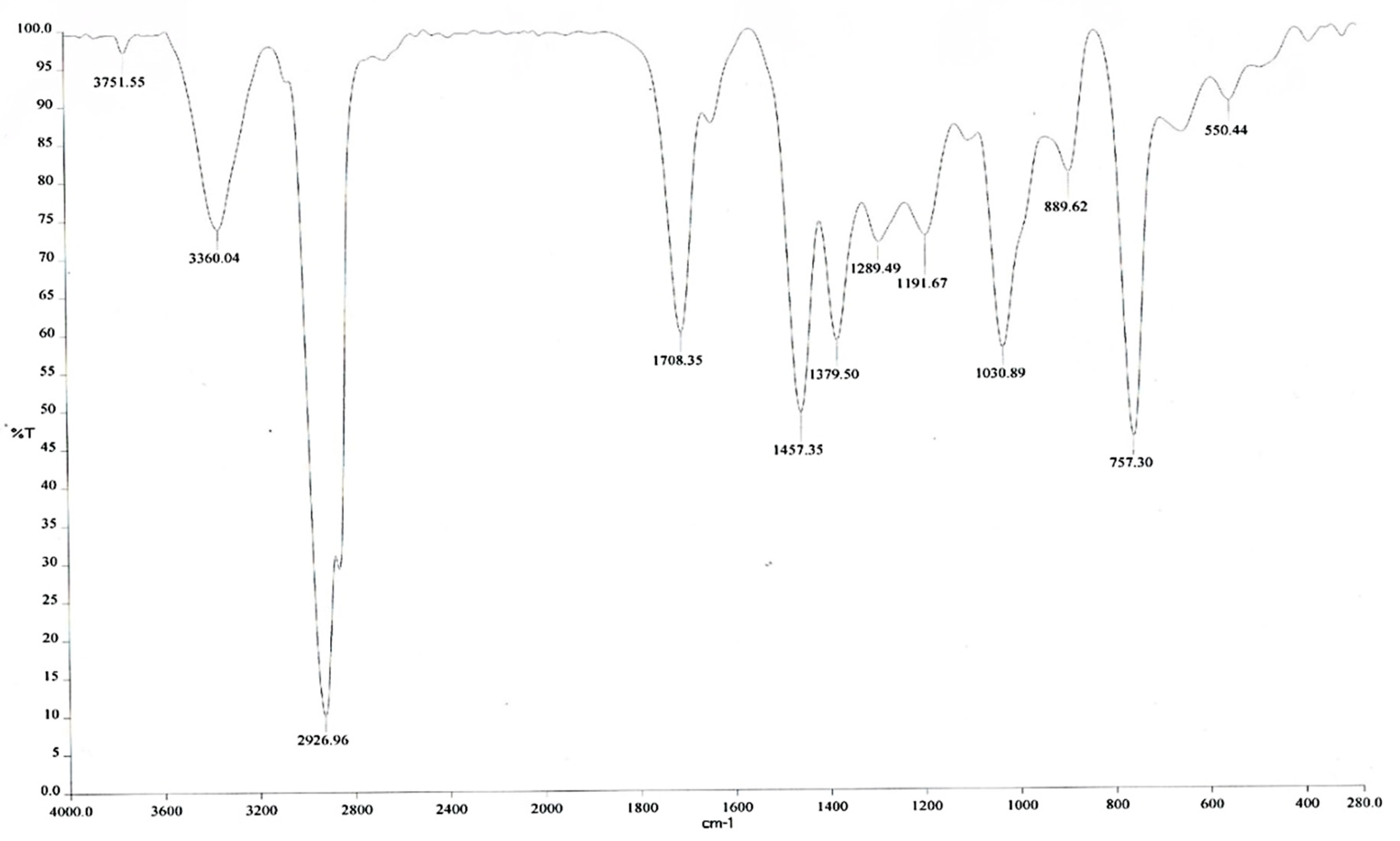
3.6. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis of the Optimized Red Pitaya Peel Extract
3.7. Evaluation of the Skin Whitening Properties of the Optimized Red Pitaya Peel Extract
3.7.1. Vitamin C Content
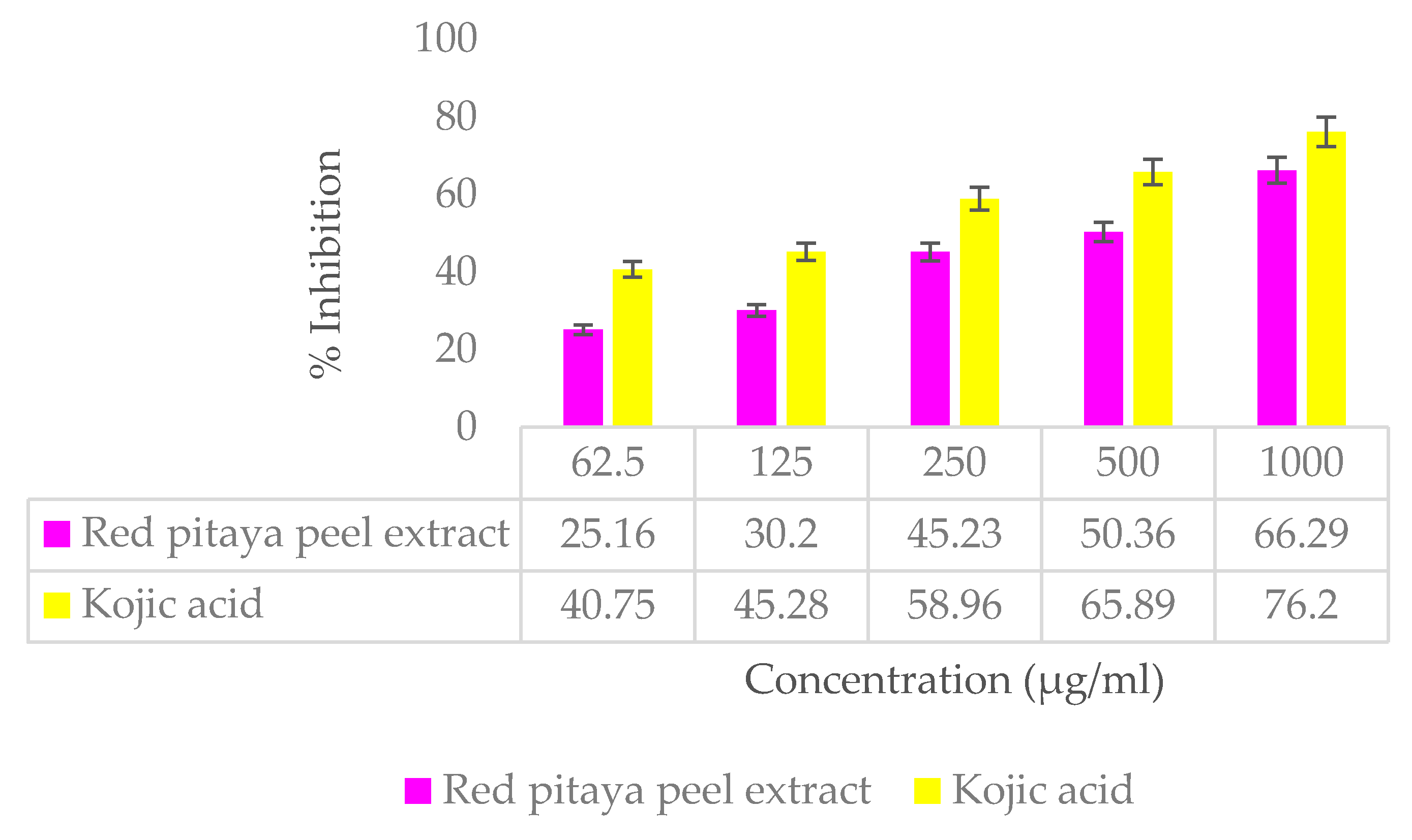
3.7.2. Anti-Tyrosinase Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RSM | Response surface methodology |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| BCB | Beta-carotene bleaching |
| FRAP | Ferric ion reducing antioxidant power |
| GC-MS | Gas chromatography-mass spectrometry |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| g | Gram |
| mg | Milligram |
| min | Minute |
| mL | Milliliter |
| mM | Millimolar |
| μL | Microliter |
| nm | Nanometer |
| PBS | Phosphate buffer saline |
| h | Hour |
| DOPA | 3,4-hydroxyphenylalanine |
| TRYP-1 | Tyrosinase-related protein-1 |
| TRYP-2 | Tyrosinase-related protein-2 |
| ROS | Reactive oxygen species |
| UV | ultraviolet |
| NO° | Nitric oxide radicals |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| β-carotene | Beta-carotene |
| ANOVA | Analysis of variance |
| R2 | Coefficient of determination |
| p | Probability |
| CV | Coefficient of variation |
| DHICA | 5,6-dihydroxyindole-2-carboxylic acid |
| mRNA | Messenger ribonucleic acid |
| EI | Electron impact. |
References
- Nerd, A.; Mizrahi, Y. Reproductive biology of cactus fruit crops. Hortic. Rev. 1997, 18, 321–346. [Google Scholar]
- Nerd, A.; Sitrita, Y.; Kaushika, R.A.; Mizrahi, Y. High summer temperatures inhibit flowering in vine pitaya crops (Hylocereus spp.). Sci. Hortic. 2002, 96, 343–350. [Google Scholar] [CrossRef]
- Hoa, T.T.; Clark, C.J.; Wadddell, B.C.; Woolf, A.B. Postharvest quality of dragon fruit (Hylocereus undatus) following disinfesting hot air treatments. Postharvest Biol. Technol. 2006, 41, 62–69. [Google Scholar] [CrossRef]
- Khalili, M.A.; Norhayati, A.H.; Rokiah, M.Y.; Asmah, R.; Muskinah, M.S.; Manaf, A.A. Hypocholesterolemic effect of red pitaya (Hylocereus sp.) on hypercholesterolemia induced rats. Int. Food Res. J. 2009, 16, 431–440. [Google Scholar]
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. In Ultraviolet Light in Human Health, Diseases and Environment. Advances in Experimental Medicine and Biology; Ahmad, S., Ed.; Springer: Cham, Switzerland, 2017; Volume 996, pp. 15–23. [Google Scholar]
- Sasaki, M.; Horikoshi, T.; Uchiwa, H.; Miyachi, Y. Up-regulation of tyrosinase gene by nitric oxide in human melanocytes. Pigment Cell Res. 2000, 13, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Farris, P. Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatol. Surg. 2005, 31, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Shibayama, H.; Hisama, M.; Ohtsuki, M.; Iwaki, M. Inhibitory effects of novel ascorbic derivative VCP-IS-2Na on melanogenesis. Chem. Pharm. Bull. 2008, 56, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Liyana-Pathirana, C.M.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Zhao, C.F.; Li, S.; Li, S.J.; Song, G.H.; Yu, L.J.; Zhang, H. Extraction optimization approach to improve accessibility of functional fraction based on combination of total polyphenol, chromatographic profiling and antioxidant activity evaluation: Pyracantha fortuneana fruit as an example. J. Funct. Foods 2013, 5, 715–728. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, R. Cosmeceuticals and silibinin. Clin. Dermatol. 2009, 27, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, K.W.; Mat Junit, S.; Aminudin, N.; Ismail, A.; Abdul Aziz, A. Antioxidant activities and polyphenolics from the shoots of Barringtonia racemosa (L.) Spreng in a polar to apolar medium system. Food Chem. 2012, 134, 324–332. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1999, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Juntachote, T.; Berghofer, E. Antioxidative properties and stability of ethanolic extracts of Holy basil and Galangal. Food Chem. 2005, 92, 193–202. [Google Scholar] [CrossRef]
- Mang, D.Y.; Abdou, A.B.; Njintang, N.Y. Application of desirability-function and RSM to optimize antioxidant properties of mucuna milk. J. Food Meas. Charact. 2015, 4, 495–507. [Google Scholar] [CrossRef]
- Lillian, B.; Maria-João, F.; Bruno, Q.; Ferreira, I.C.F.R.F.; Paula, B. Total phenols, ascorbic acid, B-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar]
- Chiari, M.E.; Joray, M.B.; Ruiz, G.; Palacios, S.M.; Carpinella, M.C. Tyrosinase inhibitory activity of native plants from central Argentina: Isolation of an active principle from Lithraea molleoides. Food Chem. 2010, 120, 10–14. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Chirinos, R.; Rogez, H.; Campos, D.; Pedreschi, R.; Larondelle, Y. Optimization of extraction conditions of antioxidant phenolic compounds from mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers. Sep. Purif. Technol. 2007, 55, 217–225. [Google Scholar]
- Kuljarachanan, T.; Devahastin, S.; Chiewchan, N. Evolution of antioxidant compounds in lime residues during drying. Food Chem. 2009, 113, 944–949. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical water technology for extraction of phenolic compounds from Chlorella sp. microalgae and assessment on its antioxidant activity. Molecules 2017, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Girennavar, B.; Patil, B.S. Radical scavenging activities of Rio red grapefruits and sour orange fruit extracts in different in vitro model systems. Bioresour. Technol. 2008, 99, 4484–4494. [Google Scholar] [CrossRef] [PubMed]
- Hor, S.Y.; Ahmad, M.; Farsi, E.; Yam, M.F.; Hashim, M.A.; Lim, C.P.; Sadikun, A.; Asmawi, M.Z. Safety assessment of methanol extract of red dragon fruit (Hylocereus polyrhizus): Acute and subchronic toxicity studies. Regul. Toxicol. Pharmacol. 2012, 63, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent. J. 2014, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, G.O.; Olagunju, J.A.; Ademuyiwa, O.; Martins, O.C. Gas chromatography–mass spectrometry analysis and phytochemical screening of ethanolic root extract of Plumbago zeylanica Linn. J. Med. Plants Res. 2011, 5, 1756–1761. [Google Scholar]
- Mou, Y.; Meng, J.; Fu, X.; Wang, X.; Tian, J.; Wang, M.; Peng, Y.; Zhou, L. Antimicrobial and antioxidant activities and effect of 1-hexadecene addition on palmarumycin C2 and C3 yields in liquid culture of endophytic fungus Berkleasmium sp. Dzf12. Molecules 2013, 18, 15587–15599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Sivakumar, T.; Arulmozhi, K.T.; Mythili, N. Gas chromatography-mass spectroscopy analysis evaluation of bioactive phytochemicals of commercial green teas (Camellia sinensis) of India. Asian J. Pharm. Clin. Res. 2015, 8, 278–282. [Google Scholar]
- Syeda, F.A.; Choudahry, M.I. Gas chromatography mass spectrometry (GC-MS) analysis of petroleum ether extract (oil) and bioassays of crude extract of Iris germanica. Int J. Genet. Mol. Biol. 2011, 3, 95–100. [Google Scholar]
- McGaw, L.J.; Jäger, A.K.; Van Staden, J. Isolation of antibacterial fatty acids from Schotia brachypetala. Fitoterapia 2002, 73, 431–433. [Google Scholar] [CrossRef]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- Adnan, L.; Azizah Osman, A.; Abdul Hamid, A. Antioxidant activity of different extracts of red pitaya (Hylocereus polyrhizus) seed. Int. J. Food Prop. 2011, 14, 1171–1181. [Google Scholar] [CrossRef]
- Kristanto, D. Dragon Fruit Cultivation in Pots and in the Garden; Penebar Swadaya: Jakarta, Indonesia, 2003. [Google Scholar]
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J. Agric. Food Chem. 2002, 50, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Kim, H.J.; Wang, Y.; Wang, A.; Mitts, T.F. Sodium l-ascorbate enhances elastic fibers deposition by fibroblasts from normal and pathologic human skin. J. Dermatol. Sci. 2014, 75, 173–182. [Google Scholar] [CrossRef] [PubMed]
- The Dermatology Review. l-ascorbic Acid. 2015. Available online: http://www.thedermreview.com/l-ascorbic-acid-acid/ (accessed on 1 February 2017).
- Ross, D. Ascorbate 6-palmitate protects human erythrocytes from oxidative damage. Free Radic. Biol. Med. 1999, 26, 81–89. [Google Scholar] [CrossRef]
- Austria, R. Stability of vitamin C derivatives in solution and in topical formulations. J. Pharm. Biomed. Anal. 1997, 15, 795–801. [Google Scholar] [CrossRef]
- Draelos, Z.D. Skin lightening preparations and the hydroquinone controversy. Dermatol. Ther. 2007, 20, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Inui, S.; Itami, S. Perifollicular pigment is the first target for Ascorbyl2 phosphate6palmitate. J. Dermatol. 2007, 34, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.K. Cosmeceutical vitamins: Vitamin, C. In Procedures in Cosmetic Dermatology Series: Cosmeceuticals; Draelos, Z., Ed.; Elsevier: Philadelphia, PA, USA, 2005; pp. 51–56. [Google Scholar]
- Jiménez, M.; Chazarra, S.; Escribano, J.; Cabanes, J.; García-Carmona, F. Competitive inhibition of mushroom tyrosinase by 4-substituted benzaldehydes. J. Agric. Food Chem. 2001, 49, 4060–4063. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Kinst-Hori, I. 2-Hydroxy-4-methoxy benzaldehyde: A potent tyrosinase inhibitor from African medicinal plants. Planta Med. 1999, 65, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Funasaka, Y.; Oka, M.; Ohashi, A.; Furumura, M.; Matsunaga, J.; Matsunaga, N.; Hearing, V.; Ichihashi, M. Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J. Lipid Res. 1999, 40, 1312–1316. [Google Scholar] [PubMed]
- Halaban, R.; Cheng, E.; Zhang, Y.; Moellmann, G.; Hanlon, D.; Michalak, M.; Setaluri, V.; Hebert, D. Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the differentiated phenotype of a melanotic melanoma cells. Proc. Natl. Acad. Sci. USA 1997, 94, 6210–6215. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Ryu, A.; Hashimoto, A.; Oka, M.; Ichihashi, M. Linoleic acid and α-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch. Dermatol. Res. 1998, 290, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Casañola-Martin, G.M.; Marrero-Ponce, Y.; Hassan, M.T. Dragon method for finding novel tyrosinase inhibitors: Biosilico identification and experimental in vitro assays. Eur. J. Med. Chem. 2007, 42, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Kamkaen, N.; Mulsri, N.; Treesak, C. Screening of some tropical vegetables for anti-tyrosinase activity. Thai Pharm. Health Sci. J. 2007, 2, 15–19. [Google Scholar]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007, 98, 2963–2967. [Google Scholar] [CrossRef] [PubMed]

| Standard Order | Coded | Uncoded | ||||
|---|---|---|---|---|---|---|
| Ethanol Concentration (%, v/v) | Temperature (°C) | Time (min) | Ethanol Concentration (%, v/v) | Temperature (°C) | Time (min) | |
| 1 | −1.000 | −1.000 | −1.000 | 70.00 | 45.00 | 80.00 |
| 2 | 0.000 | 0.000 | 1.682 | 70.00 | 65.00 | 80.00 |
| 3 | 0.000 | 0.000 | 0.000 | 80.00 | 55.00 | 100.00 |
| 4 | 0.000 | 0.000 | −1.682 | 63.18 | 55.00 | 100.00 |
| 5 | −1.000 | 1.000 | 1.000 | 90.00 | 45.00 | 120.00 |
| 6 | −1.682 | 0.000 | 0.000 | 80.00 | 31.18 | 100.00 |
| 7 | 0.000 | 0.000 | 0.000 | 80.00 | 55.00 | 100.00 |
| 8 | 0.000 | 0.000 | 1.682 | 96.82 | 55.00 | 100.00 |
| 9 | −1.000 | 1.000 | −1.000 | 70.00 | 45.00 | 120.00 |
| 10 | 1.000 | 1.000 | −1.000 | 70.00 | 65.00 | 120.00 |
| 11 | 1.682 | 0.000 | 0.000 | 80.00 | 71.82 | 100.00 |
| 12 | 0.000 | 0.000 | 0.000 | 80.00 | 55.00 | 100.00 |
| 13 | 1.000 | −1.000 | 1.000 | 90.00 | 65.00 | 80.00 |
| 14 | 1.000 | 1.000 | 1.000 | 90.00 | 65.00 | 120.00 |
| 15 | 0.000 | 0.000 | 0.000 | 80.00 | 55.00 | 100.00 |
| 16 | 0.000 | 0.000 | 0.000 | 80.00 | 55.00 | 100.00 |
| 17 | 0.000 | 0.000 | 0.000 | 80.00 | 55.00 | 100.00 |
| 18 | −1.000 | −1.000 | 1.000 | 90.00 | 45.00 | 80.00 |
| 19 | 0.000 | 1.682 | 0.000 | 80.00 | 55.00 | 133.64 |
| 20 | 0.000 | −1.682 | 0.000 | 80.00 | 55.00 | 66.36 |
| Std. a Order | DPPH b | FRAP c | BCB d | |||
|---|---|---|---|---|---|---|
| Exp. e | Pred. f | Exp. e | Pred. f | Exp. e | Pred. f | |
| 1 | 62.12 | 62.07 | 6.13 | 6.14 | 82.79 | 82.86 |
| 2 | 69.98 | 69.90 | 6.51 | 6.51 | 79.03 | 79.11 |
| 3 | 68.01 | 67.96 | 6.05 | 6.05 | 78.18 | 78.38 |
| 4 | 67.63 | 67.53 | 6.62 | 6.63 | 81.47 | 81.49 |
| 5 | 70.14 | 70.08 | 6.60 | 6.59 | 79.66 | 79.74 |
| 6 | 66.53 | 66.42 | 6.42 | 6.42 | 83.06 | 82.96 |
| 7 | 77.45 | 77.37 | 6.38 | 6.38 | 83.40 | 83.42 |
| 8 | 65.57 | 65.45 | 6.42 | 6.41 | 93.47 | 93.51 |
| 9 | 73.68 | 73.75 | 6.29 | 6.29 | 80.00 | 79.83 |
| 10 | 70.14 | 70.31 | 6.63 | 6.60 | 85.13 | 85.16 |
| 11 | 63.53 | 63.63 | 6.57 | 6.57 | 80.58 | 80.55 |
| 12 | 67.63 | 67.76 | 6.47 | 6.48 | 85.77 | 85.66 |
| 13 | 66.08 | 66.17 | 6.28 | 6.27 | 80.52 | 80.36 |
| 14 | 71.01 | 71.15 | 6.46 | 6.47 | 87.81 | 87.84 |
| 15 | 76.22 | 76.17 | 7.38 | 7.35 | 92.56 | 92.31 |
| 16 | 76.11 | 76.17 | 7.32 | 7.35 | 92.09 | 92.31 |
| 17 | 76.00 | 76.17 | 7.34 | 7.35 | 92.17 | 92.31 |
| 18 | 75.99 | 76.17 | 7.36 | 7.35 | 92.18 | 92.31 |
| 19 | 76.23 | 76.17 | 7.37 | 7.35 | 92.29 | 92.31 |
| 20 | 76.52 | 76.17 | 7.35 | 7.35 | 92.54 | 92.31 |
| Source | DPPH | FRAP | BCB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Square | F-Value | p-Value | Mean Square | F-Value | p-Value | Mean Square | F-Value | p-Value | |
| Model | 50.16 | 1474.51 | <0.0001 | 0.46 | 1486.99 | <0.0001 | 65.39 | 1916.64 | <0.0001 |
| A–Temperature | 14.28 | 419.69 | <0.0001 | 0.14 | 453.82 | <0.0001 | 34.20 | 1002.56 | <0.0001 |
| B–Time | 20.63 | 606.46 | <0.0001 | 8.403 × 10−3 | 27.40 | 0.0004 | 31.43 | 921.35 | <0.0001 |
| C–Ethanol concentration | 30.00 | 881.89 | <0.0001 | 0.048 | 155.09 | <0.0001 | 67.61 | 1981.67 | <0.0001 |
| AB | 34.07 | 1001.60 | <0.0001 | 0.021 | 66.84 | <0.0001 | 23.56 | 690.58 | <0.0001 |
| AC | 65.95 | 1938.75 | <0.0001 | 0.15 | 492.19 | <0.0001 | 24.29 | 711.87 | <0.0001 |
| BC | 0.99 | 29.01 | 0.0003 | 8.001 × 10−3 | 26.08 | 0.0005 | 33.34 | 977.15 | <0.0001 |
| A2 | 30.96 | 910.14 | <0.0001 | 1.45 | 4733.10 | <0.0001 | 173.51 | 5085.76 | <0.0001 |
| B2 | 197.69 | 5811.29 | <0.0001 | 1.26 | 4097.01 | <0.0001 | 152.63 | 4473.69 | <0.0001 |
| C2 | 101.62 | 2987.23 | <0.0001 | 1.76 | 5723.86 | <0.0001 | 121.45 | 3559.65 | <0.0001 |
| Lack of fit | 0.029 | 0.76 | 0.6137 * | 1.468 × 10−3 | 0.31 | 0.8849 * | 0.028 | 0.70 | 0.6468 * |
| No | Name of the Compound | Formula | Retention Time (min) | Peak Area (%) |
|---|---|---|---|---|
| 1. | 1-Nonadecene | C19H38 | 6.841 | 1.40 |
| 2. | 2-Isopropyl-5-methylcyclohexanol | C10H20O | 7.408 | 1.16 |
| 3. | Benzenecarboxylic acid | C7H6O2 | 7.635 | 0.90 |
| 4. | Undecane | C11H24 | 7.682 | 0.47 |
| 5. | 1-Dodecanol | C12H26O | 8.730 | 0.98 |
| 6. | Nonanoic acid | C9H18O2 | 8.978 | 0.29 |
| 7. | Benzenemethanol | C7H8O | 9.124 | 3.17 |
| 8. | 3-Allyl-2-methoxyphenol | C10H12O2 | 9.175 | 0.94 |
| 9. | Tetradecane | C14H30 | 9.813 | 0.76 |
| 10. | Decyltrifluoroacetate | C12H21F3O2 | 9.978 | 0.88 |
| 11. | Phenol, 2,4-bis(1,1-dimethylethyl) | C14H22O | 10.180 | 0.59 |
| 12. | Lauric acid | C12H24O2 | 10.273 | 0.35 |
| 13. | Lauric acid, methyl ester | C13H26O2 | 10.407 | 4.93 |
| 14. | 5-Phenylundecane | C17H28 | 11.170 | 0.77 |
| 15. | Benzophenone | C13H10O | 11.346 | 0.58 |
| 16. | Ethylhexyl benzoate | C15H22O2 | 11.554 | 1.65 |
| 17. | Heptadecane | C17H36 | 11.714 | 1.04 |
| 18. | Myristic acid | C14H28O2 | 11.819 | 0.87 |
| 19. | N-Hexadecanoic acid | C17H34O2 | 12.244 | 10.50 |
| 20. | l-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 12.410 | 14.66 |
| 21. | Benzaldehyde | C7H6O | 12.592 | 0.27 |
| 22. | Pentadecanoic acid | C15H30O2 | 12.758 | 0.27 |
| 23. | 9-Octadecenoic acid (Z) | C19H36O2 | 13.154 | 11.36 |
| 24. | 1-Hexadecene | C16H32 | 13.190 | 8.82 |
| 25. | 1-Tetradecene | C14H28 | 13.376 | 8.89 |
| 26. | Cyclopentacycloheptene | C10H8 | 13.408 | 9.96 |
| 27. | Behenic alcohol | C22H46O | 13.514 | 1.62 |
| 28. | Linoleic acid | C18H32O2 | 13.550 | 3.07 |
| 29. | Oleic acid | C18H34O2 | 14.167 | 6.17 |
| 30. | Octadecanoic acid | C18H36O2 | 15.535 | 2.69 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayakumar, R.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E. Optimization of the Antioxidant Potentials of Red Pitaya Peels and Its In Vitro Skin Whitening Properties. Appl. Sci. 2018, 8, 1516. https://doi.org/10.3390/app8091516
Vijayakumar R, Abd Gani SS, Zaidan UH, Halmi MIE. Optimization of the Antioxidant Potentials of Red Pitaya Peels and Its In Vitro Skin Whitening Properties. Applied Sciences. 2018; 8(9):1516. https://doi.org/10.3390/app8091516
Chicago/Turabian StyleVijayakumar, Ramya, Siti Salwa Abd Gani, Uswatun Hasanah Zaidan, and Mohd Izuan Effendi Halmi. 2018. "Optimization of the Antioxidant Potentials of Red Pitaya Peels and Its In Vitro Skin Whitening Properties" Applied Sciences 8, no. 9: 1516. https://doi.org/10.3390/app8091516




