Optimization of AZ91D Process and Corrosion Resistance Using Wire Arc Additive Manufacturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. WAAM (Wire and Arc Additive Manufacturing)
2.2. Experimental Setup
2.3. Sample Preparation
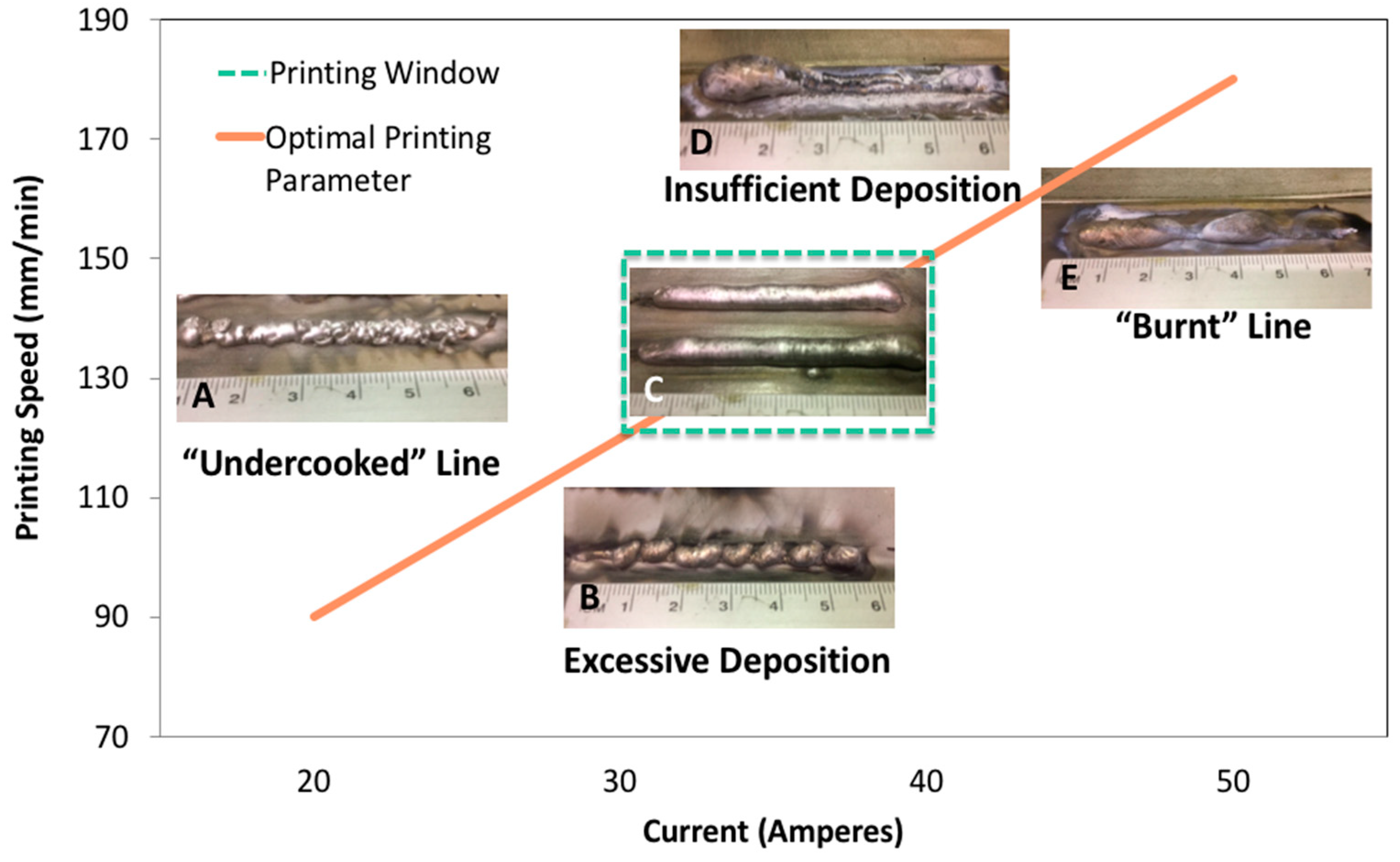
2.4. Experimental Design
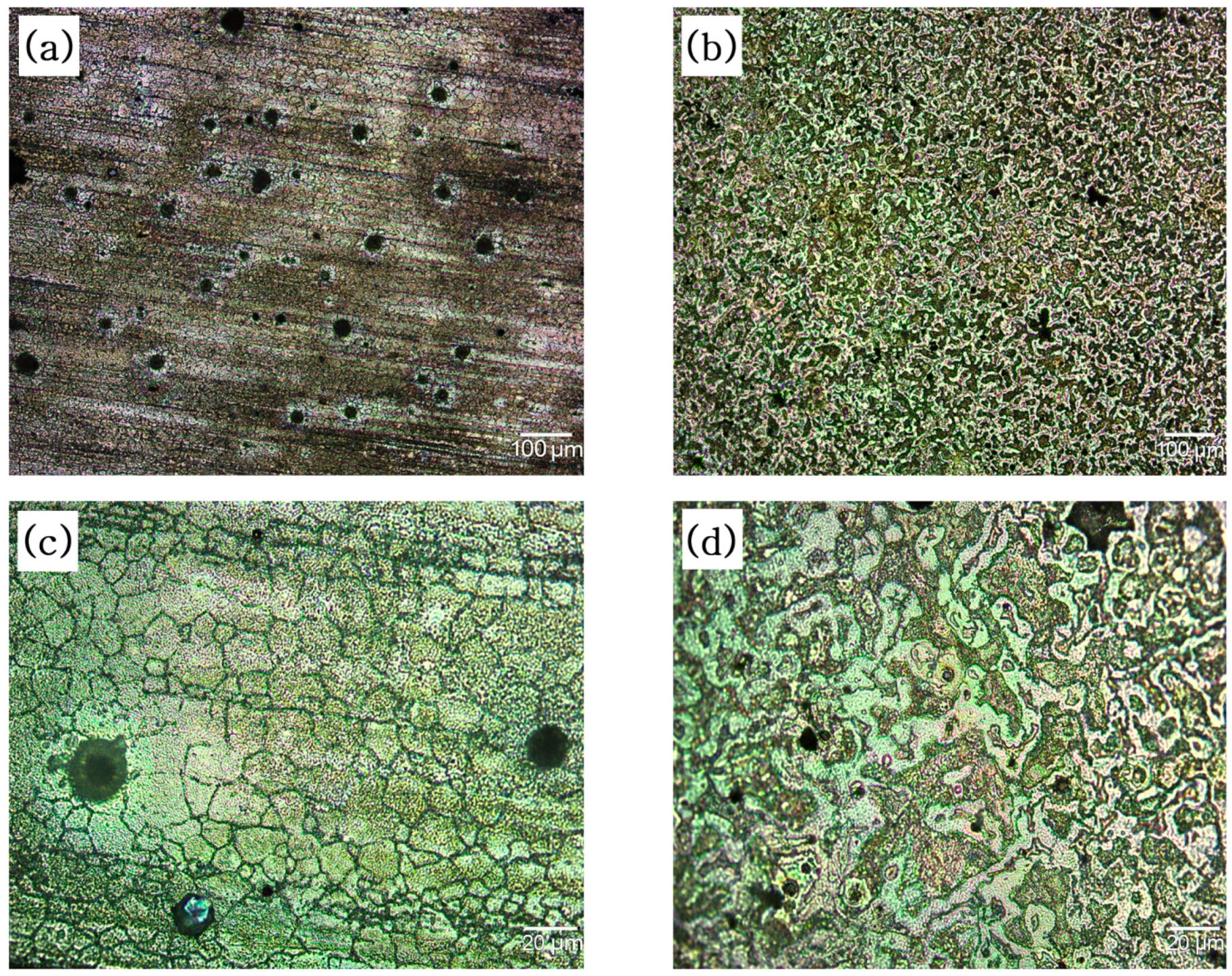
2.4.1. Optical Microscope
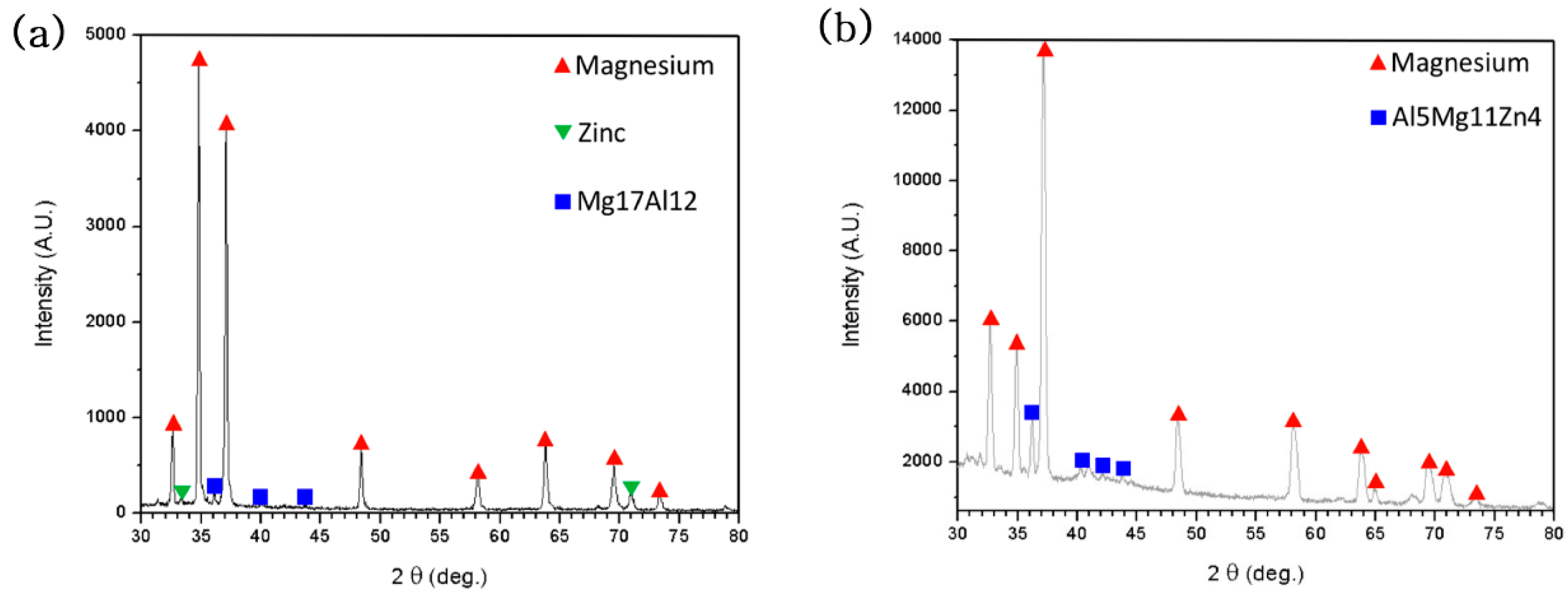
2.4.2. X-ray Diffraction (XRD)
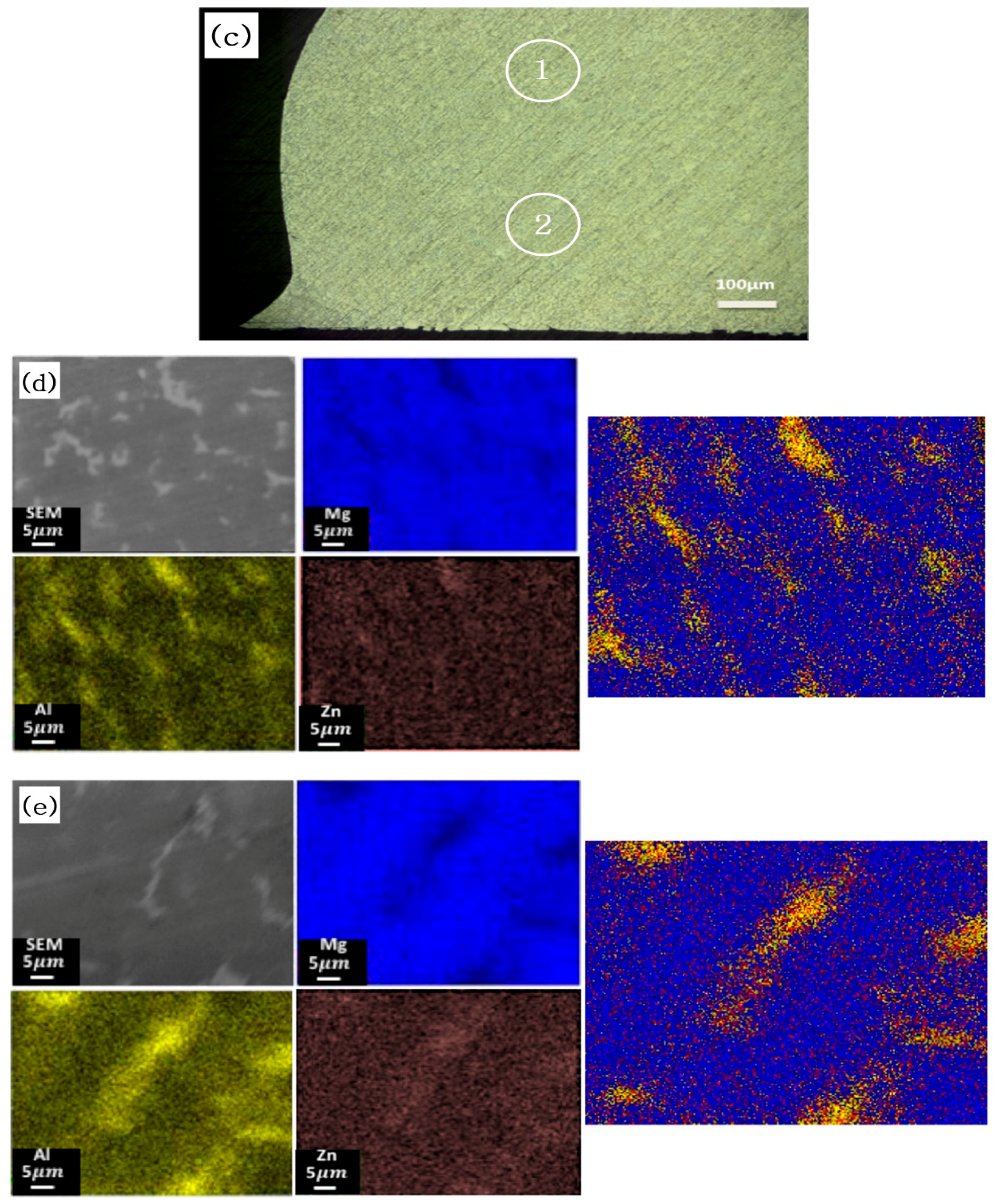
2.4.3. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
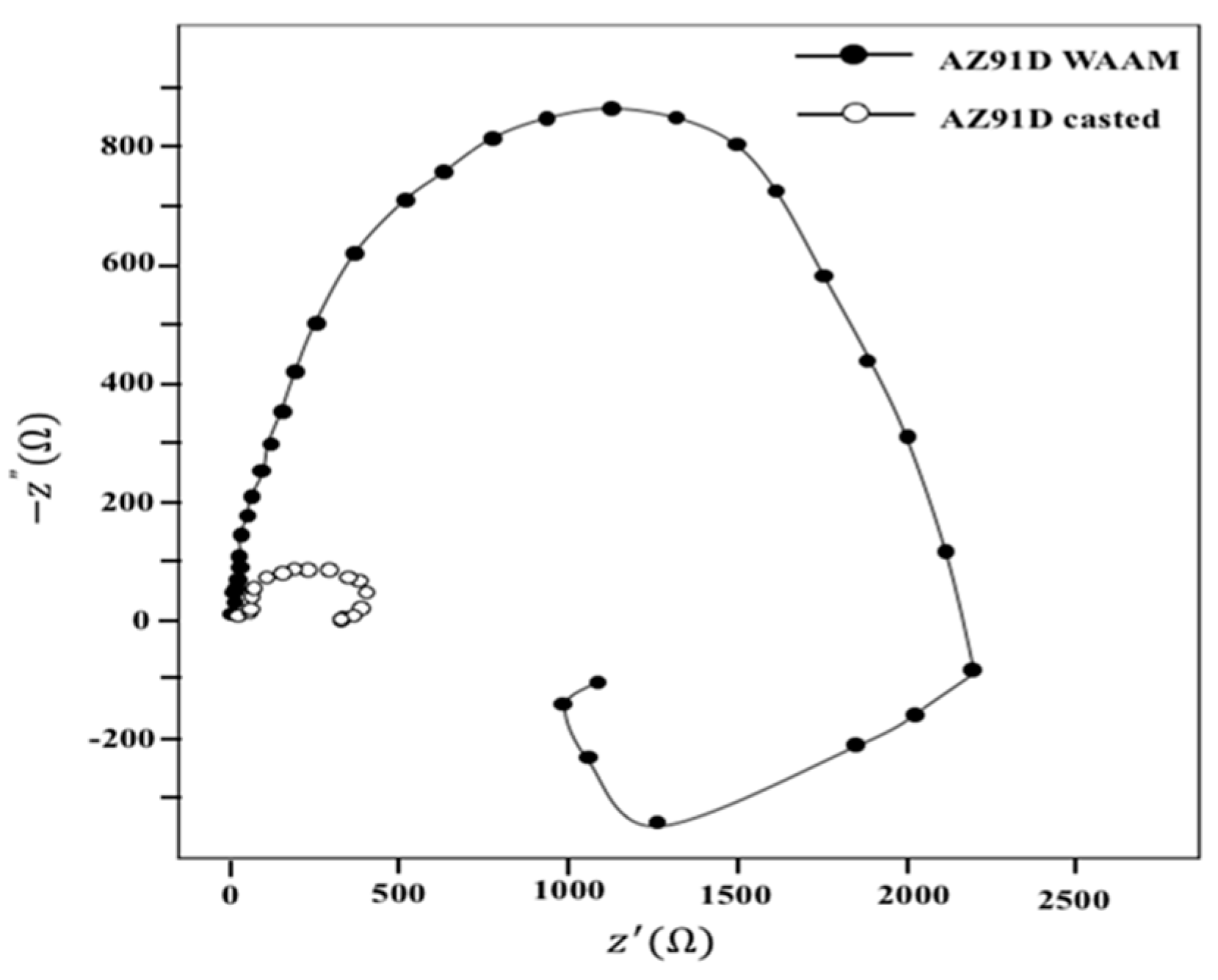
2.4.4. Electrochemical Impedance Spectroscopy (EIS)
3. Results and Discussion
3.1. Sample Preparation
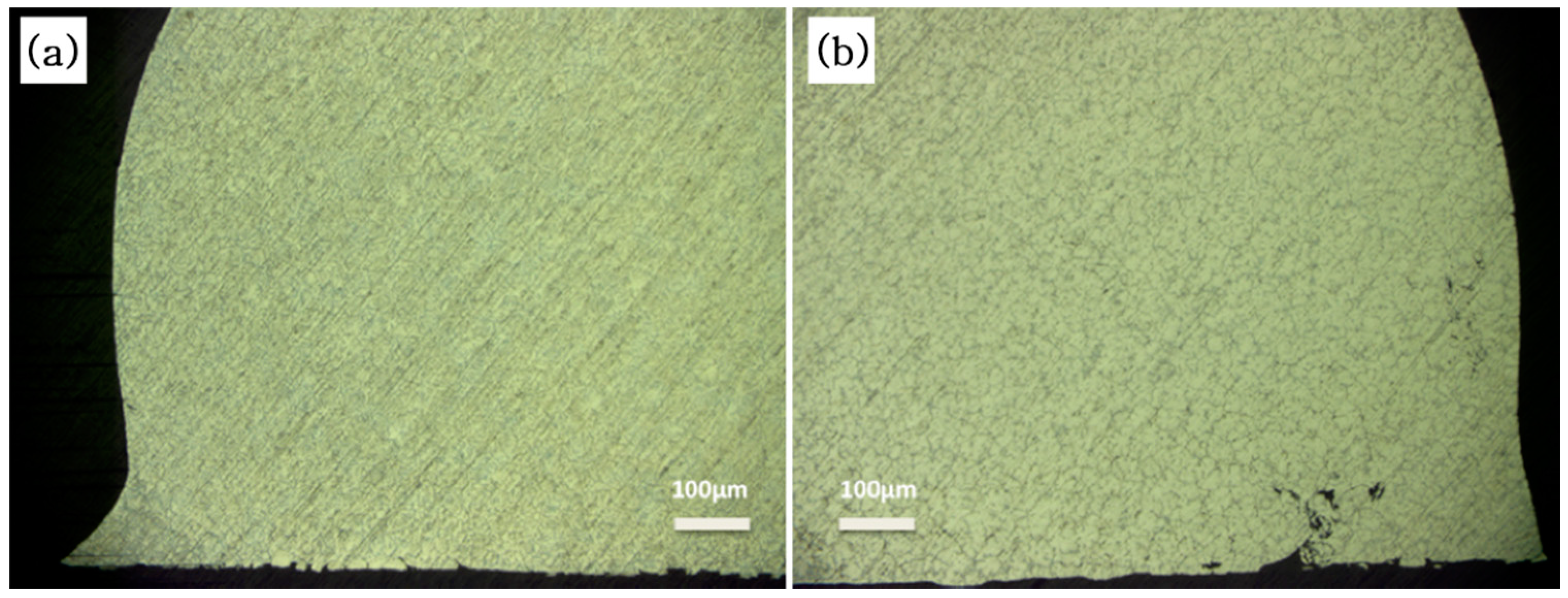
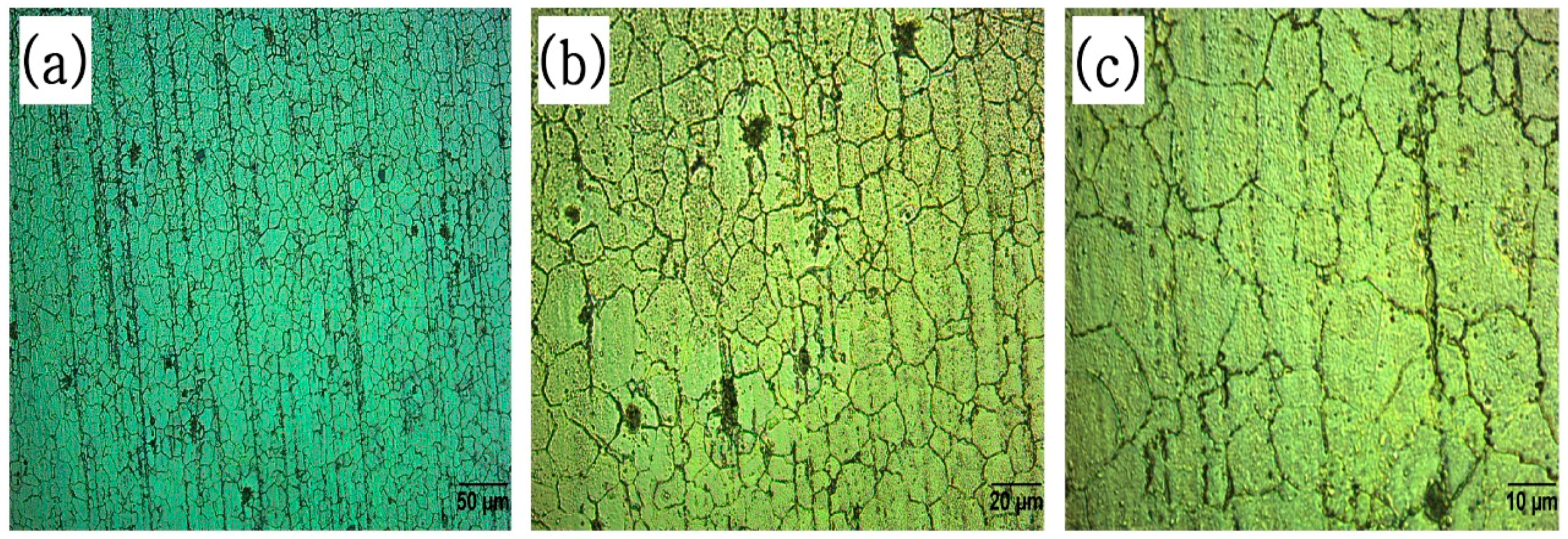
3.2. Microscopic Analysis
3.3. Electrochemical Impedance Spectroscopy (EIS)
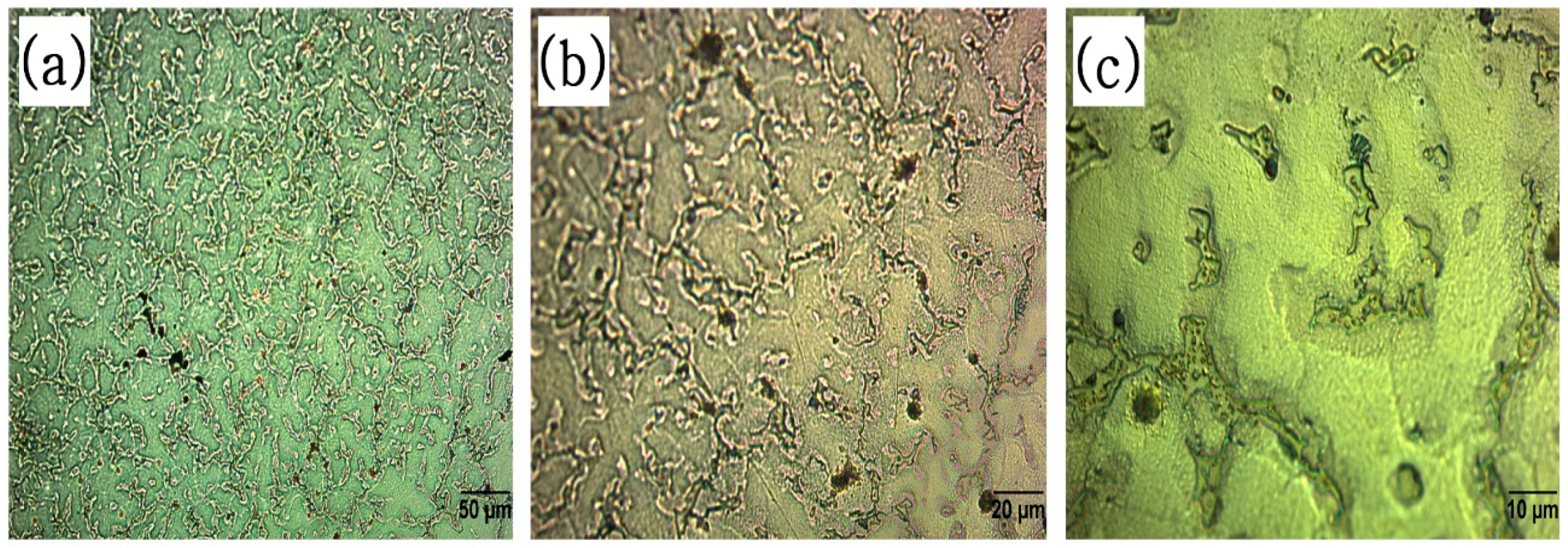
3.4. Microstructure after Corrosion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Nájera, S.E.; Michel, M.; Kim, K.-S. 3D Printed PLA/PCL/TiO2 Composite for Bone Replacement and Grafting. MRS Adv. 2018, 1–6. [Google Scholar] [CrossRef]
- Niinomi, M.; Hattori, T.; Morikawa, K.; Kasuga, T.; Suzuki, A.; Fukui, H.; Niwa, S. Development of Low Rigidity β-Type Titanium Alloy for Biomedical Applications. Mater. Trans. 2002, 43, 2970–2977. [Google Scholar] [CrossRef]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Ibrahim, M.N.M. Problem of stress shielding and improvement to the hip implant designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef]
- Lhotka, C.; Szekeres, T.; Steffan, I.; Zhuber, K.; Zweymüller, K. Four-Year Study of Cobalt and Chromium Blood Levels in Patients Managed with Two Different Metal-on-Metal Total Hip Replacements. J. Orthop. Res. 2003, 21, 189–195. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Eliezer, A. Biodegradable Metals. In Degradation of Implant Materials; Eliaz, N., Ed.; Springer: New York, NY, USA, 2012; pp. 93–109. ISBN 978-1-4614-3941-7. [Google Scholar]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo Corrosion Measurements of Magnesium Alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Levesque, J.; Dube, D.; Fiset, M.; Mantovani, D. Investigation of Corrosion Behaviour of Magnesium Alloy AM60B-F under Pseudo-Physiological Conditions. Mater. Sci. Forum 2003, 424–426, 521–526. Available online: https://www.cheric.org/research/tech/periodicals/view.php?seq=1254436 (accessed on 16 July 2018). [CrossRef]
- Xin, Y.; Liu, C.; Zhang, X.; Tang, G.; Tian, X.; Chu, P.K. Corrosion Behavior of Biomedical AZ91 Magnesium Alloy in Simulated Body Fluids. J. Mater. Res. 2007, 22, 2004–2011. [Google Scholar] [CrossRef]
- Gao, M.; Wei, R.P. A ‘Hydrogen Partitioning’ Model for Hydrogen Assisted Crack Growth. Metall. Trans. A 1985, 16, 2039–2050. [Google Scholar] [CrossRef]
- Regev, M.; Aghion, E.; Rosen, A.; Bamberger, M. Creep Studies of Coarse-Grained AZ91D Magnesium Castings. Mater. Sci. Eng. A 1998, 252, 6–16. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. ISRN Mech. Eng. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef] [Green Version]
- Han, K.N.; Kim, N.S. Challenges and Opportunities in Direct Write Technology Using Nano-Metal Particles. KONA Powder Part. J. 2009, 27, 73–83. [Google Scholar] [CrossRef]
- Amert, A.K.; Oh, D.-H.; Kim, N.-S. A Simulation and Experimental Study on Packing of Nanoinks to Attain Better Conductivity. J. Appl. Phys. 2010, 108, 102806. [Google Scholar] [CrossRef]
- Kim, N.S.; Han, K.N.; Church, K.H. Direct Writing Technology for 21th Century Industries—Focus on Micro—Dispensing Deposition Write Technology. In Proceedings of the Korean Society of Machine Tool Engineers Spring Conference 2007, Seoul, Korea, 14–19 May 2007. [Google Scholar]
- Taminger, K.M.B.; Hafley, R.A. Electron Beam Freeform Fabrication: A Rapid Metal Deposition Process. In Proceedings of the 3rd Annual Automotive Composites Conference, Troy, MI, USA, 9–10 September 2003. [Google Scholar]
- George, B.; Voort, V. Metallography of Magnesium and Its Alloys. Buehler Tech-Notes 2015, 4, 1–5. [Google Scholar]
- Huo, H.; Li, Y.; Wang, F. Corrosion of AZ91D Magnesium Alloy with a Chemical Conversion Coating and Electroless Nickel Layer. Corros. Sci. 2004, 46, 1467–1477. [Google Scholar] [CrossRef]
- West, E.G. The Welding of Non-Ferrous Metals, 1st ed.; John Wiley & Sons: New York, NY, USA, 1951. [Google Scholar]
- Humpherys, F.J. Recrystallization and Related Annealing Phenomena, 1st ed.; Elsevier B. V.: Kidlington, Oxford, UK, 1995. [Google Scholar]
- Martinez, D.A.; Han, S.; Kim, N.P. Magnesium Alloy 3D Printing by Wire and Arc Additive Manufacturing (WAAM). MRS Adv. 2018, 1–6. [Google Scholar] [CrossRef]
- Cao, C.-N. On the Impedance Plane Displays for Irreversible Electrode Reactions Based on the Stability Conditions of the Steady-State—I. One State Variable besides Electrode Potential. Electrochim. Acta 1990, 35, 831–836. [Google Scholar] [CrossRef]
- Cao, C.-N. On the Impedance Plane Displays for Irreversible Electrode Reactions Based on the Stability Conditions of the Steady-State—II. Two State Variables besides Electrode Potential. Electrochim. Acta 1990, 35, 837–844. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Feliú, S. Influence of Microstructure and Composition on the Corrosion Behaviour of Mg/Al Alloys in Chloride Media. Electrochim. Acta 2008, 53, 7890–7902. [Google Scholar] [CrossRef]

| Density | Elastic Modulus (GPa) | Compressive Strength (MPa) | Fracture Toughness (MPa/m2) | |
|---|---|---|---|---|
| Natural Bone | 1.8–2.1 | 3–20 | 130–180 | 3–6 |
| Ti alloy | 4.4–4.5 | 110–170 | 758–1117 | 55–115 |
| Co-Cr Alloy | 8.3–9.2 | 230 | 450–1000 | N/A |
| Stainless Steel | 7.9–8.1 | 189–205 | 170–310 | 50–200 |
| Magnesium | 1.74–2.0 | 41–45 | 64–100 | 15–40 |
| Element | Wt % |
|---|---|
| Al | 8.5–9.5 |
| Zn | 0.45–0.9 |
| Si | 0.05 max |
| Mn | 0.17 max |
| Cu | 0.015 max |
| Fe | 0.004 max |
| Ni | 0.001 max |
| Other | 0.01 max |
| Mg | Balance |
| Current (Ampere) | Printing Speed (mm/min) | Heat Input (kJ/mm) |
|---|---|---|
| 30 | 120 | 0.225 |
| 30 | 135 | 0.200 |
| 30 | 150 | 0.180 |
| 32.5 | 120 | 0.244 |
| 32.5 | 135 | 0.217 |
| 32.5 | 150 | 0.195 |
| 35 | 120 | 0.263 |
| 35 | 135 | 0.233 |
| 35 | 150 | 0.210 |
| 37.5 | 120 | 0.281 |
| 37.5 | 135 | 0.250 |
| 37.5 | 150 | 0.225 |
| 40 | 120 | 0.300 |
| 40 | 135 | 0.267 |
| 40 | 150 | 0.240 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Zielewski, M.; Martinez Holguin, D.; Michel Parra, M.; Kim, N. Optimization of AZ91D Process and Corrosion Resistance Using Wire Arc Additive Manufacturing. Appl. Sci. 2018, 8, 1306. https://doi.org/10.3390/app8081306
Han S, Zielewski M, Martinez Holguin D, Michel Parra M, Kim N. Optimization of AZ91D Process and Corrosion Resistance Using Wire Arc Additive Manufacturing. Applied Sciences. 2018; 8(8):1306. https://doi.org/10.3390/app8081306
Chicago/Turabian StyleHan, Seungkyu, Matthew Zielewski, David Martinez Holguin, Monica Michel Parra, and Namsoo Kim. 2018. "Optimization of AZ91D Process and Corrosion Resistance Using Wire Arc Additive Manufacturing" Applied Sciences 8, no. 8: 1306. https://doi.org/10.3390/app8081306
APA StyleHan, S., Zielewski, M., Martinez Holguin, D., Michel Parra, M., & Kim, N. (2018). Optimization of AZ91D Process and Corrosion Resistance Using Wire Arc Additive Manufacturing. Applied Sciences, 8(8), 1306. https://doi.org/10.3390/app8081306





