Effect of Platinum Doping on the Morphology and Sensing Performance for CuO-Based Gas Sensor
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental
2.1. Preparation of CuO
2.2. Pt-Doped CuO Flowers
2.3. Characterization
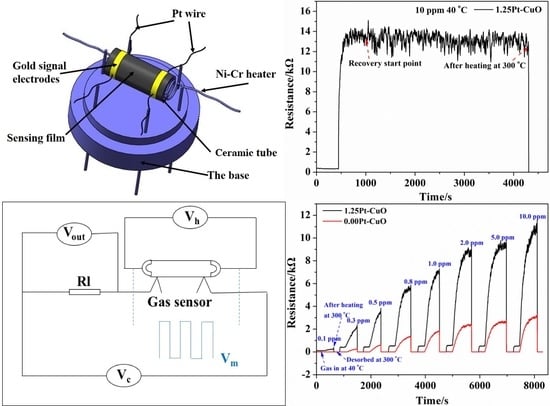
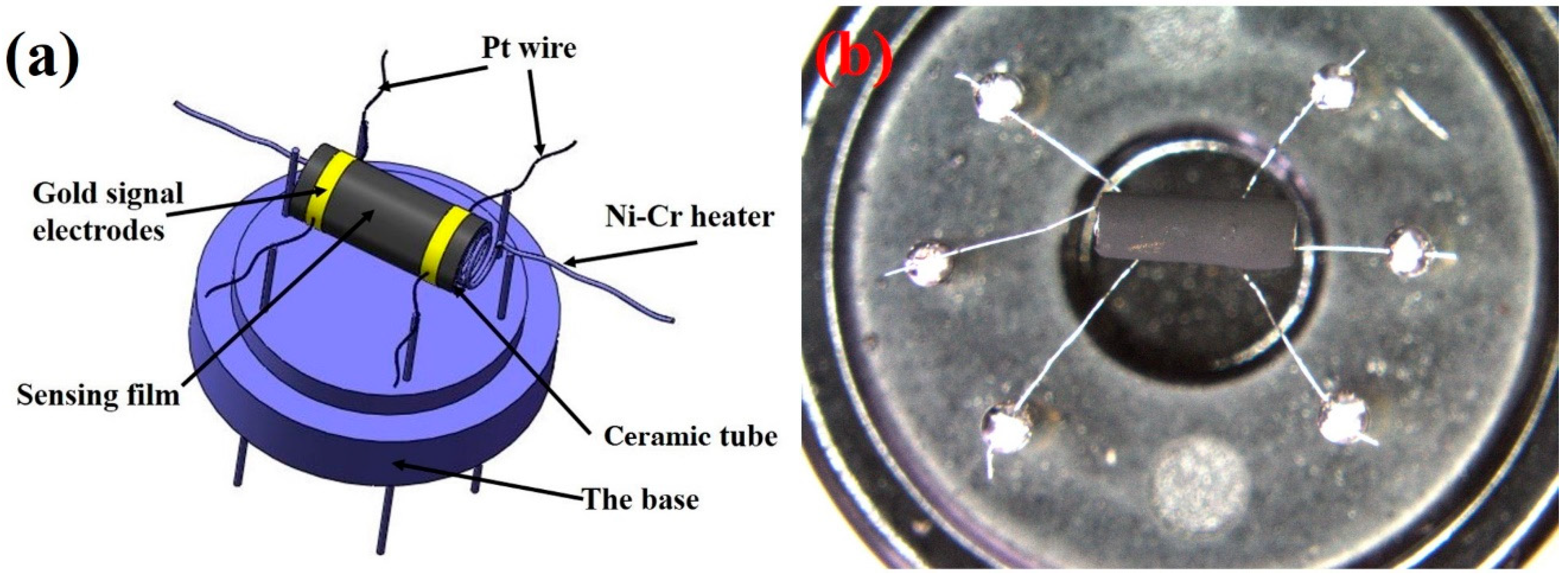
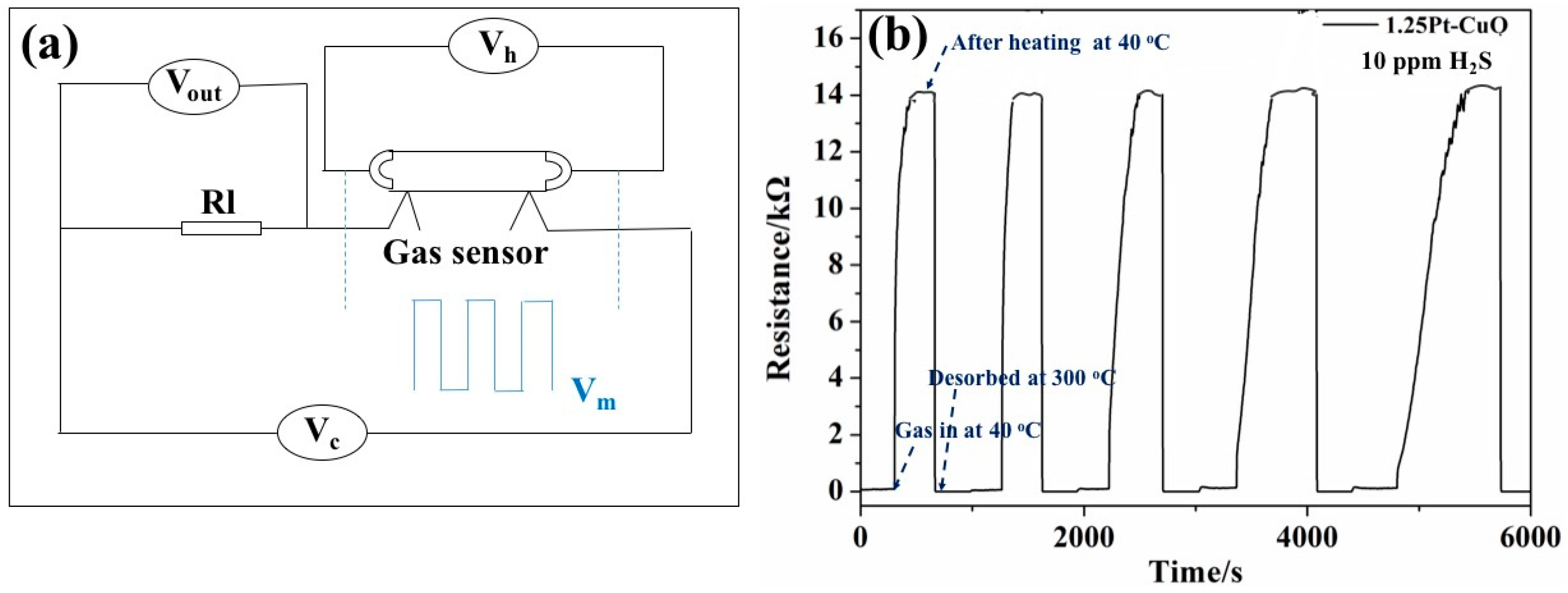
2.4. Fabrication and Characterization of Gas Sensors
3. Results and Discussions
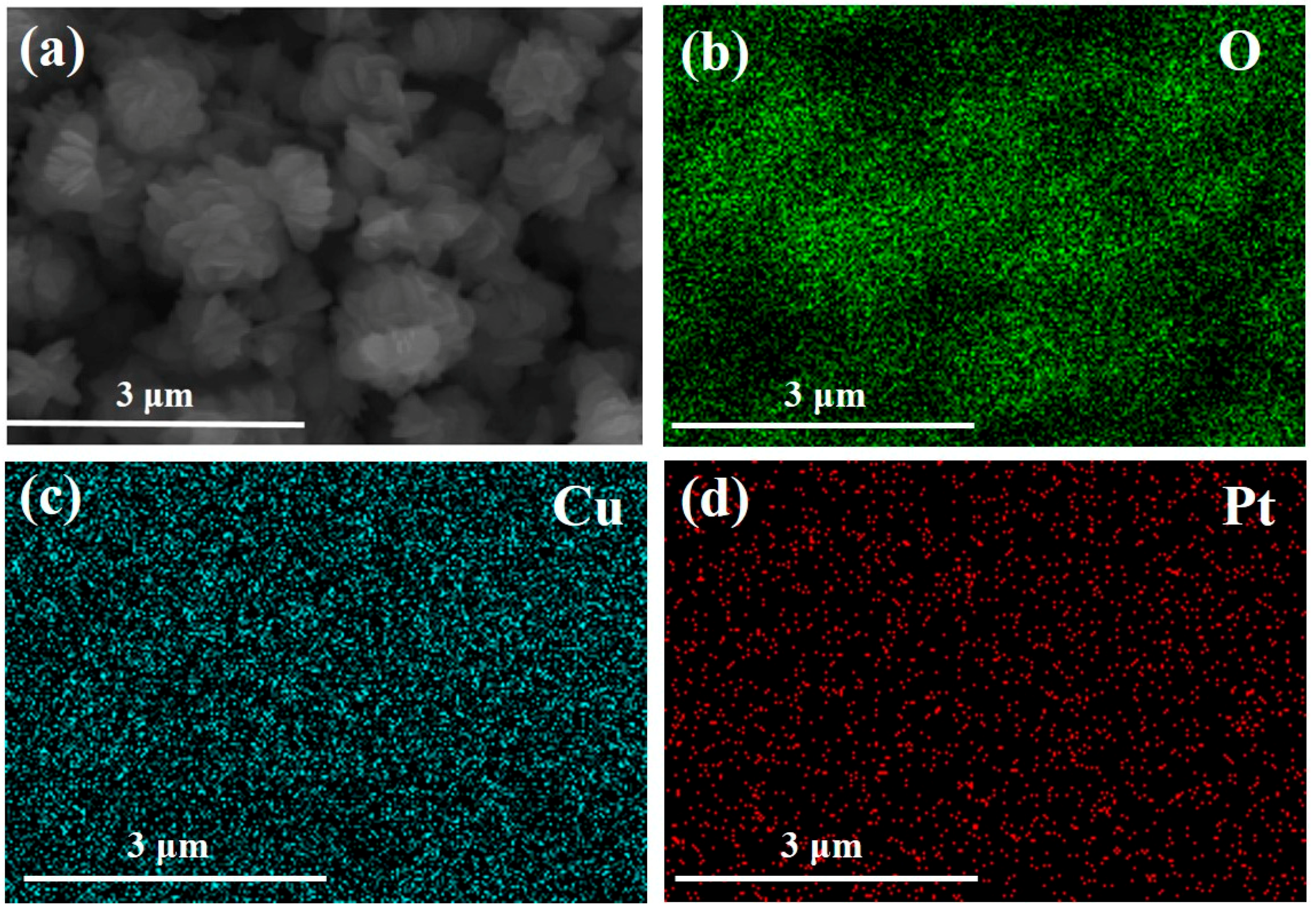
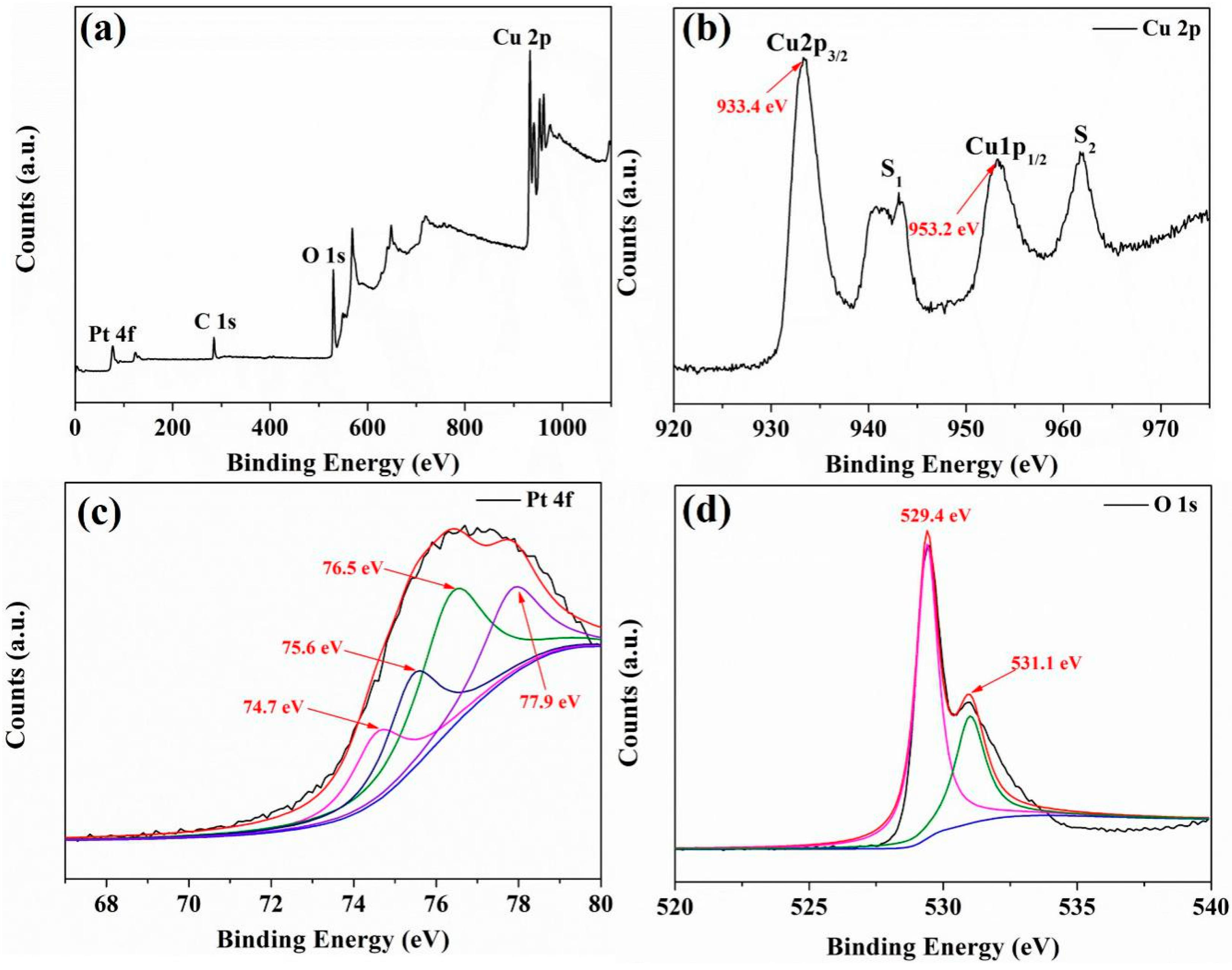
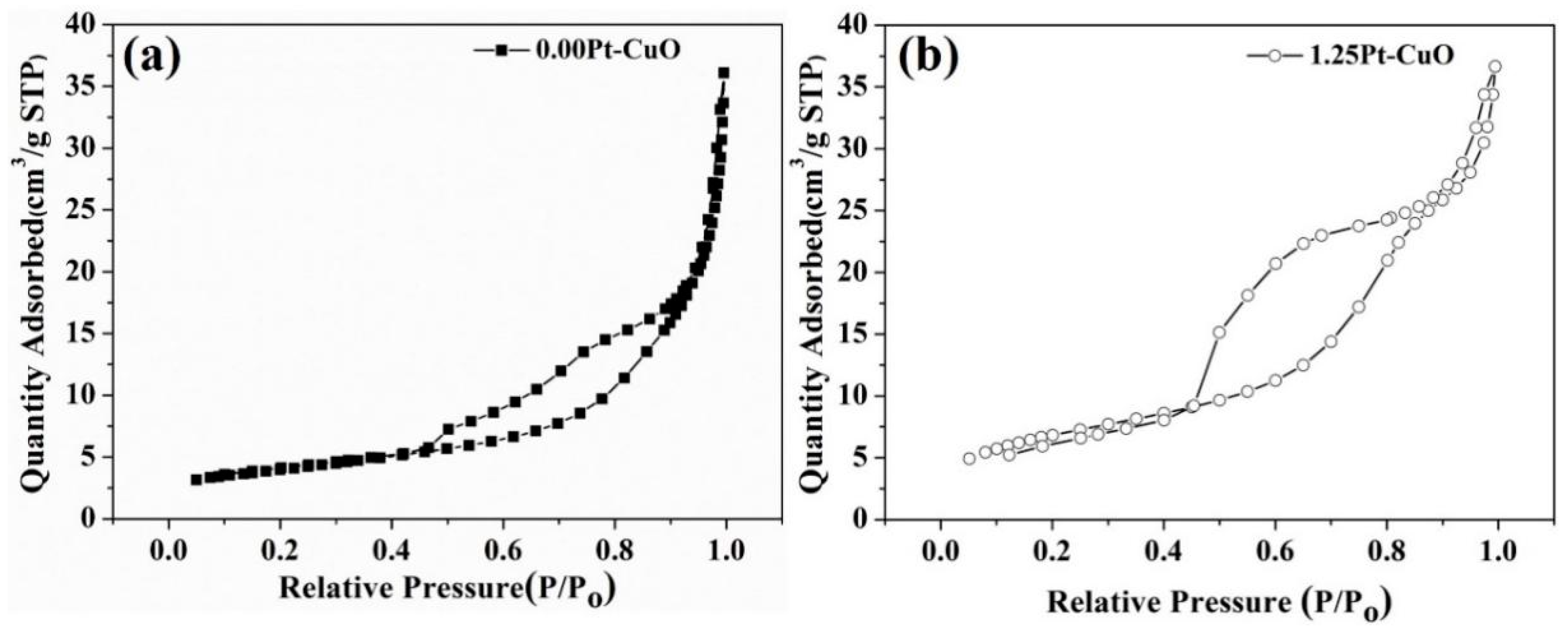
3.1. Structure and Morphology of Sensing Materials
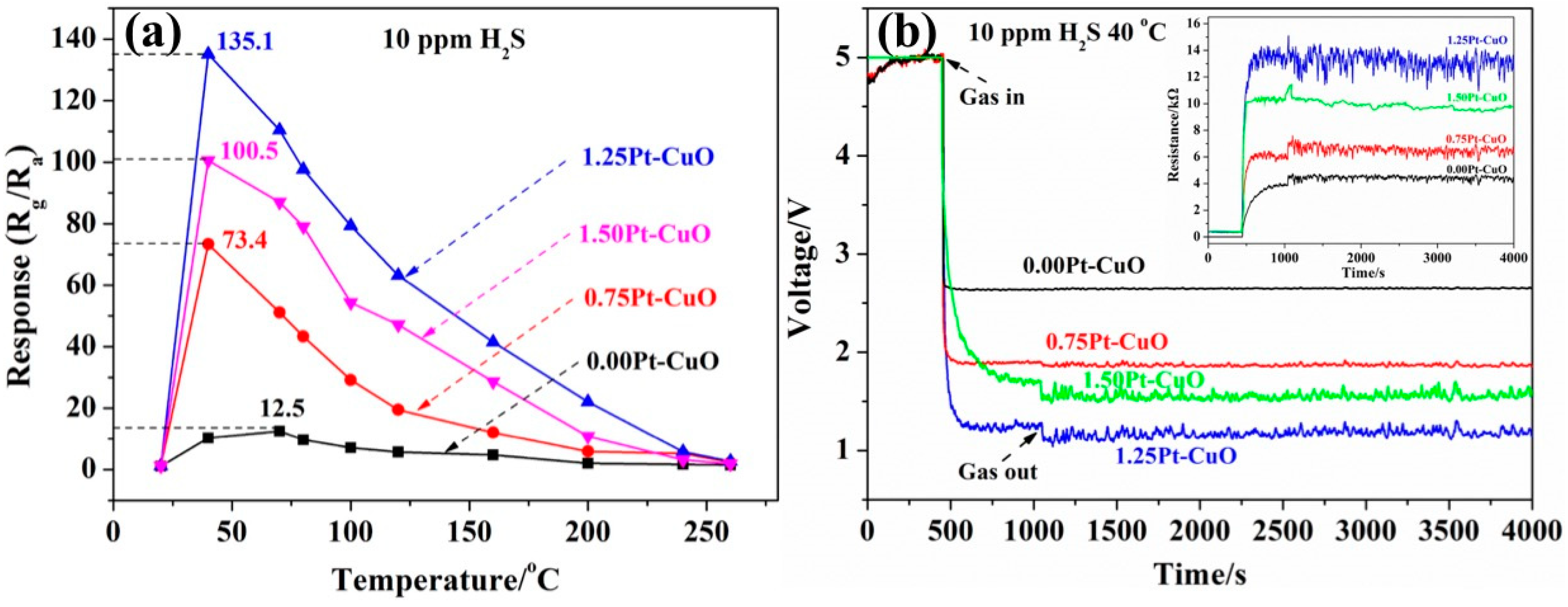
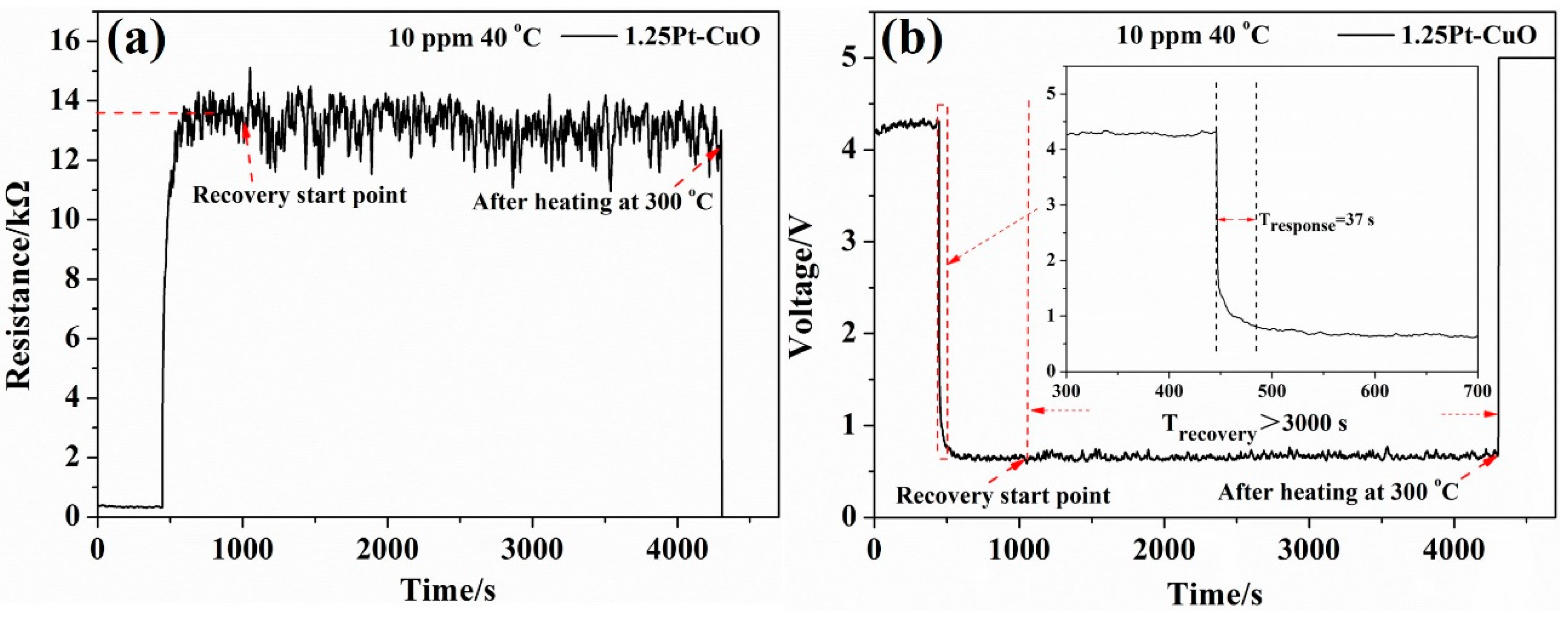
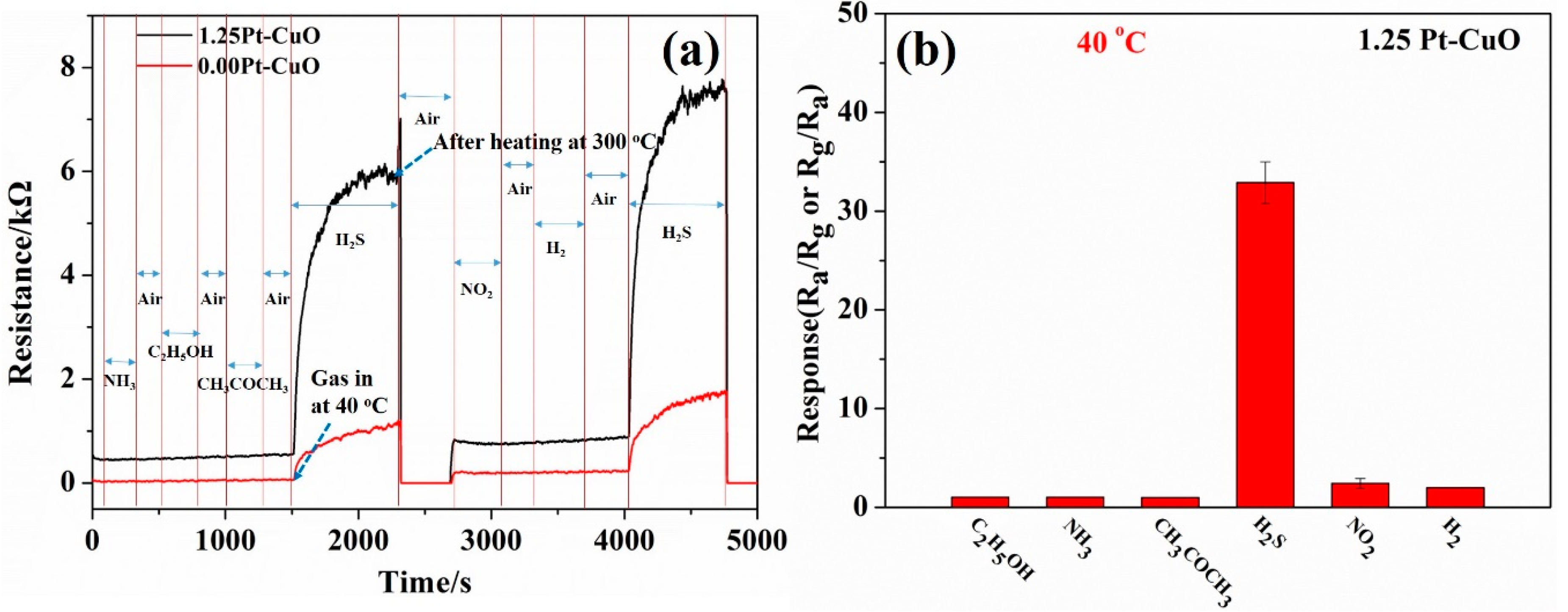
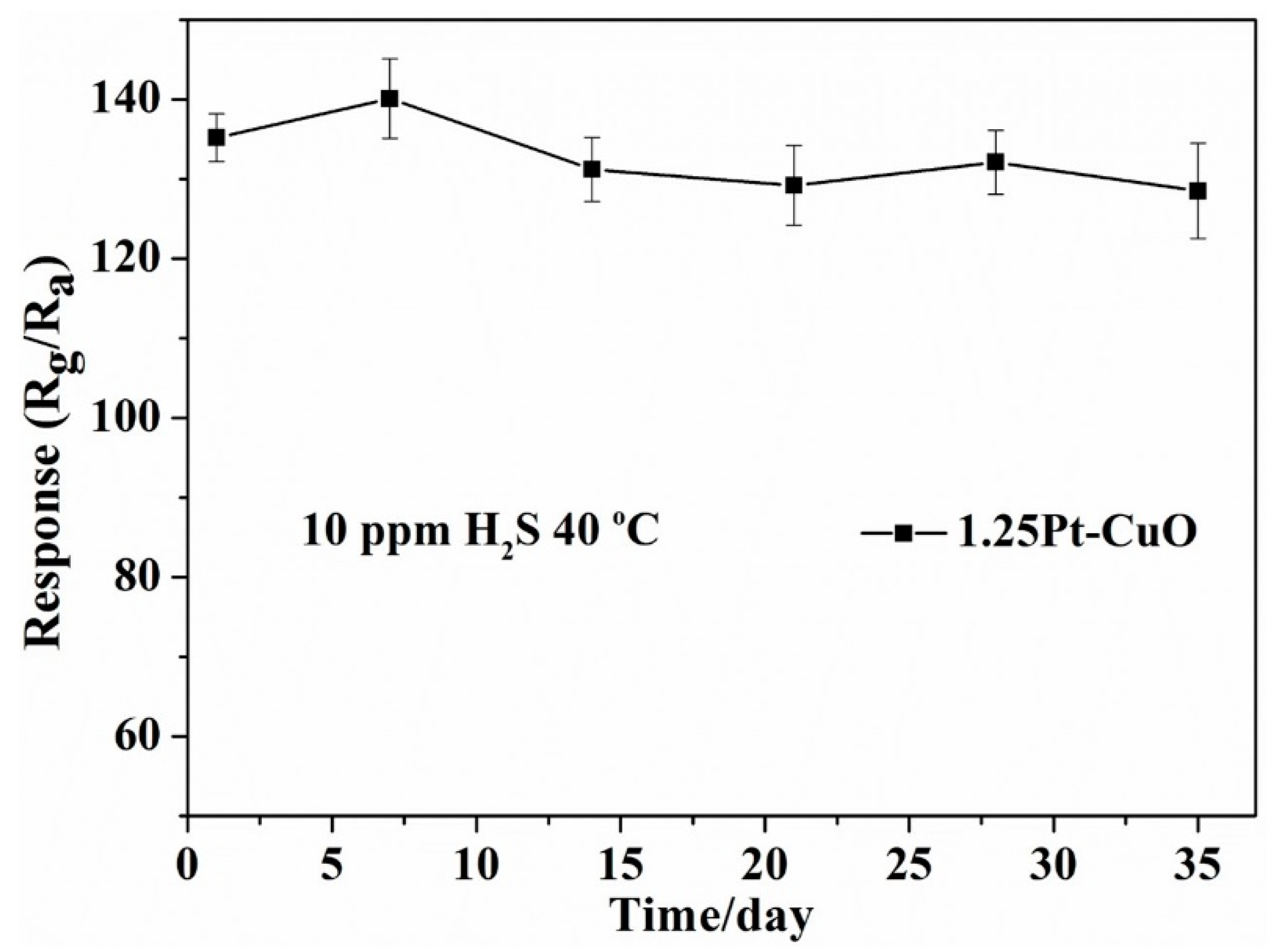
3.2. Gas Sensing Characteristics
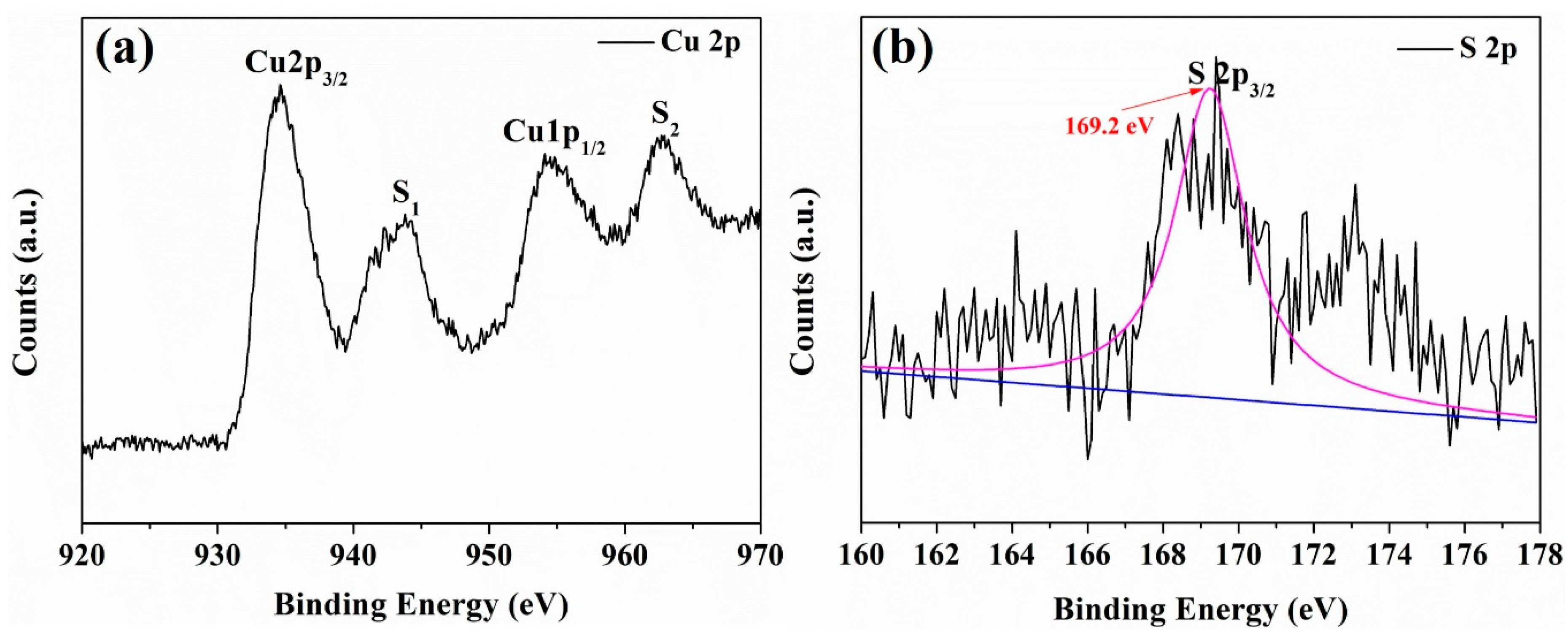
3.3. The Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hendrickson, R.G.; Chang, A.; Hamilton, R. Co-worker fatalities from hydrogen sulfide. Am. J. Ind. Med. 2004, 45, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.N.; Gong, M.M.; Li, Y.S.; Liu, Y.L.; Dou, X.C. Sensitive, selective, and fast detection of ppb-level H2S gas boosted by ZnO-CuO mesocrystal. Nanoscale Res. Lett. 2016, 475, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.B.; Zhu, Z.G.; Chen, C.; Wen, T.Y.; Zhao, X.L.; Xie, L.L. Highly sensitive H2S gas sensors based on Pd-doped CuO nanoflowers with low operating temperature. Sens. Actuators B Chem. 2017, 253, 809–817. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. DFT studies on interaction of H2S gas with α-Fe2O3 nanostructures. J. Inorg. Organomet. Polym. Mater. 2016, 26, 394–404. [Google Scholar] [CrossRef]
- Li, W.H.; Sun, W.; Xu, X.Q.; Du, L.P.; Li, M.Y. Coumarin-based fluorescent probes for H2S detection. J. Fluoresc. 2013, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Zhang, H.; Wu, P.; Zhang, H.R.; Xu, J.J.; Chen, H.Y. In situ activation of CdS electrochemiluminescene film and its application in H2S detection. Anal. Chem. 2014, 86, 8657–8664. [Google Scholar] [CrossRef] [PubMed]
- Henthorn, H.A.; Pluth, M.D. Mechanistic insights into the H2S-mediated reduction of aryl azides commonly used in H2S detection. J. Am. Chem. Soc. 2015, 135, 15330–15336. [Google Scholar] [CrossRef] [PubMed]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Righettoni, M.; Teleki, A. Semiconductor gas sensors: Dry synthesis and application. Angew. Chem. Int. Ed. 2010, 49, 7632–7659. [Google Scholar] [CrossRef] [PubMed]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. In depth study on the notable room-temperature NO2 gas sensor based on CuO nanoplatelets prepared by sonochemical method: Comparison of various bases. Sens. Actuators B Chem. 2018, 266, 761–772. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Ganapathi, S.K.; Kaur, M.; Datta, N.; Muthe, K.P.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. Sub-ppm H2S sensing at room temperature using CuO thin films. Sens. Actuators B Chem. 2010, 151, 90–96. [Google Scholar] [CrossRef]
- Xu, J.Q.; Wang, X.H.; Shen, J.N. Hydrothermal synthesis of In2O3 for detecting H2S in air. Sens. Actuators B Chem. 2006, 115, 642–646. [Google Scholar] [CrossRef]
- Wang, C.H.; Chu, X.F.; Wu, M.M. Detection of H2S down to ppb levels at room temperature using sensors based on ZnO nanorods. Sens. Actuators B Chem. 2006, 113, 320–323. [Google Scholar] [CrossRef]
- Song, Z.L.; Wei, Z.; Wang, B.C.; Luo, Z.; Xu, S.M.; Zhang, W.K.; Yu, H.X.; Li, M.; Huang, Z.; Zang, J.F.; et al. Sensitive room-temperature H2S gas sensors employing SnO2 quantum wire/reduced graphene oxide nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, Q.; Xu, J.Q.; Xu, P.C.; Pan, Q.Y.; Li, F. Self-assemblies of Pd nanoparticles on the surface of single crystal ZnO nanowires for chemical sensors with enhanced performances. J. Mater. Chem. 2009, 27, 4701–4706. [Google Scholar] [CrossRef]
- Tao, W.H.; Tsai, C.H. H2S sensing properties of noble metal doped WO3 thin film sensor fabricated by micromachining. Sens. Actuators B Chem. 2002, 81, 237–247. [Google Scholar] [CrossRef]
- Demel, J.; Zhigunov, A.; Jirka, I.; Klementova, M.; Lang, K. Facile synthesis of CuO nanosheets via the controlled delamination of layered copper hydroxide acetate. J. Colloid Interface Sci. 2015, 452, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.J.; Wang, X.L.; Xiang, J.Y.; Qiao, Y.Q.; Zhang, D.; Gu, J.P.; Tu, J.P. CuO/graphene composite as anode materials for lithium-ion batteries. Electrochim. Acta 2011, 56, 2306–2311. [Google Scholar] [CrossRef]
- Wang, G.L.; Huang, J.C.; Chen, S.L.; Gao, Y.Y.; Cao, D.X. Preparation and supercapacitance of CuO nanosheet arrays grown on nickel foam. J. Power Sources 2011, 196, 5756–5760. [Google Scholar] [CrossRef]
- Nezamzadeh, E.A.; Hushmandrad, S. Solar photodecolorization of methylene blue by CuO/X zeolite as a heterogeneous catalyst. Appl. Catal. A Gen. 2010, 388, 149–159. [Google Scholar] [CrossRef]
- Capone, S.; Forleo, A.; Francioso, L.; Rella, R.; Siciliano, P.; Spadavecchia, J.; Presicce, D.S.; Taurino, A.M. Solid state gas sensors: State of the art and future activities. J. Optoelectron. Adv. Mater. 2003, 5, 1335–1348. [Google Scholar] [CrossRef]
- Kim, K.M.; Jeong, H.M.; Kim, H.R.; Choi, K.I.; Kim, H.J.; Lee, J.H. Selective detection of NO2 using Cr-doped CuO nanorods. Sensors 2012, 12, 8013–8025. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jin, C.; Park, S.; Kim, S.; Lee, C.M. H2S gas sensing properties of bare and Pd-functionalized CuO nanorods. Sens. Actuators B Chem. 2012, 161, 594–599. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B Chem. 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Ivanov, P.; Llobet, E.; Vilanova, X.; Brezmes, J.; Hubalek, J.; Correig, X. Development of high sensitivity ethanol gas sensors based on Pt-doped SnO2 surface. Sens. Actuators B Chem. 2004, 99, 201–206. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.R.; Zhao, Y.Q.; Zhu, B.L.; Kong, F.H.; Wang, D.; Wu, S.H. H2S sensing characteristics of Pt-doped α-Fe2O3 thick film sensors. Sens. Actuators B Chem. 2007, 125, 79–84. [Google Scholar] [CrossRef]
- Miller, D.; Akbar, S.; Morris, P. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Kayestha, R.; Sumati; Hajela, K. ESR studies on the effect of ionic radii on displacement of Mn2+ bound to a soluble β-galactoside binding hepatic lectin. FEBS Lett. 1995, 368, 285–288. [Google Scholar] [CrossRef]
- Sheppard, L.R.; Lamo, M.B.; Holik, J.; Mayfield, K.J.; Nelson, D.R. Solute diffusion of platinum in rutile titanium dioxide. J. Am. Ceram. Soc. 2013, 96, 407–411. [Google Scholar] [CrossRef]
- Wang, D.G.; Wang, Y.Q.; Jiang, T.T.; Jia, H.X.; Yu, M.H. The preparation of M (M: Mn2+, Cd2+, Zn2+)-doped CuO nanostructures via the hydrothermal method and their properties. J. Mater. Sci. Mater. Electron. 2016, 27, 2138–2145. [Google Scholar] [CrossRef]
- Petr, V.; Václav, Š.; Jirˇí, H.; Martin, K. Shape-controlled synthesis of Sn-doped CuO nanoparticles for catalytic degradation of Rhodamine B. J. Colloid Interface Sci. 2016, 481, 28–38. [Google Scholar]
- Zhang, Y.W.; Si, R.; Liao, C.S.; Yan, C.H. Facile alcohothermal synthesis, size-dependent ultraviolet absorption, and enhanced CO conversion activity of ceria nanocrystals. J. Phys. Chem. B 2003, 107, 10159–10167. [Google Scholar] [CrossRef]
- Hu, X.B.; Zhu, Z.G.; Li, Z.H.; Xie, L.L.; Wu, Y.H.; Zheng, L.Y. Heterostructure of CuO microspheres modified with CuFe2O4 nanoparticles for highly sensitive H2S gas sensor. Sens. Actuators B Chem. 2018, 264, 139–149. [Google Scholar] [CrossRef]
- Liu, L.Q.; Zhou, F.; Wang, L.G.; Qi, X.J.; Shi, F.; Deng, Y.Q. Low-temperature CO oxidation over supported Pt, Pd catalysts: Particular role of FeOx support for oxygen supply during reactions. J. Catal. 2010, 274, 1–10. [Google Scholar] [CrossRef]
- Wang, S.R.; Zhang, J.X.; Yang, J.D.; Gao, X.L.; Zhang, H.X.; Wang, Y.S.; Zhu, Z.Y. Spinel ZnFe2O4 nanoparticle-decorated rod-like ZnO nanoheterostructures for enhanced gas sensing performances. RSC Adv. 2015, 5, 10048–10057. [Google Scholar] [CrossRef]
- Hu, D.; Han, B.Q.; Deng, S.J.; Feng, Z.P.; Wang, Y.; Popovic, J.; Nuskol, M.; Wang, Y.; Djerdj, I. Novel mixed phase SnO2 nanorods assembled with SnO2 nanocrystals for enhancing gas-sensing performance toward isopropanol gas. J. Phys. Chem. C 2014, 118, 9832–9840. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Rodriguez, J.A.; Hrbek, J. Reaction of S2 with ZnO and Cu/ZnO surfaces: Photoemission and molecular orbital studies. J. Phys. Chem. B 1997, 101, 10860–10869. [Google Scholar] [CrossRef]
- Cho, Y.H.; Liang, X.; Kang, Y.C.; Lee, J.H. Ultrasensitive detection of trimethylamine using Rh-doped SnO2 hollow spheres prepared by ultrasonic spray pyrolysis. Sens. Actuators B Chem. 2015, 207, 330–337. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A Review. Sensors 2012, 1, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Zhang, J.; Kang, Y.F.; Wu, S.H.; Wang, S.R. Brochantite tabular microspindles and their conversion to wormlike CuO structures for gas sensing. CrystEngComm 2012, 14, 620–625. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kheel, H.; Hyun, S.K.; Jin, C.; Lee, C. Enhanced H2S gas sensing perfoemane of networked CuO-ZnO composite nanoparticle sensor. Mater. Res. Bull. 2016, 82, 130–135. [Google Scholar] [CrossRef]
- Sonia, S.; Kumar, P.S.; Jayram, N.D.; Masuda, Y.; Manqalaraj, D.; Lee, C. Superhydrophobic and H2S gas sensing properties of CuO nanostructured thin films through a successive ionic layered adsorption reaction process. RSC Adv. 2016, 6, 24290–24298. [Google Scholar] [CrossRef]
- Kappler, J.; Barsan, N.; Weimar, U.; Dieguez, A.; Alay, J.L.; Rodriguez, A.R.; Morante, J.R.; Gopel, W. Correlation between XPS, Raman and TEM measurements and the gas sensitivity of Pt and Pd doped SnO2 based gas sensors. Fresenius’ J. Anal. Chem. 1998, 361, 110–114. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Boris, I.; Brinzari, V.; Han, S.H.; Cho, B.K. The role of doping effect on the response of SnO2-based thin film gas sensors: Analysis based on the results obtained for Co-doped SnO2 films deposited by spray pyrolysis. Sens. Actuators B Chem. 2013, 182, 112–124. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Hu, X.-B.; He, M.; Xie, L.-L.; Zhu, Z.-G.; Wu, J.-Q. Effect of Platinum Doping on the Morphology and Sensing Performance for CuO-Based Gas Sensor. Appl. Sci. 2018, 8, 1091. https://doi.org/10.3390/app8071091
Tang Q, Hu X-B, He M, Xie L-L, Zhu Z-G, Wu J-Q. Effect of Platinum Doping on the Morphology and Sensing Performance for CuO-Based Gas Sensor. Applied Sciences. 2018; 8(7):1091. https://doi.org/10.3390/app8071091
Chicago/Turabian StyleTang, Qi, Xiao-Bing Hu, Meng He, Li-Li Xie, Zhi-Gang Zhu, and Jian-Qing Wu. 2018. "Effect of Platinum Doping on the Morphology and Sensing Performance for CuO-Based Gas Sensor" Applied Sciences 8, no. 7: 1091. https://doi.org/10.3390/app8071091
APA StyleTang, Q., Hu, X.-B., He, M., Xie, L.-L., Zhu, Z.-G., & Wu, J.-Q. (2018). Effect of Platinum Doping on the Morphology and Sensing Performance for CuO-Based Gas Sensor. Applied Sciences, 8(7), 1091. https://doi.org/10.3390/app8071091