Antimicrobial Effect of Titanium Hydroxyapatite in Denture Base Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Specimens
2.2. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
2.3. Bacterial Strains and Culture Conditions
2.4. Evaluation of the Antimicrobial Effect of TiHA against Planktonic Bacteria
2.5. Evaluation of the Antimicrobial Effect of TiHA against Biofilms
2.6. Inductively Coupled Plasma Atomic Emission Spectroscopy Analysis (ICPA)
2.7. Statistical Analysis
2.8. Ethics Statement
3. Results
3.1. SEM and EDX Evaluation of TiHA-Containing Resin Specimens
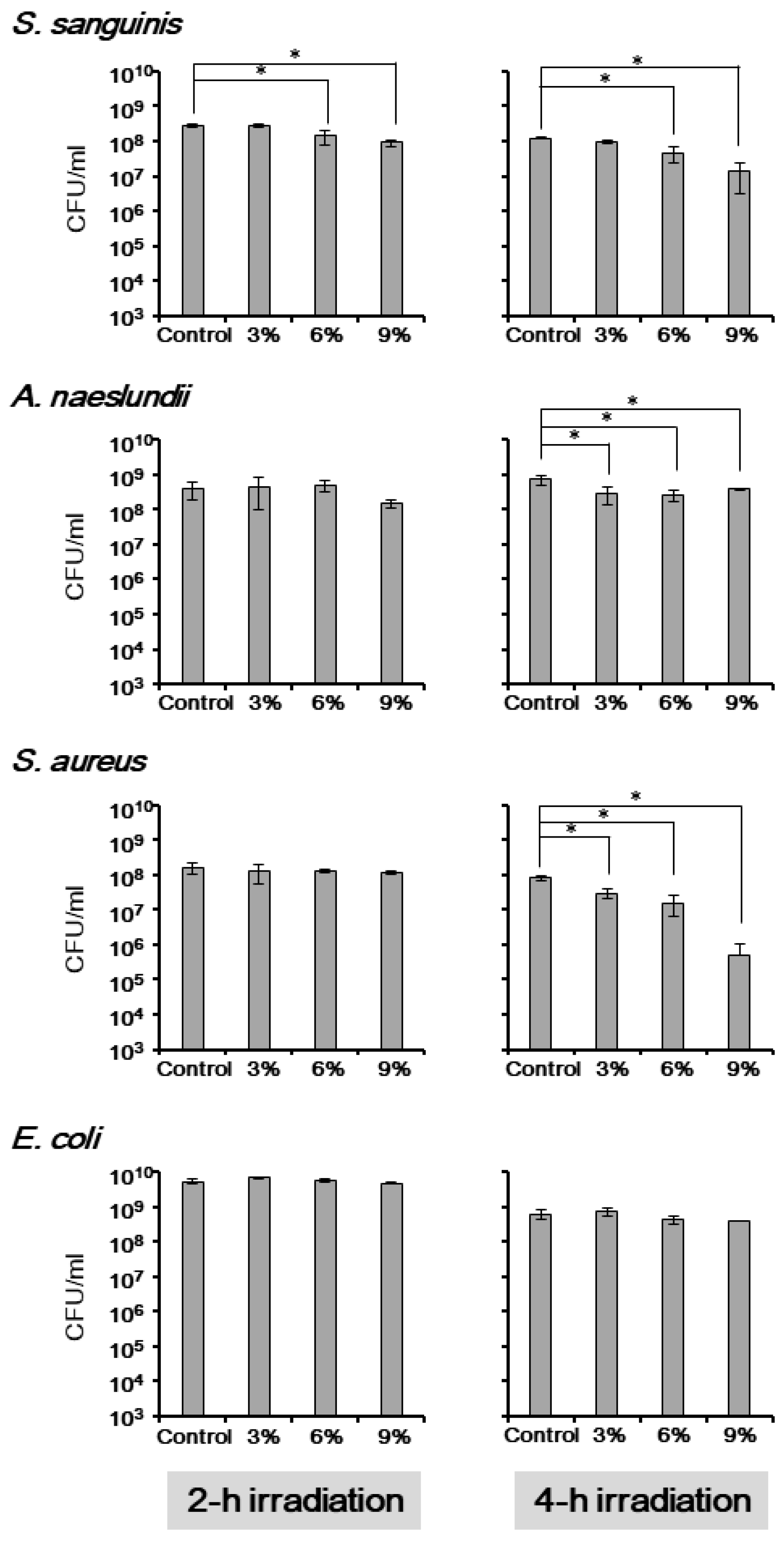
3.2. The Antimicrobial Effect of TiHA against Planktonic Bacteria
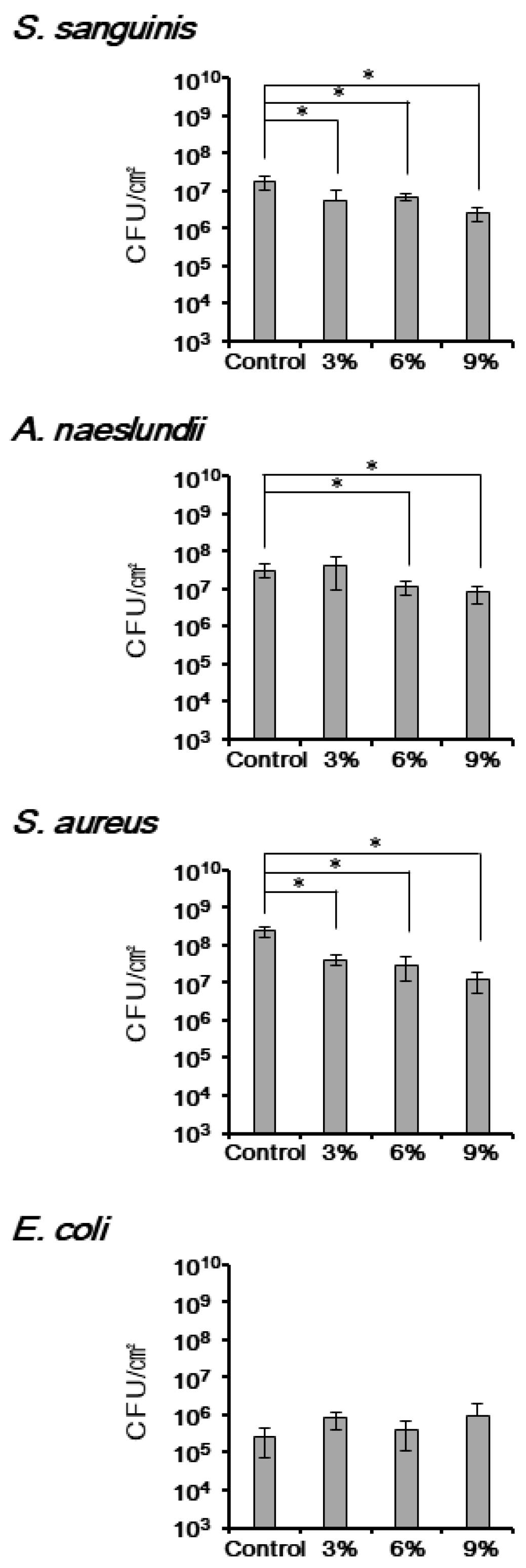
3.3. The Antimicrobial Effect of TiHA against Single-Species Bacterial Biofilms
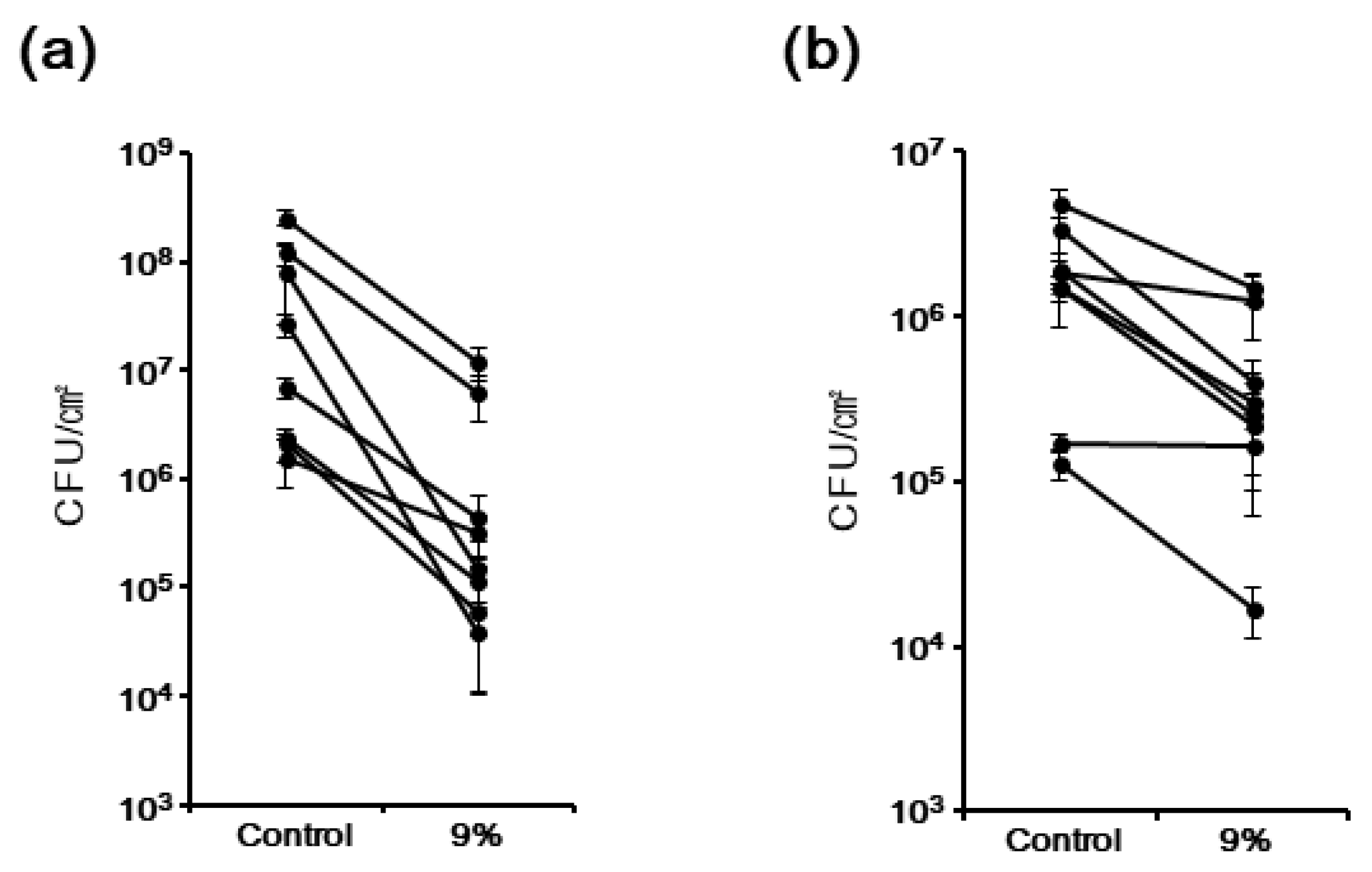
3.4. The Antimicrobial Effect of TiHA against Biofilms Formed by Multiple Species of Bacteria from the Human Saliva
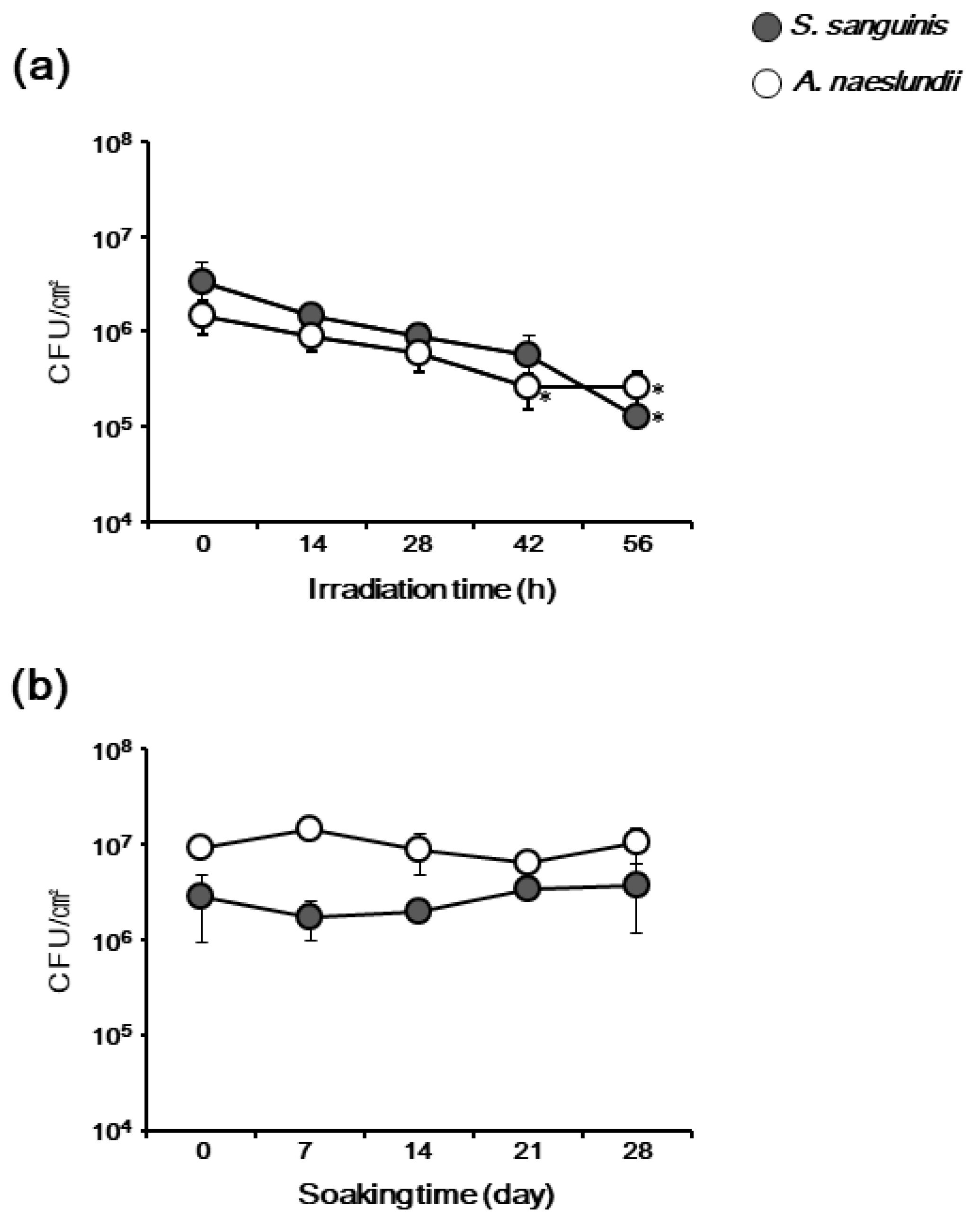
3.5. TiHA Resistance to Long-Time Irradiation and Soaking
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shibata, T.; Hamada, N.; Kimoto, K.; Sawada, T.; Sawada, T.; Kumada, H.; Umemoto, T.; Toyoda, M. Antifungal effect of acrylic resin containing apatite-coated TiO2 photocatalyst. Dent. Mater. J. 2007, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Neill, D.J. A study of materials and methods employed in cleaning dentures. Br. Dent. J. 1968, 124, 107–115. [Google Scholar]
- Busscher, H.J.; Cowan, M.M.; van der Mei, H.C. On the relative importance of specific and non-specific approaches to oral microbial adhesion. FEMS Microbiol. Rev. 1992, 8, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Bellon-Fontaine, M.N.; Mozes, N.; van der Mei, H.C.; Sjollema, J.; Cerf, O.; Rouxhet, P.G.; Busscher, H.J. A comparison of thermodynamic approaches to predict the adhesion of dairy microorganisms to solid substrata. Cell Biophys. 1990, 17, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Kulak-Ozkan, Y.; Kazazoglu, E.; Arikan, A. Oral hygiene habits, denture cleanliness, presence of yeasts and stomatitis in elderly people. J. Oral Rehabil. 2009, 29, 300–304. [Google Scholar] [CrossRef]
- El-Solh, A.A. Association between pneumonia and oral care in nursing home residents. Lung 2011, 189, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Papandonatos, G.D.; Dunford, R.G. Associations between oral conditions and respiratory disease in a national sample survey population. Ann. Periodontol. 1998, 3, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, H.F.; Harvey, I.; Newcombe, R.G. Oral health care among nursing home residents in Avon. Gerodontology 2000, 17, 33–38. [Google Scholar] [CrossRef] [PubMed]
- De Visschere, L.M.; Grooten, L.; Theuniers, G.; Vanobbergen, J.N. Oral hygiene of elderly people in long-term care institutions a cross-sectional study. Gerodontology 2006, 23, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Catalán, A.; Pacheco, J.G.; Martínez, A.; Mondaca, M.A. In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Geerts, G.A.; Stuhlinger, M.E.; Basson, N.J. Effect of an antifungal denture liner on the saliva yeast count in patients with denture stomatitis: A pilot study. J. Oral Rehabil. 2008, 35, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Kulak-Ozkan, Y.; Kazazoglu, E.; Arikan, A. Use of microwave energy to disinfect a long-term soft lining material contaminated with Candida albicans or Staphylococcus aureus. J. Prosthet. Dent. 1998, 79, 454–458. [Google Scholar]
- Radford, D.R.; Challacombe, S.J.; Walter, J.D. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit. Rev. Oral Biol. Med. 1999, 10, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wei, S.S.; Hu, S.Q.; Tang, J.X. Preparation of TiO2/Ag colloids with ultraviolet resistance and antibacterial property using short chain polyethylene glycol. J. Hazard Mater. 2009, 172, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ohko, Y.; Sekiguchi, Y.; Fujishima, A.; Kubota, Y. Self-sterilization using silicone catheters coated with Ag and TiO2 nanocomposite thin film. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Sawada, T.; Kumasaka, T.; Hamada, N.; Shibata, T.; Nonami, T.; Kimoto, K. Self-cleaning effects of acrylic resin containing fluoridated apatite-coated titanium dioxide. Gerodontology 2014, 31, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Kado, D.; Sakurai, K.; Sugiyama, T.; Ueda, T. Evaluation of Cleanability of a Titanium Dioxide (TiO2)-coated Acrylic Resin Denture Base. Prosthodont. Res. Pract. 2005, 4, 69–76. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, Y.; Ai, X.; Liu, Z.; Zhang, D. Corrosion resistance and biological activity of TiO2 implant coatings produced in oxygen-rich environments. Proc. Inst. Mech. Eng. Part H 2017, 231, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, N.; Negishi, H.; Okada, S.; Nonami, T.; Kimoto, K. Response of human fibroblasts to implant surface coated with titanium dioxide photocatalytic films. J. Prosthodont. Res. 2010, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lilja, M.; Forsgren, J.; Welch, K.; Astrand, M.; Engqvist, H.; Strømme, M. Photocatalytic and antimicrobial properties of surgical implant coatings of titanium dioxide deposited though cathodic arc evaporation. Biotechnol. Lett. 2012, 34, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical evidence for the mechanism of the primary stage of photosynthesis. Bull. Chem. Soc. Jpn. 1971, 44, 1148–1150. [Google Scholar] [CrossRef]
- Hisanaga, T.; Harada, K.; Tanaka, K. Photocatalytic degradation of organochlorine compounds in suspended TiO2. J. Photochem. Photobiol. A Chem. 1990, 54, 113–118. [Google Scholar] [CrossRef]
- Wakamura, M.; Hashimoto, K.; Watanabe, T. Photocatalysis by Calcium Hydroxyapatite Modified with Ti (IV): Albumin Decomposition and Bactericidal Effect. Langmuir 2003, 19, 3428–3431. [Google Scholar] [CrossRef]
- Zeng, H.; Chittur, K.K.; Lacefield, W.R. Dissolution reprecipitation of calcium phosphate thin films produced by ion beam sputter deposition technique. Biomaterials 1999, 20, 443–451. [Google Scholar] [CrossRef]
- Cheng, Y.; Sakai, T.; Moroi, R.; Nakagawa, M.; Sakai, H.; Ogata, T.; Terada, Y. Self-cleaning ability of a photocatalyst-containing denture base material. Dent. Mater. J. 2008, 27, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Uchimaru, M.; Sakai, T.; Moroi, R.; Shiota, S.; Shibata, Y.; Deguchi, M.; Sakai, H.; Yamashita, Y.; Terada, Y. Antimicrobial and antifungal effects of tissue conditioners containing a photocatalyst. Dent. Mater. J. 2011, 30, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, Y.; Konno, H.; Nagano, K.; Abiko, Y.; Nakamura, Y.; Tanaka, Y.; Yoshimura, F. The influence of a glucosyltransferase, encoded by gtfP, on biofilm formation by Streptococcus sanguinis in a dual-species model. APMIS 2014, 122, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Cisar, J.O.; Sandberg, A.L.; Abeygunawardana, C.; Reddy, G.P.; Bush, C.A. Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology 1995, 5, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Sugai, M.; Komatsuzawa, H.; Ooku-Inomata, K.; Miyake, Y.; Ishida, E.; Suginaka, H. Isolation and characterization of Staphylococcus aureus mutants which form altered cell clusters. Microbiol. Immunol. 1994, 38, 995–999. [Google Scholar] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tong, Z.; Huo, L.; Yang, F.; Su, W. Antibacterial and biological properties of biofunctionalized nanocomposites on titanium for implant application. J. Biomater. Appl. 2016, 31, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Haenle, M.; Fritsche, A.; Zietz, C.; Bader, R.; Heidenau, F.; Mittelmeier, W.; Gollwitzer, H. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: In vitro effectiveness against MRSA and mechanical properties. J. Mater. Sci. Mater. Med. 2011, 22, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Kanie, T.; Arikawa, H.; Fujii, K.; Inoue, K. Physical and mechanical properties of PMMA resins containing γ-methacryloxypropyltrimethoxysilane. J. Oral Rehabil. 2004, 31, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Pesci-Bardon, C.; Fosse, T.; Madinier, I.; Serre, D. In vitro new dialysis protocol to assay the antiseptic properties of a quaternary ammonium compound polymerized with denture acrylic resin. Lett. Appl. Microbiol. 2004, 39, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal activity of copper-deposited TiO2 thin film under weak UV light illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Denepitiya, L.; Kleinberg, I. A comparison of the microbial compositions of pooled human dental plaque and salivary sediment. Arch. Oral Biol. 1982, 27, 739–745. [Google Scholar] [CrossRef]
- Mager, D.L.; Haffajee, A.D.; Socransky, S.S. Effects of periodontitis and smoking on the microbiota of oral mucous membranes and saliva in systemically healthy subjects. J. Clin. Periodontol. 2003, 30, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Kononen, E.; Paju, S.; Pussinen, PJ.; Hyvonen, M.; Di Tella, P.; Suominen-Taipale, L.; Knuuttila, M. Population-based study of salivary carriage of periodontal pathogens in adults. J. Clin. Microbiol. 2007, 45, 2446–2451. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Wakamura, M.; Yoshida, N.; Watanabe, T. Band gap and photocatalytic properties of Ti-substituted hydroxyapatite: Comparison with anatase-TiO2. J. Mol. Catal. A Chem. 2011, 338, 18–23. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, W.; Yoshida, Y.; Komasa, S.; Hasegawa, Y.; Okazaki, J. Antimicrobial Effect of Titanium Hydroxyapatite in Denture Base Resin. Appl. Sci. 2018, 8, 963. https://doi.org/10.3390/app8060963
Sato W, Yoshida Y, Komasa S, Hasegawa Y, Okazaki J. Antimicrobial Effect of Titanium Hydroxyapatite in Denture Base Resin. Applied Sciences. 2018; 8(6):963. https://doi.org/10.3390/app8060963
Chicago/Turabian StyleSato, Wataru, Yasuo Yoshida, Satoshi Komasa, Yoshiaki Hasegawa, and Joji Okazaki. 2018. "Antimicrobial Effect of Titanium Hydroxyapatite in Denture Base Resin" Applied Sciences 8, no. 6: 963. https://doi.org/10.3390/app8060963




