A Comparative Study of Fungal Community Structure, Diversity and Richness between the Soil and the Phyllosphere of Native Grass Species in a Copper Tailings Dam in Shanxi Province, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Research Methods
2.2.1. Sample Collection
2.2.2. Soil Physical and Chemical Properties
2.2.3. DNA Extraction and High-Throughput Sequencing of Soil and Phyllosphere Samples
2.3. Statistical Analysis
3. Results
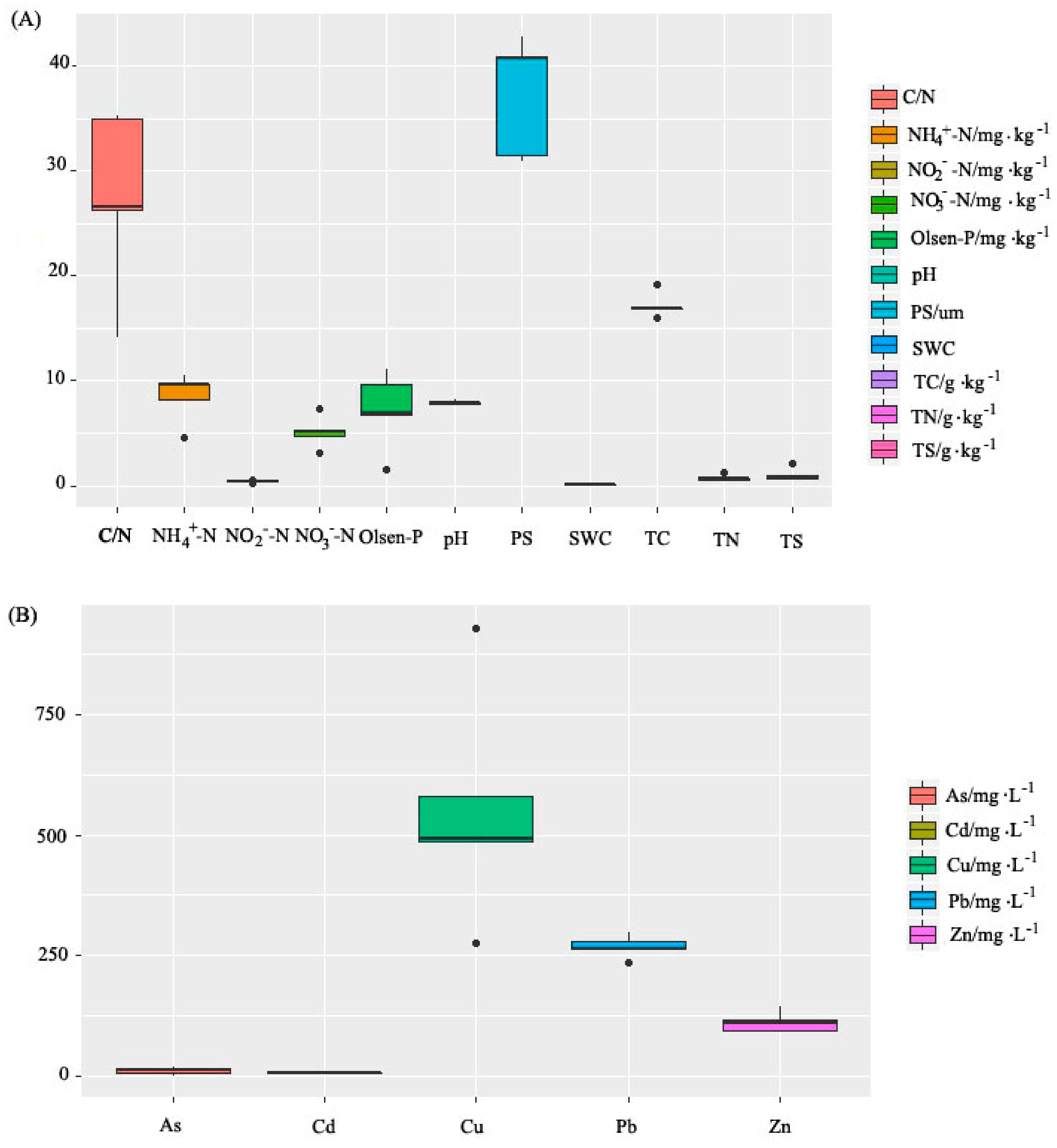
3.1. Soil Physicochemical Properties
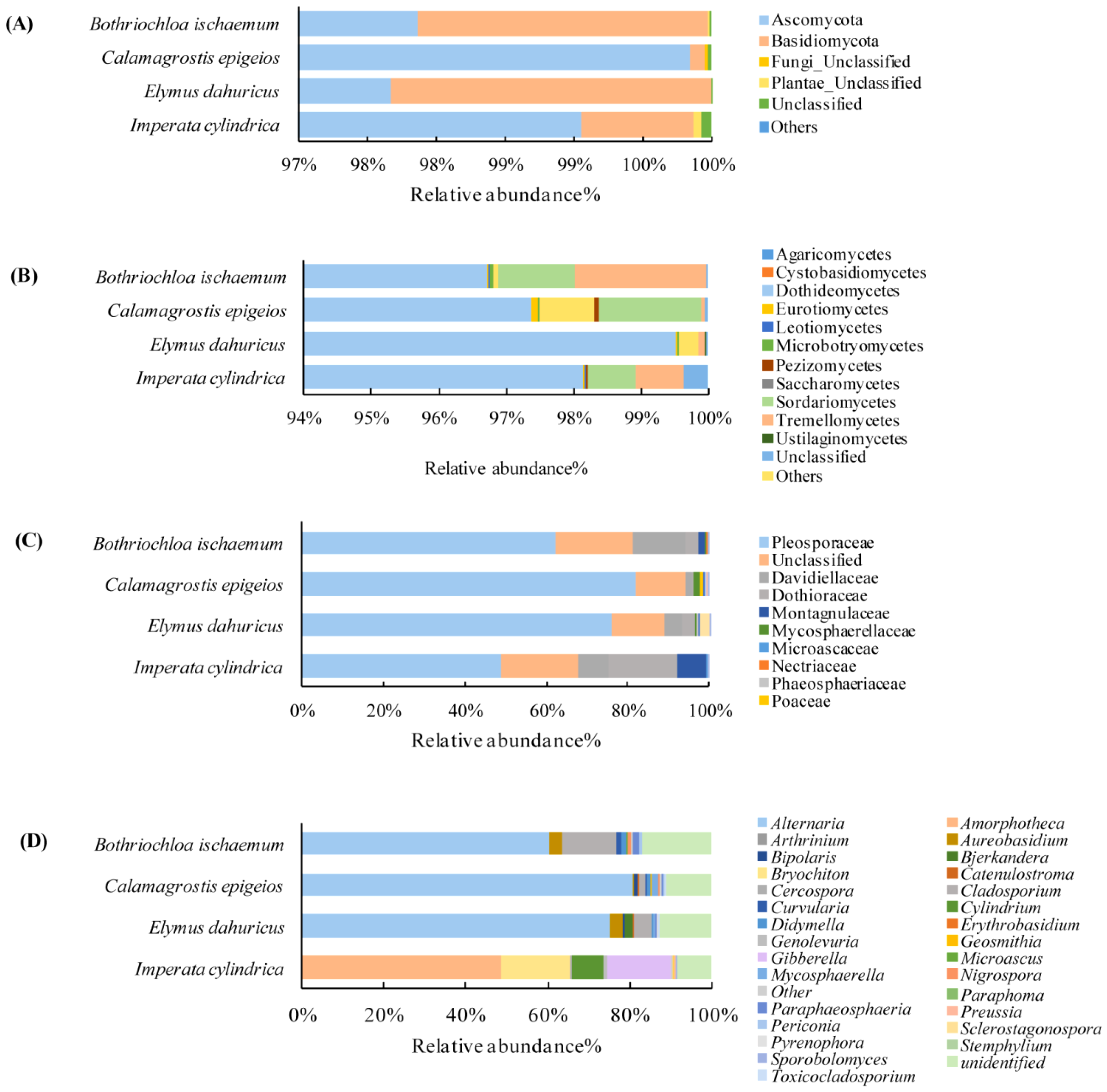
3.2. Community Composition of the Soil and Phyllosphere
3.2.1. Soil Fungal Communities
3.2.2. Phyllosphere Fungal Community Diversity and Richness
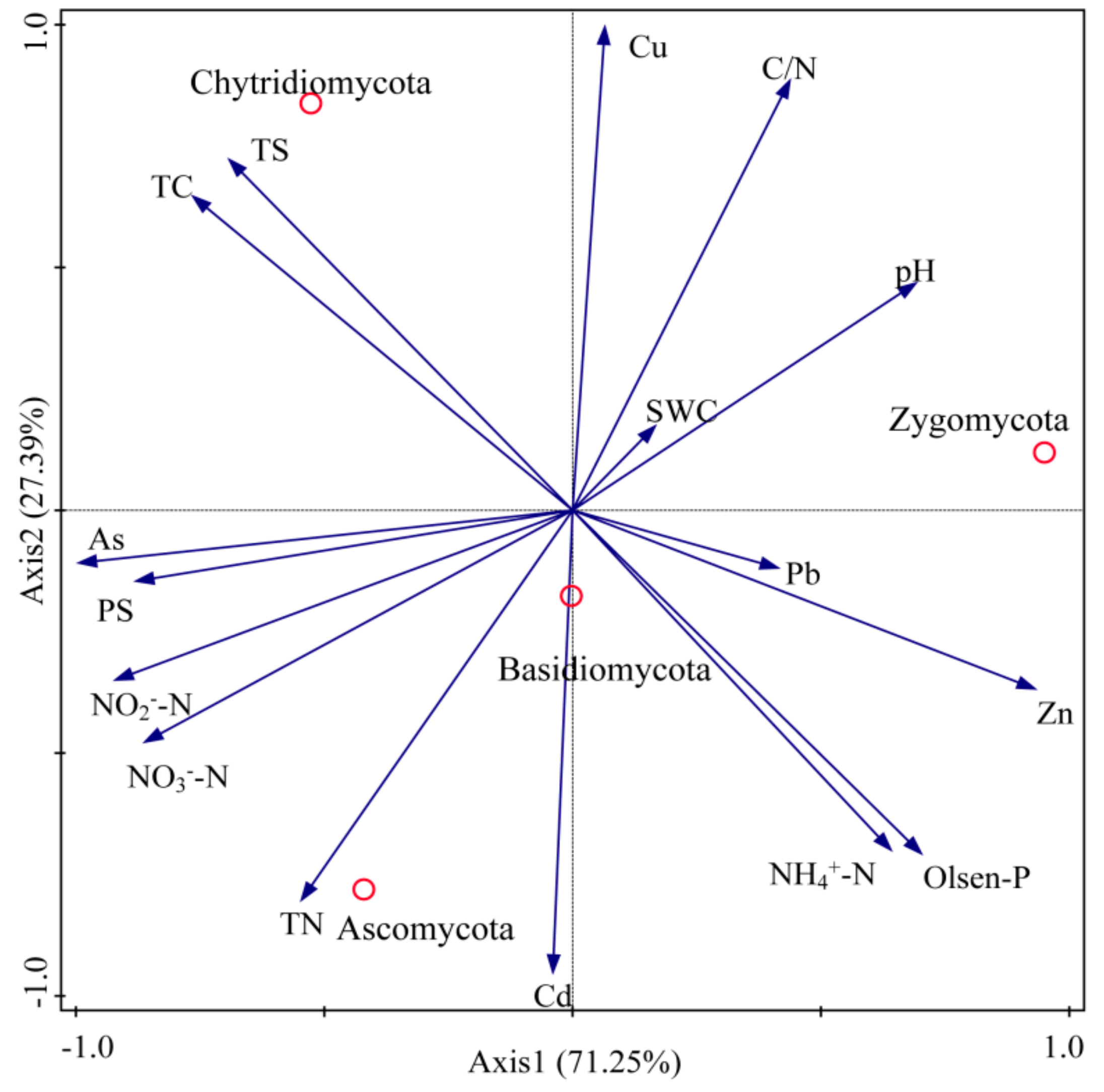
3.3. Properties of Soil Fungi and the Phyllosphere Fungal Communities in Native Grass Species
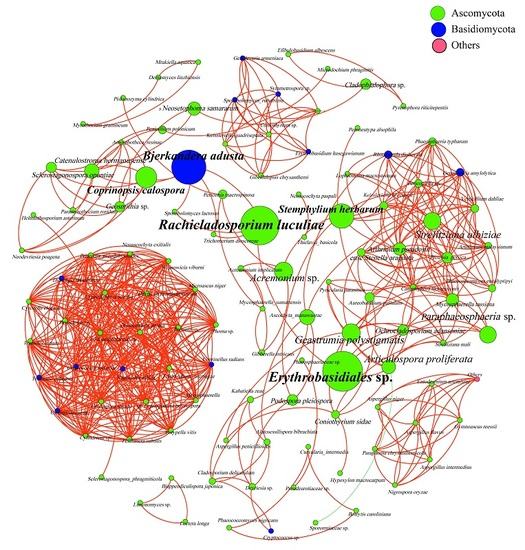
3.4. Analysis of Phyllosphere Fungal Communities Using Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Men, H.; Li, F.; Ba, L. Research progress of soil microbe in Ningxia. J. Anhui Agric. Sci. 2012, 40, 11264–11267. [Google Scholar]
- Liu, D.; Huang, Y.; Sun, H.; An, S. The restoration age of Robinia pseudoacacia, plantation impacts soil microbial biomass and microbial community structure in the loess plateau. Catena 2018, 165, 192–200. [Google Scholar] [CrossRef]
- Kezia, G.; Ingo, S.; François, B.; Tesfaye, W. Forest management type influences diversity and community composition of soil fungi across temperate forest ecosystems. Front. Microbiol. 2015, 6, 1300. [Google Scholar]
- Intanon, S.; Hulting, A.G.; Myrold, D.D.; Mallory-Smith, C.A. Short-term effects of soil amendment with meadowfoam seed meal on soil microbial composition and function. Appl. Soil Ecol. 2015, 89, 85–92. [Google Scholar] [CrossRef]
- Rossi, G.; Beni, C. Effects of medium-term amendment with diversely processed sewage sludge on soil humification-mineralization processes and on Cu, Pb, Ni, and Zn bioavailability. Plants 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, J.; Zhang, T. Advancement on soil fungal research. J. Fungal Res. 2005, 3, 52–58. [Google Scholar]
- Li, G.; Ma, K. Progress in the study of elevational patterns of soil microbial diversity. Acta Ecol. Sin. 2018, 38, 1521–1529. [Google Scholar]
- Ma, J. Research advances in effects of plant on soil microbial diversity. Biotechworld 2016, 2, 24. [Google Scholar]
- Bi, J.; He, D. Research advances in effects of plant on soil microbial diversity. Chin. Agric. Sci. Bull. 2009, 9, 244–250. [Google Scholar]
- MejíA, L.; Rojas, E.; Maynard, Z.; Svan, B.; Arnold, A.; Hebbar, P.; Samuels, G.; Robbins, N.; Herre, E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control. 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Pan, J.; Hu, Q.; Qi, H.; Zhang, H.; Zhuang, G.; Bai, Z. Advance in the research of phyllospheric microorganism. Acta Ecol. Sin. 2011, 31, 583–592. [Google Scholar]
- Ronseaux, S.; Clément, C.; Barka, E. Interaction of Ulocladium atrum, a potential biological control agent, with Botrytis cinerea and grapevine plantlets. Agronomy 2013, 3, 632–647. [Google Scholar] [CrossRef]
- Cao, M.; Jia, T.; Jing, J.; Chai, B. Distribution and rDNA-ITS sequence analysis of endophyte symbionts of Bothriochloa ischaemum at a copper tailings site. Acta Pratacult. Sin. 2017, 5, 163–172. [Google Scholar]
- Jia, T.; Cao, M.; Jing, J.; Liu, J.; Chai, B. Endophytic fungi and soil microbial community characteristics over different years of phytoremediation in a copper tailings dam of Shanxi, China. Sci. Total Environ. 2017, 574, 881–888. [Google Scholar]
- Wang, R.H; Jia, T.; Cao, M.W.; Chai, B.F. Characteristics of soil physicochemical properties and enzyme activities over different reclaimed years in a copper tailings dam. Environ. Sci. 2018, 39, 3339–3348. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Lee, S.M.; Jeng, S.L. Estimating population size for capture recapture data when capture probabilities vary by time and individual animal. Biometrics 1992, 48, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Shen, T.J. Nonparametric estimation of Shannon’s index of diversity when there are unseen species in sample. Environ. Ecol. Stat. 2003, 10, 429–443. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, R.; Xu, N.; Liu, Y.; Chai, C.; Wang, J.; Fu, X.; Zhong, H.; Ni, H. Fungal community structure of different degeneration deyeuxia angustifolia wetlands in Sanjiang Plain. Environ. Sci. 2016, 37, 3598–3605. [Google Scholar]
- Wang, N.; Yu, J.; Chang, Z.; Huang, H.; Gu, K.; Zhang, Z. Responses of fungal community diversity and composition in paddy soils to straw return. Soils 2017, 49, 1115–1120. [Google Scholar]
- Ju, T.; Chen, Y.; Chang, C.; An, L. The diversity of soil fungi and its relations with fertility factors in Taxus chinenesis (Pilg.) Rehd community of Xiaolongshan of Tianshui City. Res. Environ. Sci. 2008, 21, 128–132. [Google Scholar]
- Yelle, D.; Ralph, J.; Lu, F.; Hammel, K. Evidence for cleavage of lignin by a brown rot basidiomycete. Environ. Microbiol. 2008, 10, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Dorfelt, H.; Gube, M.; Jackson, D.; Reitner, J.; Seyfullah, L.; Schmidt, A. Estimating the Phanerozoic history of the Ascomycota lineages: combining fossil and molecular data. Mol. Phylogenet. Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shi, S.; Lin, F.; Jiang, P. Response of the soil fungal community to multi-factor environmental changes in a temperate forest. Appl. Soil Ecol. 2014, 81, 45–56. [Google Scholar] [CrossRef]
- Jiang, H.; Yan, W.; Li, X.; Fan, Y. Diversity and community structure of soil fungi in Larix gmelinii Forest. J. Northwest Forest. Univ. 2010, 25, 100–103. [Google Scholar]
- Wan, X.; Huang, Z.; He, Z.; Yu, Z.; Wang, M.; Davis, M.; Yang, Y. Soil C: N ratio is the major determinant of soil microbial community structure in subtropical coniferous and broadleaf forest plantations. Plant Soil 2015, 387, 103–116. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, Y.; Wang, B.; Wang, W.; Li, X.; Liu, J. Characteristics of soil microorganisms and soil nutrients in different sand-fixation shrub plantations in Kubuqi Desert, China. Chin. J. Appl. Ecol. 2017, 28, 3871–3880. [Google Scholar]
- Han, S.; Gao, R.; Li, A.; Ma, H.; Yin, Y.; Si, Y.; Chen, S.; Zheng, Q. Soil microbial community structure of two types of forests in the mid-subtropics of China. Chin J. Appl. Ecol. 2015, 26, 2151–2158. [Google Scholar]
- Xing, Y.; Si, Y.; Hong, C.; Li, Y. Impact of long-term heavy metal pollution on microbial community in iron mine soil. Res. Environ. Sci. 2013, 26, 1201–1211. [Google Scholar]
- Su, C. Studies on Lead and Cadmium adsorption by exopolysaccharide and extracelluar protein of natural biofilm. J. Jilin Univ. (Sci. Ed.) 2005, 43, 121–125. [Google Scholar]
- Wang, L.; Chen, G.; Zeng, G.; Zhang, W.; Chen, Y. Fungal extracellular polymeric substances and their interaction mechanisms with heavy metals. Environ. Pollut. Control 2010, 32, 74–80. [Google Scholar]
- Antízar-Ladislao, B.; Lopez-Real, J.; Beck, A.J. Investigation of organic matter dynamics during in-vessel composting of an aged coal-tar contaminated soil using fluorescence excitation-emission spectroscopy. Chemosphere 2006, 64, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Chitrakar, R.; Obulareddy, N.; Panchal, S.; Williams, P.; Melotto, M. Molecular battles between plant and pathogenic bacteria in the phyllosphere. Braz. J. Med. Microbiol. Res. 2010, 43, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W. Taxonomy and related studies on the Nectrioid fungi from China. Chin. Bull. Life Sci. 2010, 22, 1083–1085. [Google Scholar]
- Gao, L.; Yu, X.; Xue, Y.; Cui, X.; Li, R.; Yang, Q.; Li, S.; Wang, P. Factors affecting the number of soil microbes in the coastal terrace. J. Anhui Agric. Sci. Univ. 2017, 44, 71–80. [Google Scholar]
- Yang, B.; Jia, N.; Xiong, P. Review on the Secondary Metabolites from Sporormiella sp. Nat. Prod. Res. Dev. 2016, 5, 810–819. [Google Scholar]
- Qiao, S.; Zhou, Y.; Chao, B.; Jia, T.; Li, C. Characteristics of fungi community structure and genetic diversity of forests in Guandi Mountains. Environ. Sci. 2017, 38, 2502–2512. [Google Scholar]

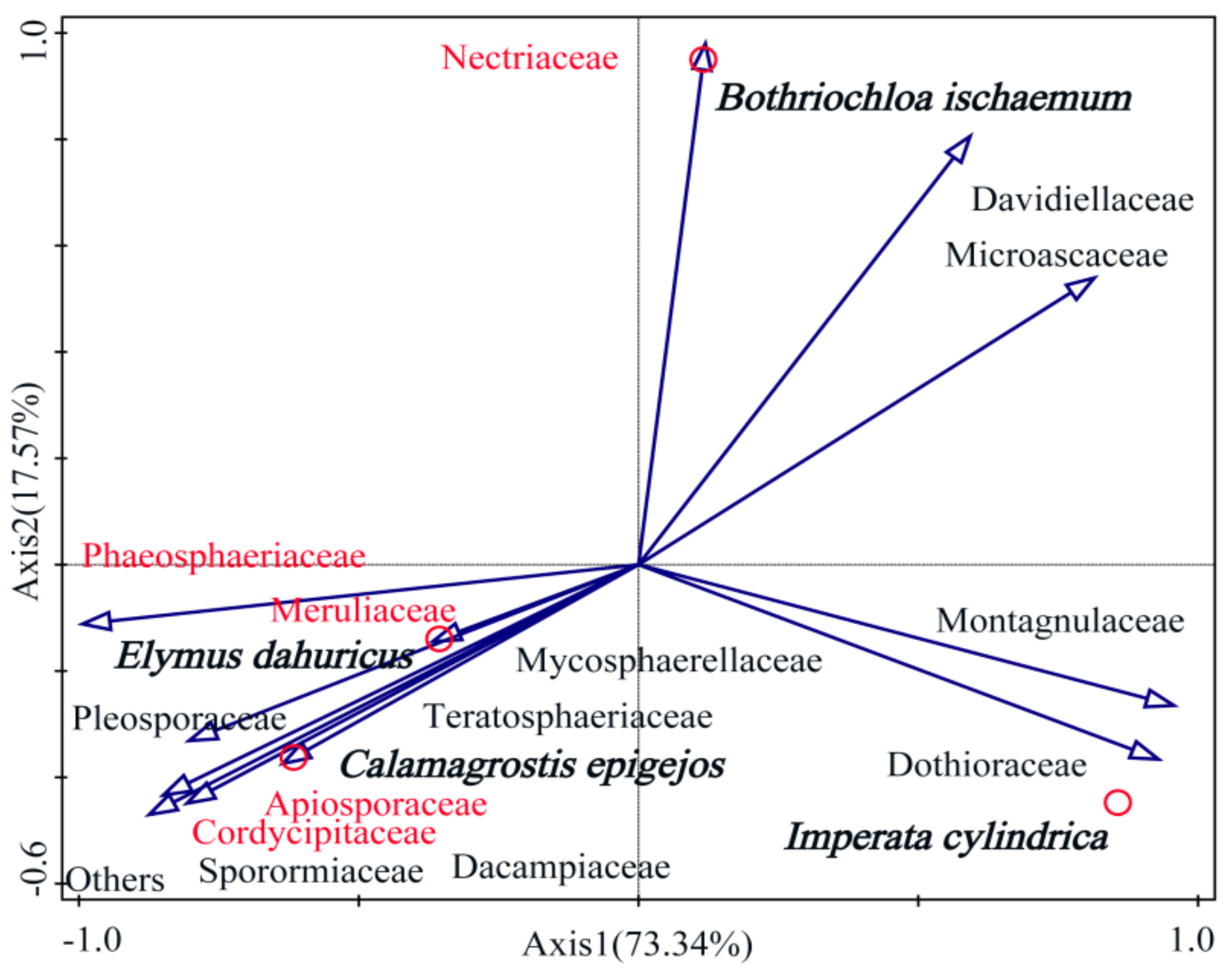
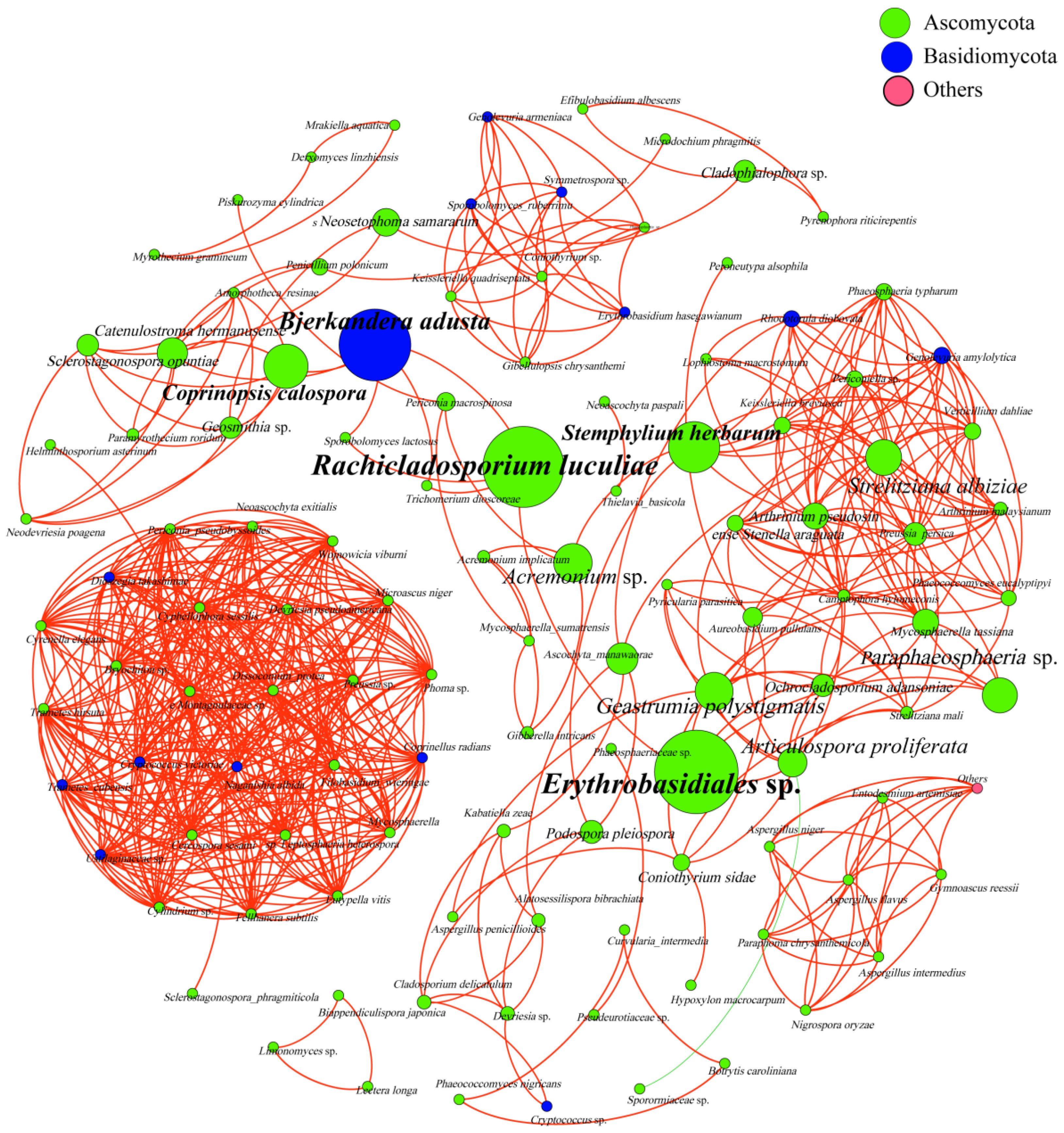
| Sample | ACE | Chao1 | Shannon | Simpson | Coverage % |
|---|---|---|---|---|---|
| Bothriochloa ischaemum | 110.85 | 108.57 | 1.50 | 0.40 | 99.96% |
| Imperata cylindrica | 104.59 | 107.00 | 1.66 | 0.30 | 99.95% |
| Elymus dahuricus | 70.50 | 67.27 | 1.10 | 0.58 | 99.98% |
| Calamagrostis epigejos | 117.25 | 115.05 | 0.95 | 0.66 | 99.94% |
| Type | Nodes | Edges | Average Degree | Network Diameter | Network Density | Modularity | Average Clustering Coefficient | Average Path Length | Co-Presence |
|---|---|---|---|---|---|---|---|---|---|
| Index | 116 | 587 | 10.121 | 14 | 0.088 | 0.605 | 0.788 | 4.016 | 99.83% |
| Erythrobasidiales sp. | Rachicladosporium luculiae | Bjerkandera adusta | Stemphylium herbarum | Coprinopsis calospora | |
|---|---|---|---|---|---|
| Phylum | Ascomycota | Ascomycota | Basidiomycota | Ascomycota | Ascomycota |
| Betweenness Centrality | 697.43 | 672.50 | 585.50 | 387.89 | 323.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, T.; Wang, R.; Fan, X.; Chai, B. A Comparative Study of Fungal Community Structure, Diversity and Richness between the Soil and the Phyllosphere of Native Grass Species in a Copper Tailings Dam in Shanxi Province, China. Appl. Sci. 2018, 8, 1297. https://doi.org/10.3390/app8081297
Jia T, Wang R, Fan X, Chai B. A Comparative Study of Fungal Community Structure, Diversity and Richness between the Soil and the Phyllosphere of Native Grass Species in a Copper Tailings Dam in Shanxi Province, China. Applied Sciences. 2018; 8(8):1297. https://doi.org/10.3390/app8081297
Chicago/Turabian StyleJia, Tong, Ruihong Wang, Xiaohui Fan, and Baofeng Chai. 2018. "A Comparative Study of Fungal Community Structure, Diversity and Richness between the Soil and the Phyllosphere of Native Grass Species in a Copper Tailings Dam in Shanxi Province, China" Applied Sciences 8, no. 8: 1297. https://doi.org/10.3390/app8081297
APA StyleJia, T., Wang, R., Fan, X., & Chai, B. (2018). A Comparative Study of Fungal Community Structure, Diversity and Richness between the Soil and the Phyllosphere of Native Grass Species in a Copper Tailings Dam in Shanxi Province, China. Applied Sciences, 8(8), 1297. https://doi.org/10.3390/app8081297