Impact of Pore Geometry and Water Saturation on Gas Effective Diffusion Coefficient in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pore Diameter and Water Distribution
2.3. The Pore-Scale Model
3. Results and Discussions
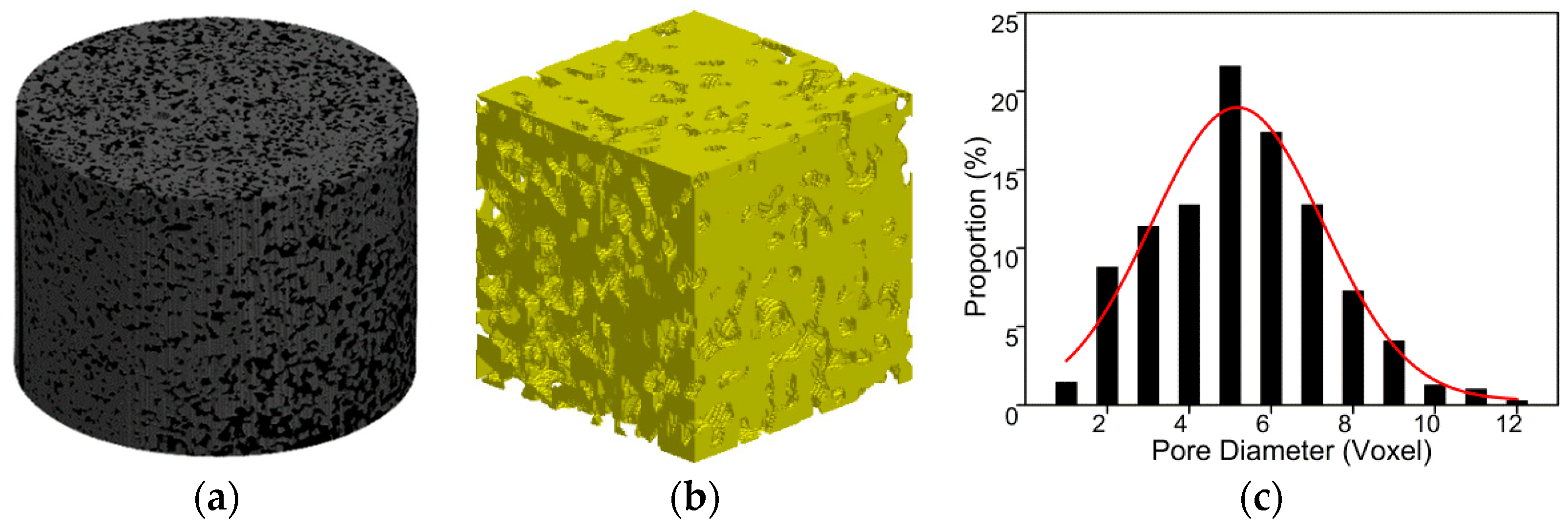
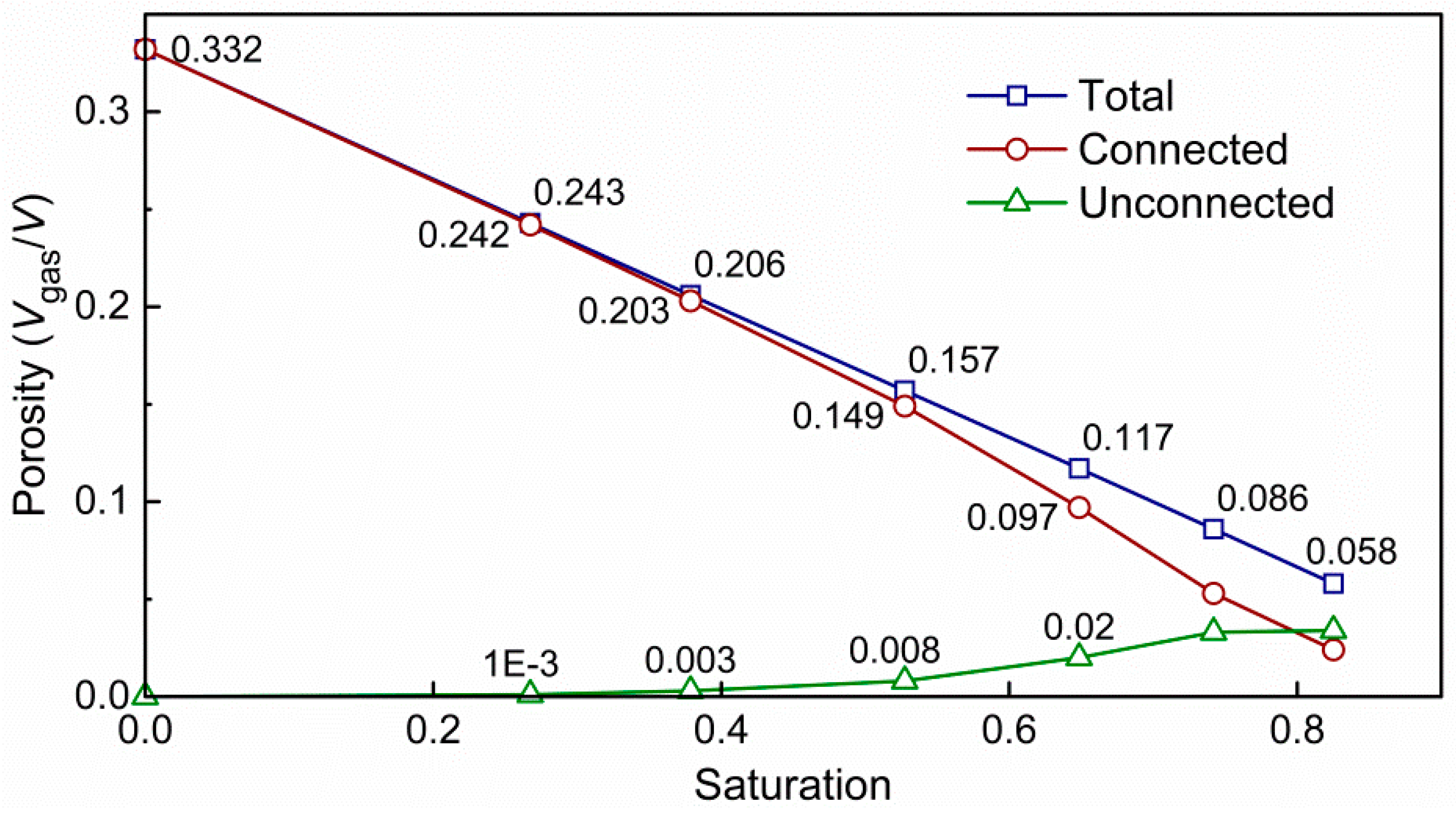
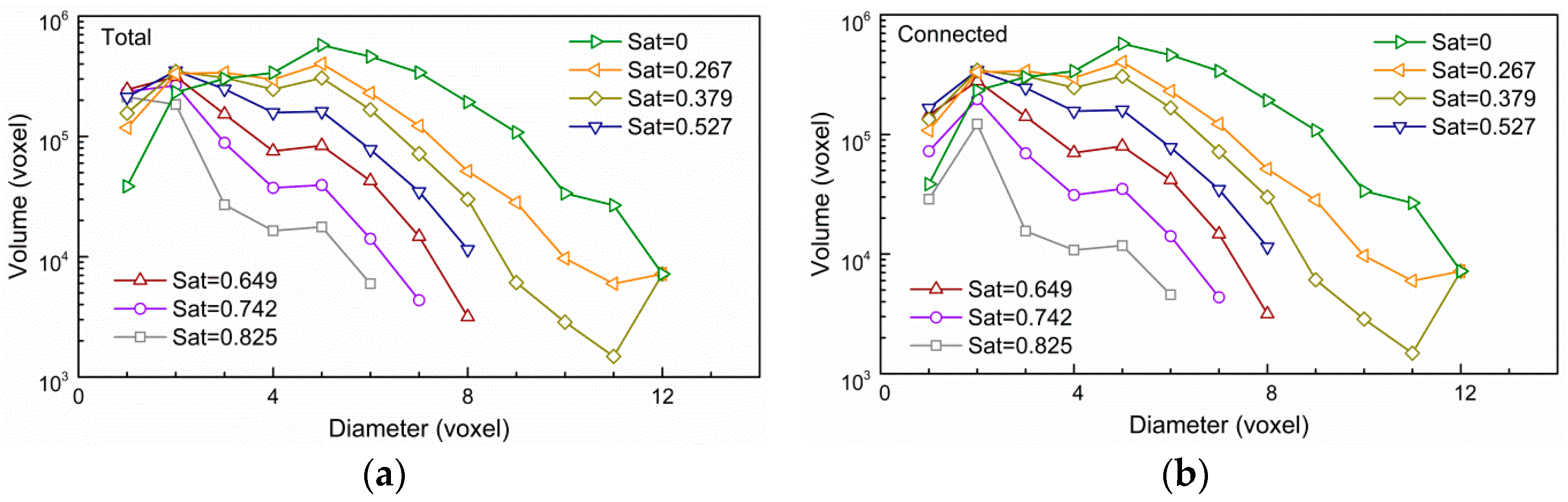
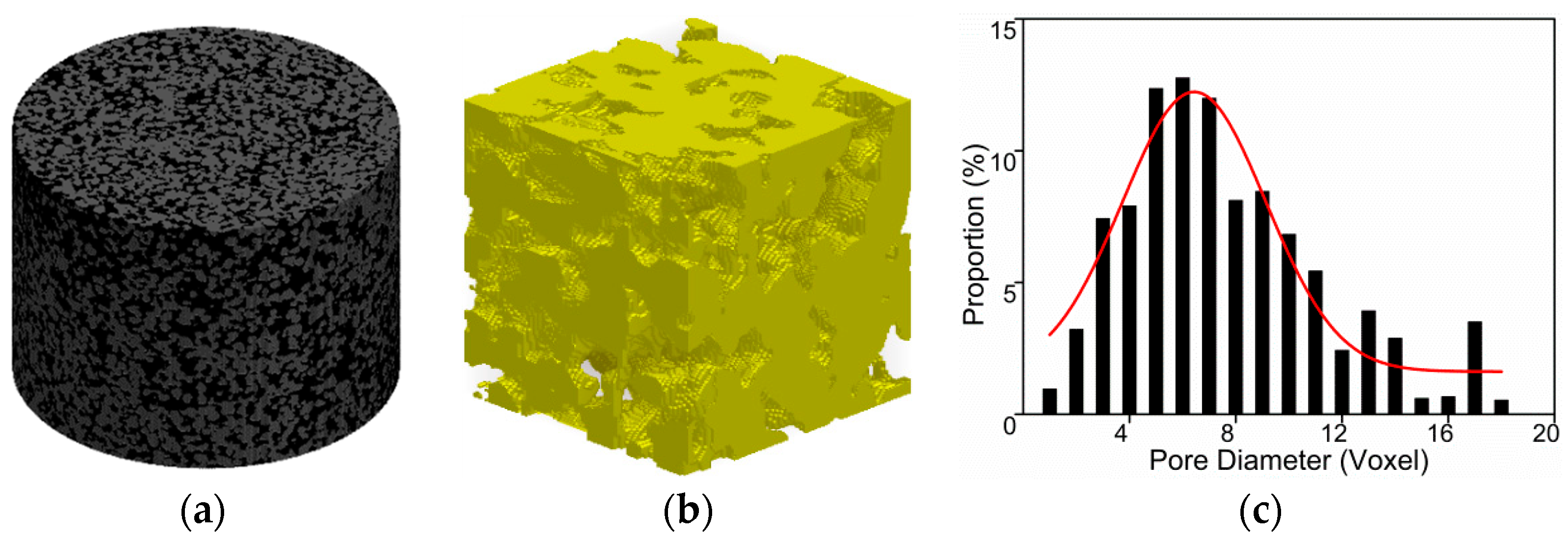
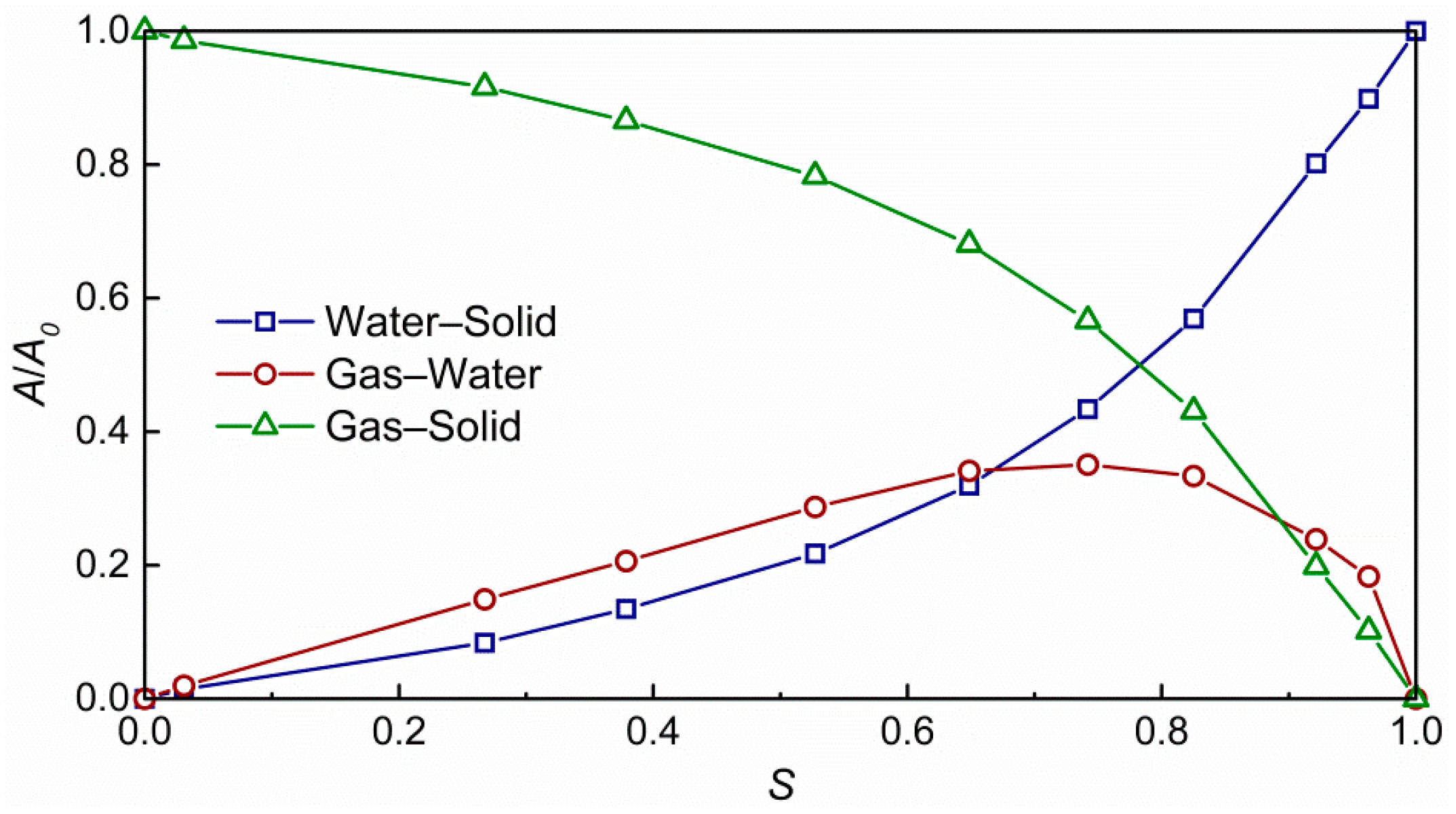
3.1. Impact of Saturation on Pore Structure
3.2. Impact of Saturation on Effective Diffusion Coefficient
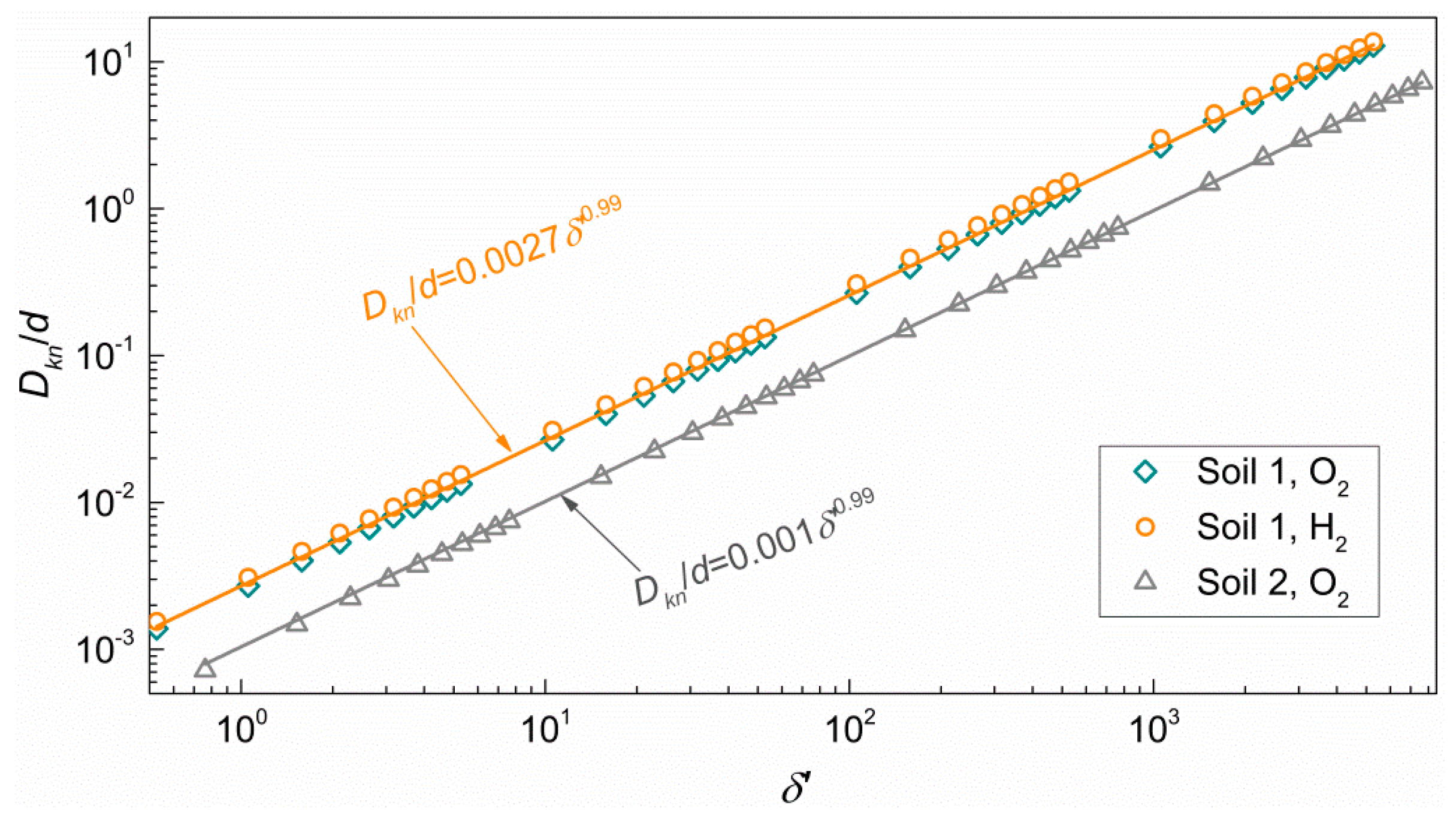
3.3. Effective Knudsen Diffusion Coefficient
4. Conclusions
- (1)
- The pore geometry including porosity, connectivity, pore size and interface strongly depend on water distribution and saturation, and the relationship between average pore diameter and gas saturation can be fitted into a power-law;
- (2)
- The present approach can be used to calculate the effective diffusion coefficient, but needs to know the pore structure;
- (3)
- The effective diffusion coefficient in the Knudsen region and in the continuum region approximates the effective Knudsen diffusion coefficient and the effective bulk diffusion coefficient, respectively. In the transition region, it can be calculated by the dusty-gas model under certain accuracy.
- (4)
- Pore diameter is essential in calculating the effective Knudsen diffusion coefficient which increases with gas saturation following a power-law.
Author Contributions
Funding
Conflicts of Interest
References
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J.D. Impact of shale gas development on regional water quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef] [PubMed]
- Stamford, L.; Azapagic, A. Life cycle environmental impacts of UK shale gas. Appl. Energy 2014, 134, 506–518. [Google Scholar] [CrossRef]
- Zavalaaraiza, D.; Alvarez, R.A.; Lyon, D.R.; Allen, D.T.; Marchese, A.J.; Zimmerle, D.J.; Hamburg, S.P. Super-emitters in natural gas infrastructure are caused by abnormal process conditions. Nat. Commun. 2017, 8, 14012. [Google Scholar] [CrossRef] [PubMed]
- Bjørnarå, T.I.; Nordbotten, J.M.; Park, J. Vertically integrated models for coupled two-phase flow and geomechanics in porous media. Water Resour. Res. 2016, 52, 1398–1417. [Google Scholar] [CrossRef] [Green Version]
- Chalbaud, C.; Robin, M.; Lombard, J.M.; Martin, F.; Egermann, P.; Bertin, H. Interfacial tension measurements and wettability evaluation for geological CO2 storage. Adv. Water Resour. 2009, 32, 98–109. [Google Scholar] [CrossRef]
- Obi, E.O.I.; Blunt, M.J. Streamline-based simulation of carbon dioxide storage in a North Sea aquifer. Water Resour. Res. 2006, 42, 1–13. [Google Scholar] [CrossRef]
- Juanes, R.; Spiteri, E.J.; Orr, F.M., Jr.; Blunt, M.J. Impact of relative permeability hysteresis on geological CO2 storage. Water Resour. Res. 2006, 42, 1–13. [Google Scholar] [CrossRef]
- Kim, T.H.; Cho, J.; Lee, K.S. Evaluation of CO2 injection in shale gas reservoirs with multi-component transport and geomechanical effects. Appl. Energy 2017, 190, 1195–1206. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, M.; Miao, H.; Huang, Z.; Gao, S.; Ruan, W. In situ volatile fatty acids influence biogas generation from kitchen wastes by anaerobic digestion. Bioresour. Technol. 2014, 163, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Komilis, D.P.; Ham, R.K.; Park, J.K. Emission of volatile organic compounds during composting of municipal solid wastes. Water Res. 2004, 38, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, S.A.; Sleep, B.E. Knudsen diffusion, gas permeability, and water content in an unconsolidated porous medium. Water Resour. Res. 2002, 38. [Google Scholar] [CrossRef]
- Blunt, M.J.; Bijeljic, B.; Dong, H.; Gharbi, O.; Iglauer, S.; Mostaghimi, P.; Paluszny, A.; Pentland, C. Pore-scale imaging and modelling. Adv. Water Resour. 2013, 51, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Raoof, A.; Hassanizadeh, S.M. Saturation-dependent solute dispersivity in porous media: Pore-scale processes. Water Resour. Res. 2013, 49, 1943–1951. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.; Liu, Z.S.; Huang, C.; Djilali, N. Determination of the effective diffusion coefficient in porous media including knudsen effects. Microfluid. Nanofluid. 2008, 4, 257–260. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Liu, Y. Pore-scale simulation of gas diffusion in unsaturated soil aggregates: Accuracy of the dusty-gas model and the impact of saturation. Geoderma 2017, 303, 196–203. [Google Scholar] [CrossRef]
- Edwards, R.W.J.; Doster, F.; Celia, M.A.; Bandilla, K.W. Numerical modeling of gas and water flow in shale gas formations with a focus on the fate of hydraulic fracturing fluid. Environ. Sci. Technol. 2017, 51, 13779–13787. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.J. The diffusion of gases through porous media. J. Soil Sci. 1959, 10, 79–82. [Google Scholar] [CrossRef]
- Millington, R.J. Gas diffusion in porous media. Science 1959, 130, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Penman, H.L. Gas and vapour movements in the soil: I. The diffusion of vapours through porous solids. J. Agric. Sci. 1940, 30, 437–462. [Google Scholar] [CrossRef]
- Penman, H.L. Gas and vapour movements in the soil: II. The diffusion of carbon dioxide through porous solids. J. Agric. Sci. 1940, 30, 570–581. [Google Scholar] [CrossRef]
- Deepagoda, T.K.K.C.; de Jonge, L.W.; Kawamoto, K.; Komatsu, T.; Moldrup, P. The water-induced linear reduction gas diffusivity model extended to three pore regions. Vadose Zone J. 2015, 14, 1–9. [Google Scholar]
- Deepagoda, T.K.K.C.; Moldrup, P.; Schjønning, P.; Kawamoto, K.; Komatsu, T.; de Jonge, L.W. Variable pore connectivity model linking gas diffusivity and air-phase tortuosity to soil matric potential. Vadose Zone J. 2012, 11, 120–128. [Google Scholar] [CrossRef]
- Moldrup, P.; Deepagoda, T.K.K.C.; Hamamoto, S.; Komatsu, T.; Kawamoto, K.; Rolston, D.E.; de Jonge, L.W. Structure-dependent water-induced linear reduction model for predicting gas diffusivity and tortuosity in repacked and intact soil. Vadose Zone J. 2013, 12, 1–11. [Google Scholar] [CrossRef]
- Thorbjørn, A.; Moldrup, P.; Blendstrup, H.; Komatsu, T.; Rolston, D.E. A gas diffusivity model based on air, solid, and water-phase resistance in variably saturated soil. Vadose Zone J. 2008, 7, 1230–1240. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Ostadi, H.; Jiang, K.; Chen, R. Modelling water intrusion and oxygen diffusion in a reconstructed microporous layer of pem fuel cells. Int. J. Hydrog. Energy 2014, 39, 17222–17230. [Google Scholar] [CrossRef] [Green Version]
- Dullien, F.A.L. Porous Media: Fluid Transport and Pore Structure; Academic Press: Cambridge, MA, USA, 1992. [Google Scholar]
- Gomez-Soberon, J.M.V. Porosity of recycled concrete with substitution of recycled concrete aggregate—An experimental study. Cem. Concr. Res. 2002, 32, 1301–1311. [Google Scholar] [CrossRef]
- Gomez-Soberon, J.M.V. Relationship between gas adsorption and the shrinkage and creep of recycled aggregate concrete. Cem. Concr. Aggreg. 2003, 25, 42–48. [Google Scholar]
- Miguel Mendivil-Escalante, J.; Manuel Gomez-Soberon, J.; Luis Almaral-Sanchez, J.; Guadalupe Cabrera-Covarrubias, F. Metamorphosis in the porosity of recycled concretes through the use of a recycled polyethylene terephthalate (PET) additive. Correlations between the porous network and concrete properties. Materials 2017, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Bijeljic, B.; Blunt, M.J. Pore-scale imaging of geological carbon dioxide storage under in situ conditions. Geophys. Res. Lett. 2013, 40, 3915–3918. [Google Scholar] [CrossRef] [Green Version]
- Cnudde, V.; Boone, M.N. High-resolution X-ray computed tomography in geosciences: A review of the current technology and applications. Earth Sci. Rev. 2013, 123, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Thiele, S.; Zengerle, R.; Ziegler, C. Nano-morphology of a polymer electrolyte fuel cell catalyst layer-imaging, reconstruction and analysis. Nano Res. 2011, 4, 849–860. [Google Scholar] [CrossRef]
- Wildenschild, D.; Sheppard, A.P. X-ray imaging and analysis techniques for quantifying pore-scale structure and processes in subsurface porous medium systems. Adv. Water Resour. 2013, 51, 217–246. [Google Scholar] [CrossRef]
- Saif, T.; Lin, Q.; Butcher, A.R.; Bijeljic, B.; Blunt, M.J. Multi-scale multi-dimensional microstructure imaging of oil shale pyrolysis using X-ray micro-tomography, automated ultra-high resolution SEM, maps mineralogy and fib-sem. Appl. Energy 2017, 202, 628–647. [Google Scholar] [CrossRef]
- Zaretskiy, Y.; Geiger, S.; Sorbie, K.; Förster, M. Efficient flow and transport simulations in reconstructed 3d pore geometries. Adv. Water Resour. 2010, 33, 1508–1516. [Google Scholar] [CrossRef]
- Singh, K.; Bijeljic, B.; Blunt, M.J. Imaging of oil layers, curvature and contact angle in a mixed-wet and a water-wet carbonate rock. Water Resour. Res. 2016, 52, 1716–1728. [Google Scholar] [CrossRef] [Green Version]
- Raeini, A.Q.; Blunt, M.J.; Bijeljic, B. Direct simulations of two-phase flow on micro-CT images of porous media and upscaling of pore-scale forces. Adv. Water Resour. 2014, 74, 116–126. [Google Scholar] [CrossRef]
- Hu, W.L.; Huang, N.; Zhang, X.X. Impact of saturation on mass transfer rate between mobile and immobile waters in solute transport within aggregated soils. J. Hydrol. 2014, 519, 3557–3565. [Google Scholar] [CrossRef]
- Hu, W.; Liu, G.; Zhang, X. A pore-scale model for simulating water flow in unsaturated soil. Microfluid. Nanofluid. 2018, 22, 71. [Google Scholar] [CrossRef]
- Cunningham, R.E.; Williams, R.J.J. Diffusion in Gases and Porous Media; Springer: New York, NY, USA, 1980. [Google Scholar]
- Mason, E.A.; Malinauskas, A.P. Gas Transport in Porous Media: The Dusty-Gas Model; Elsevier: Amsterdam, The Netherlands, 1983. [Google Scholar]
- Hu, W. The Research of Fluid Migration in Soil Using the Pore-Scale Modelling Method. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 1 October 2015. [Google Scholar]
- Becker, J.; Wieser, C.; Fell, S.; Steiner, K. A multi-scale approach to material modeling of fuel cell diffusion media. Int. J. Heat Mass Transf. 2011, 54, 1360–1368. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Effect of pore size distribution and air flux on mass transport in direct contact membrane distillation. J. Membr. Sci. 2003, 215, 75–85. [Google Scholar] [CrossRef]
- Mason, E.A.; Evans, R.B., III. Graham’s laws: Simple demonstrations of gases in motion part 1, theory. J. Chem. Educ. 1969, 46, 358–364. [Google Scholar] [CrossRef]
- Fernandez-Pineda, C.; Izquierdo-Gil, M.A.; Garcia-Payo, M.C. Gas permeation and direct contact membrane distillation experiments and their analysis using different models. J. Membr. Sci. 2002, 198, 33–49. [Google Scholar] [CrossRef]
- Martínez, L.; Florido-Díaz, F.J.; Hernández, A.; Prádanos, P. Characterisation of three hydrophobic porous membranes used in membrane distillation: Modelling and evaluation of their water vapour permeabilities. J. Membr. Sci. 2002, 203, 15–27. [Google Scholar] [CrossRef]
- Evans, R.B., III; Watson, G.M.; Mason, E.A. Gaseous diffusion in porous media at uniform pressure. J. Chem. Phys. 1961, 35, 2076–2083. [Google Scholar] [CrossRef]
- Thorstenson, D.C.; Pollock, D.W. Gas transport in unsaturated zones multicomponent systems and the adequacy of fick’s laws. Water Resour. Res. 1989, 25, 477–507. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y. Impact of liquid water on oxygen reaction in cathode catalyst layer of proton exchange membrane fuel cell: A simple and physically sound model. J. Power Sources 2016, 318, 251–263. [Google Scholar] [CrossRef]
- Young, I.M.; Ritz, K. Tillage, habitat space and function of soil microbes. Soil Tillage Res. 2000, 53, 201–213. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Moldrup, P.; Olesen, T.; Yoshikawa, S.; Komatsu, T.; Rolston, D.E. Three-porosity model for predicting the gas diffusion coefficient in undisturbed soil. Soil Sci. Soc. Am. J. 2004, 68, 750–759. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Jiang, Y.; Chen, D.; Lin, Y.; Han, Q.; Cui, Y. Impact of Pore Geometry and Water Saturation on Gas Effective Diffusion Coefficient in Soil. Appl. Sci. 2018, 8, 2097. https://doi.org/10.3390/app8112097
Hu W, Jiang Y, Chen D, Lin Y, Han Q, Cui Y. Impact of Pore Geometry and Water Saturation on Gas Effective Diffusion Coefficient in Soil. Applied Sciences. 2018; 8(11):2097. https://doi.org/10.3390/app8112097
Chicago/Turabian StyleHu, Wulong, Yao Jiang, Daoyi Chen, Yongshui Lin, Qiang Han, and Yifei Cui. 2018. "Impact of Pore Geometry and Water Saturation on Gas Effective Diffusion Coefficient in Soil" Applied Sciences 8, no. 11: 2097. https://doi.org/10.3390/app8112097




