Removal of Fluoride and Arsenate from Aqueous Solutions by Aluminum-Modified Guava Seeds
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biosorbent
2.2. Biosorbent Characterization
2.3. Adsorption Kinetic Experiments
- q = measured sorption per unit weight of solid, (mg/g).
- V = volume of solution, (L).
- C0 = initial concentration of fluoride or arsenic, (mg/L).
- Ce = equilibrium concentration of fluoride or arsenic, (mg/L).
- M = dry weight of biosorbent (g).
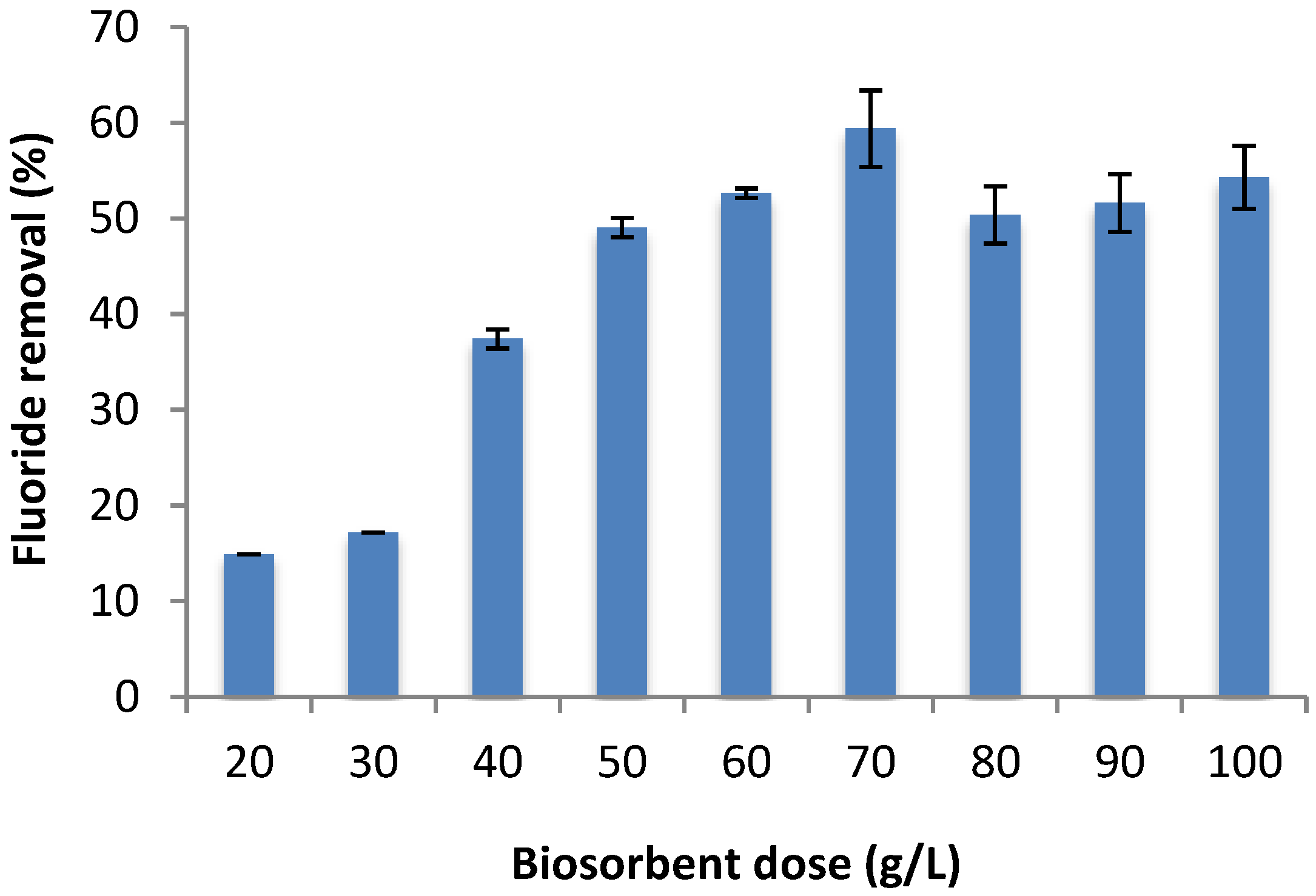
2.4. Effect of Adsorbent Dose on Fluoride Removal
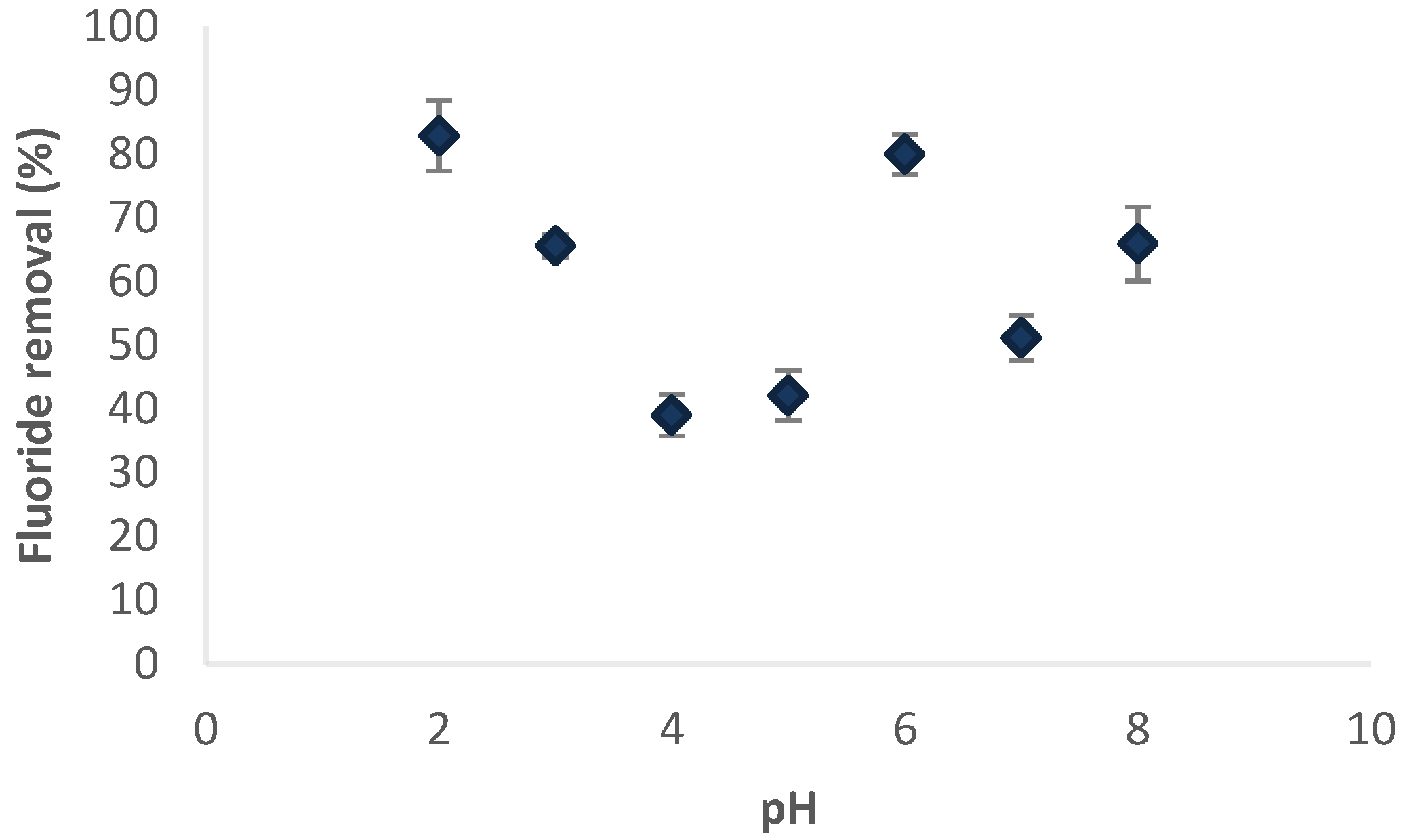
2.5. Effect of pH on Fluoride Removal
2.6. Fluoride and Arsenic Adsorption Isotherms
3. Results and Discussion
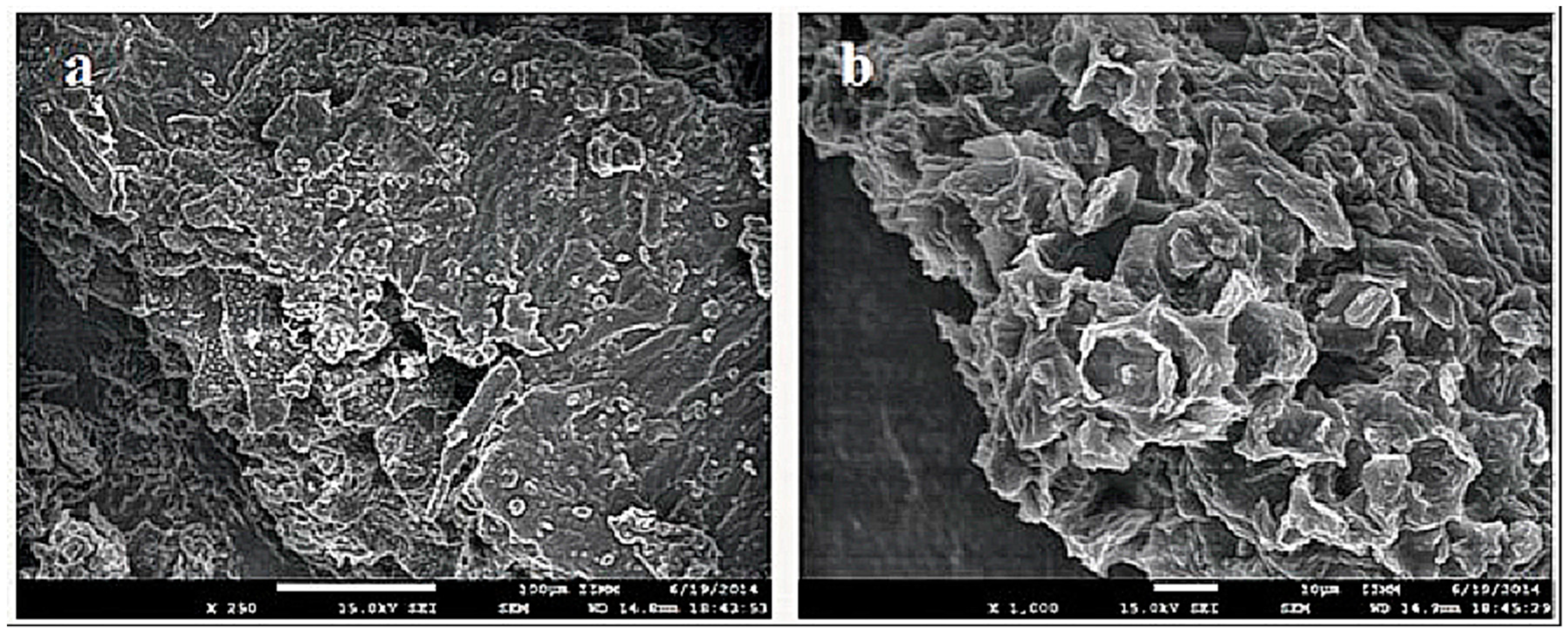
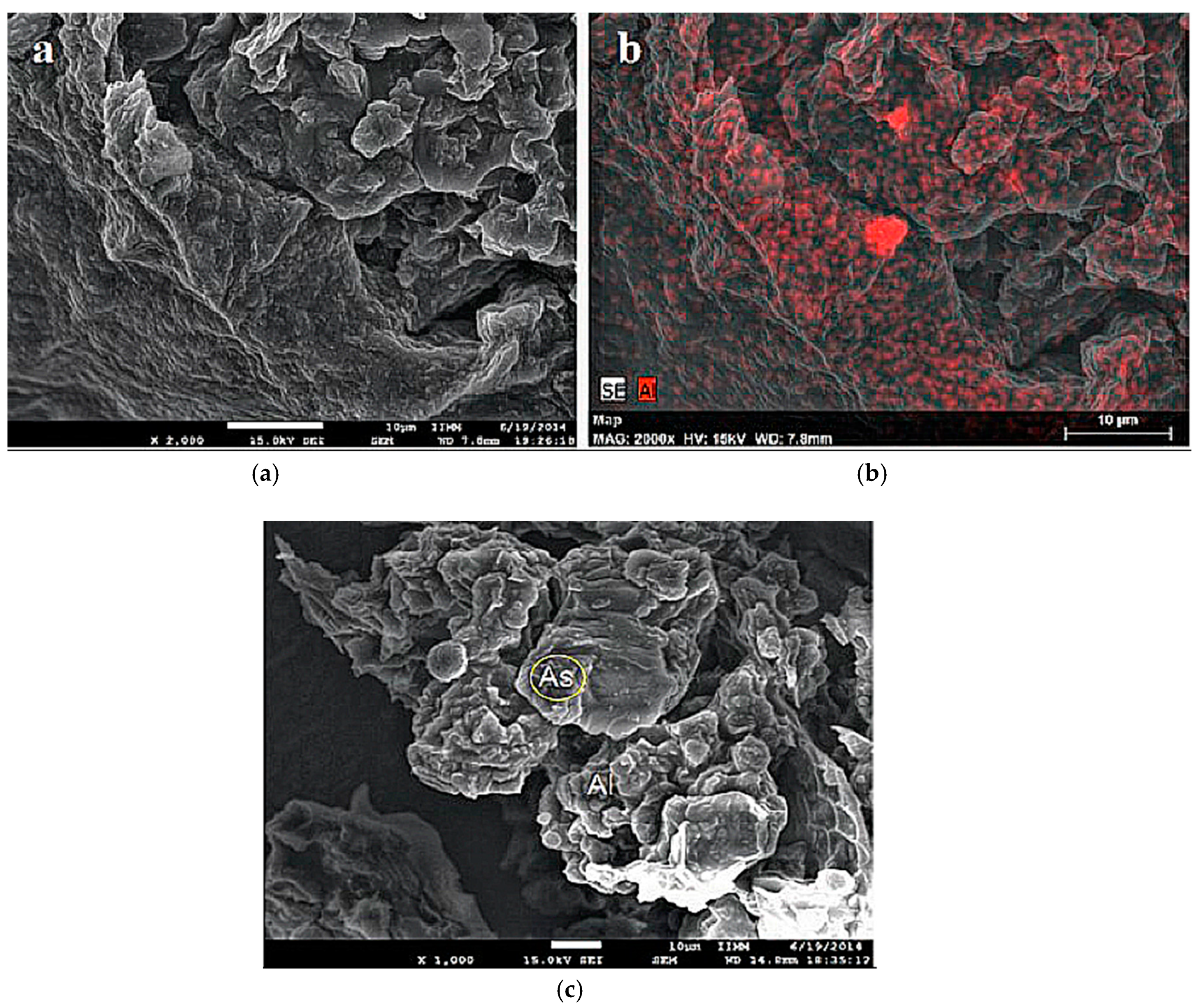
3.1. Biosorbent Characterization
3.2. Fourier Transform Infrared Spectroscopy
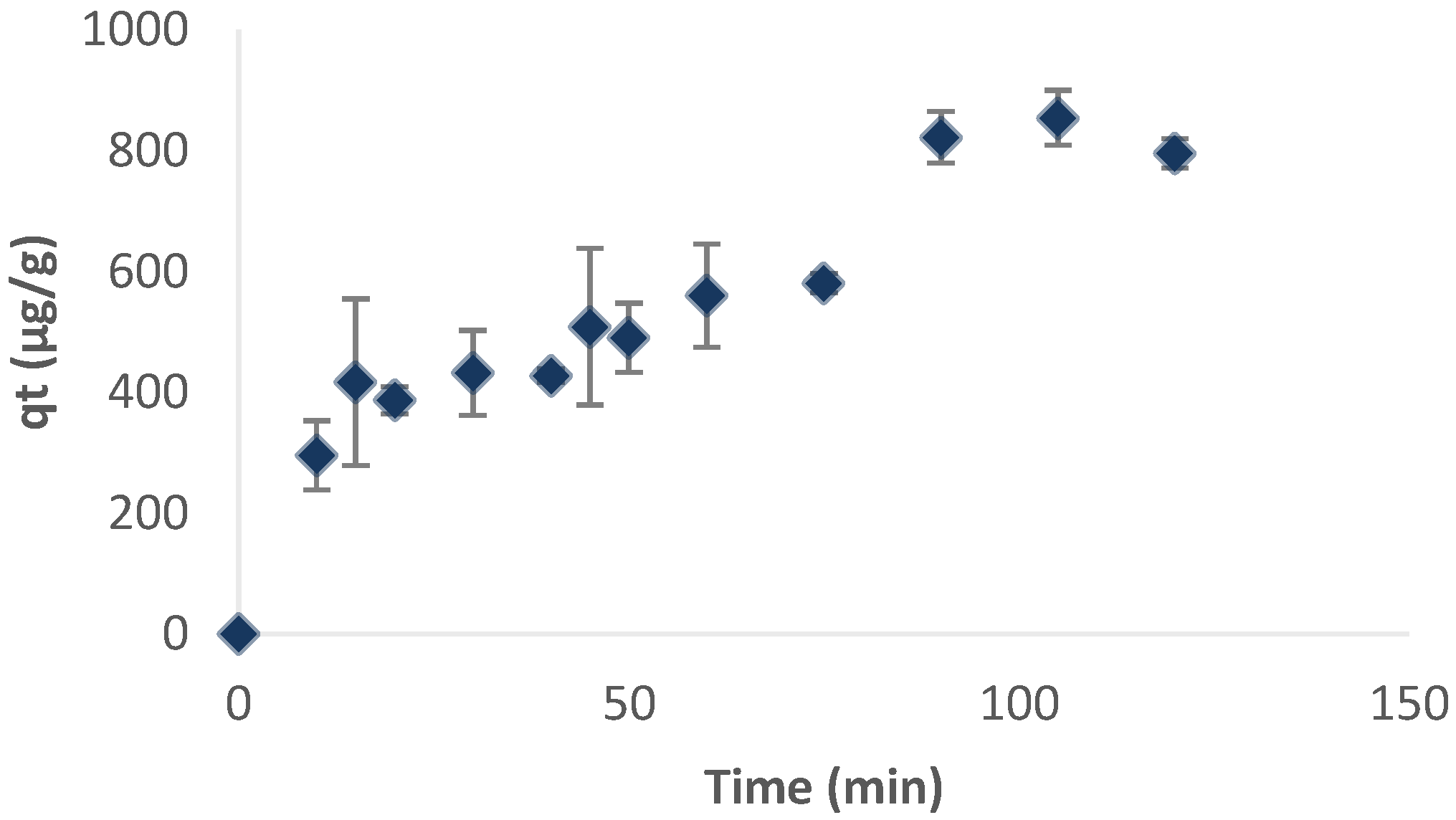
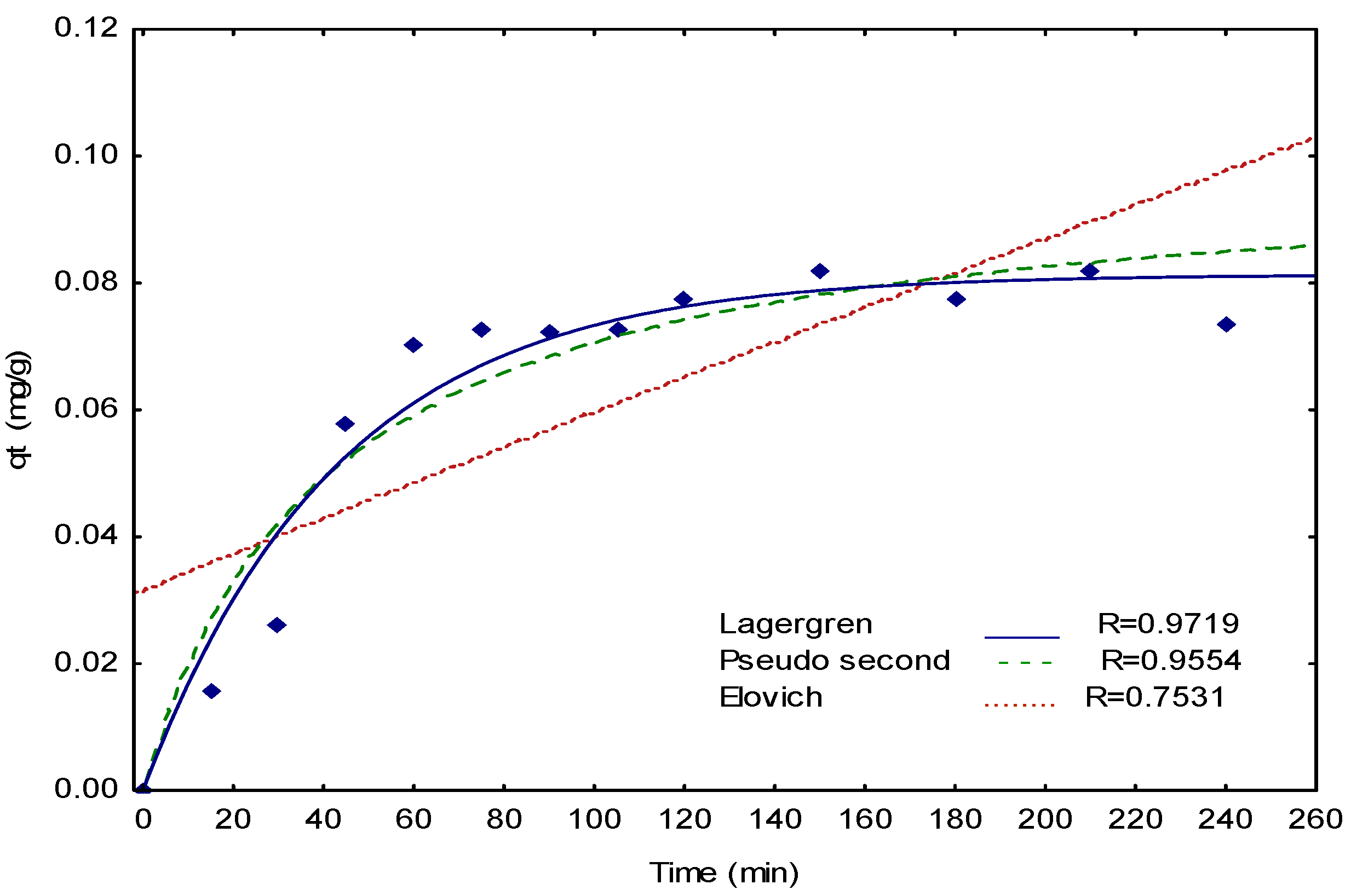
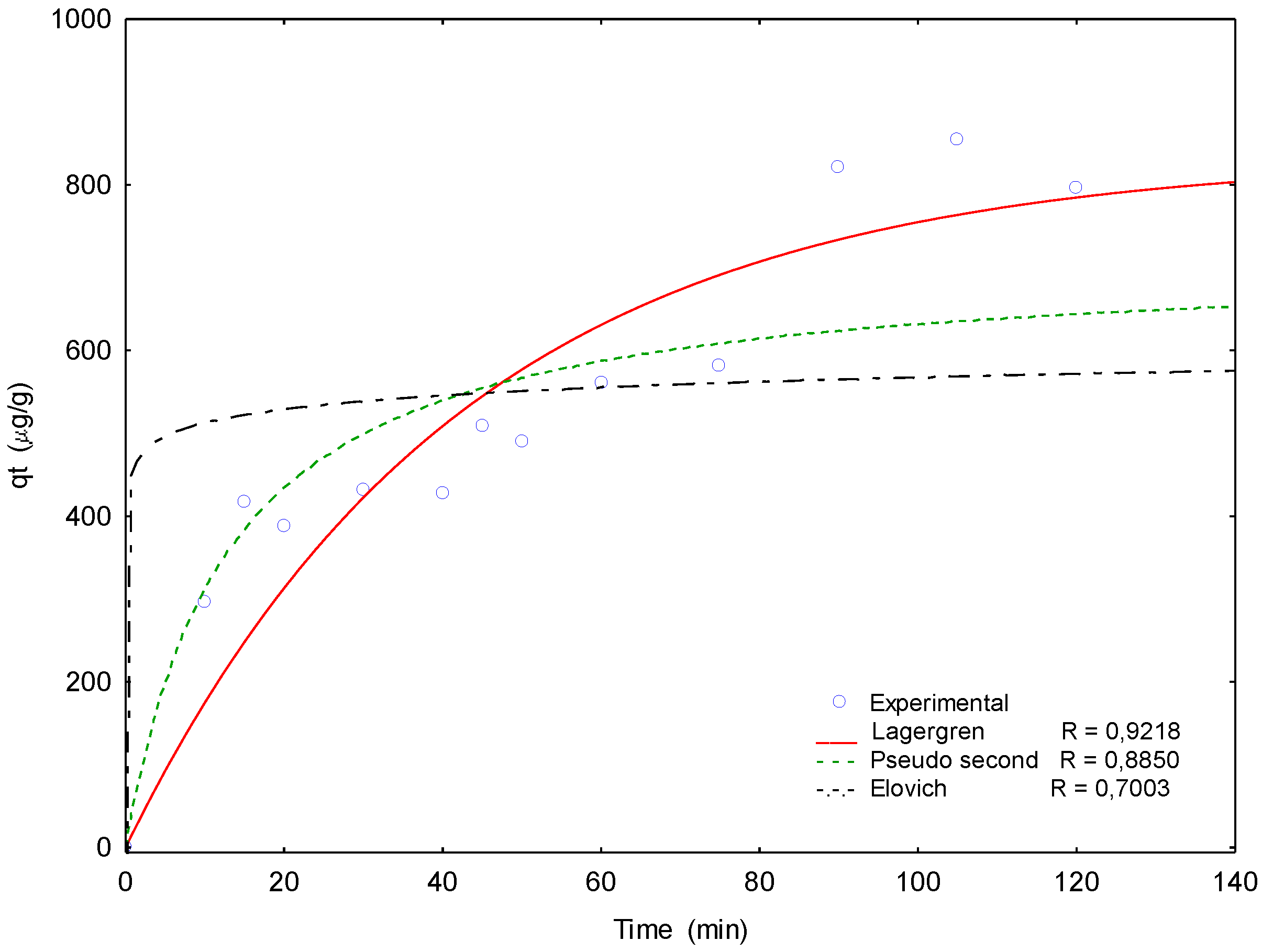
3.3. Arsenate and Fluoride Biosorption Kinetics
- KL = Lagergren rate constant, (min−1).
- qt = quantity of ion adsorbed at any given time t, (mg/g).
- qe = quantity of ion adsorbed at equilibrium, (mg/g).
- K2 = pseudo-second-order rate constant, (g/mg min).
- qt = quantity of ion adsorbed at any given time t, (mg/g).
- qe = quantity of ion adsorbed at equilibrium, (mg/g).
- qt = adsorbate concentration at any time, t, per weight of adsorbent, (mg/g).
- a = Elovich constant related to the initial adsorption rate, (mg/g min).
- b = Elovich constant related to the desorption rate, (g/mg).
3.4. Effect of Adsorbent Dose on Fluoride Removal
3.5. Effect of pH on Fluoride Removal
3.6. Fluoride and Arsenic Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barathi, M.; Kumar, A.; Santhana, K.; Rajesh, N. Efficacy of novel Al–Zr impregnated cellulose adsorbent prepared using microwave irradiation for the facile defluoridation of water. J. Environ. Chem. Eng. 2013, 1, 1325–1335. [Google Scholar] [CrossRef]
- Bechir, A.; Sirbu, R.; Bechir, E.S.; Pacurar, M.; Ghergic, D.L.; Arghir, O.C. Dental fluorosis as a result of cumulated exposure. J. Environ. Prot. Ecol. 2012, 13, 2161–2166. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Jiménez-Becerril, J.M.; Solache-Ríos, M.; García-Sosa, I. Fluoride removal from aqueous solutions by boehmite. Water Air Soil Pollut. 2012, 223, 1073–1078. [Google Scholar] [CrossRef]
- Sujana, M.G.; Pradhan, H.K.; Anand, S. Studies on sorption of some geomaterials for fluoride removal from aqueous solutions. J. Hazard. Mater. 2009, 161, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Teutli-Sequeira, A.; Solache-Ríos, M.; Balderas-Hernández, P. Modification effects of hematite with aluminum hydroxide on the removal of fluoride ion from water. Water Air Soil Pollut. 2012, 223, 319–327. [Google Scholar] [CrossRef]
- Teutli-Sequeira, A.; Solache-Ríos, M.; Martínez-Miranda, V.; Linares-Hernández, I. Comparison of aluminum modified natural materials in the removal of fluoride ions. J. Colloid Interface Sci. 2014, 418, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, H.A.; Cortés-Martínez, R.; Alfaro-Cuevas-Villanueva, R. Fluoride removal from aqueous solutions by mechanically modified guava seeds. Int. J. Sci. Basic Appl. Res. 2013, 11, 159–172. [Google Scholar]
- Dupont, L.; Jolly, G.; Aplincourt, M. Arsenic adsorption on lignocellulosic substrate loaded with ferric ion. Environ. Chem. Lett. 2007, 5, 125–129. [Google Scholar] [CrossRef]
- Mueller, B.; Hug, S.J. Climatic variations and de-coupling between arsenic and iron in arsenic contaminated ground water in the lowlands of Nepal. Chemosphere 2018, 210, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Jing, C.; Cui, J.; Huang, Y.; Li, A. Fabrication, characterization, and application of a composite adsorbent for simultaneous removal of arsenic and fluoride. ACS Appl. Mater. Interfaces 2012, 4, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gong, W.; Lan, H.; Yang, T.; Liu, H.; Qu, J. Simultaneous removal of arsenate and fluoride by iron and aluminum binary oxide: Competitive adsorption effects. Sep. Purif. Technol. 2012, 92, 100–105. [Google Scholar] [CrossRef]
- Monser, L.; Adhoum, N. Modified activated carbon for the removal of copper, zinc, chromium and cyanide from wastewater. Sep. Purif. Technol. 2002, 26, 137–146. [Google Scholar] [CrossRef]
- Diaz-Nava, C.; Olguin, M.T.; Solache-Ríos, M. Water defluoridation by Mexican heulandite–clinoptilolite. Sep. Sci. Technol. 2002, 37, 3109–3128. [Google Scholar] [CrossRef]
- Jiménez-Núñez, M.L.; Olguín, M.T.; Solache-Ríos, M. Fluoride removal from aqueous solutions by magnesium, nickel, and cobalt calcined hydrotalcite-like compounds. Sep. Sci. Technol. 2007, 42, 3623–3639. [Google Scholar] [CrossRef]
- Ghorai, S.; Pant, K.K. Equilibrium, kinetics and breakthrough studies for adsorption of fluoride on activated alumina. Sep. Purif. Technol. 2005, 42, 265–271. [Google Scholar] [CrossRef]
- Amin, F.; Talpur, F.N.; Balouch, A.; Surhio, M.A.; Bhutto, M.A. Biosorption of fluoride from aqueous solution by white—rot fungus Pleurotus eryngii ATCC 90888. Environ. Nanotechnol. Monit. Manag. 2015, 3, 30–37. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, J.; Gao, N. Characteristics and model studies for fluoride and arsenic adsorption on goethite. J. Environ. Sci. 2010, 22, 1689–1694. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Wang, D.; Lin, X.; Liu, W.; Ma, L.; Li, Y.; Huang, Y. Modified native cellulose fibers—A novel efficient adsorbent for both fluoride and arsenic. J. Hazard. Mater. 2011, 185, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2018. [Google Scholar] [CrossRef]
- Cai, H.; Chen, G.; Peng, C.; Xu, L.; Zhu, X.; Zhang, Z.; Dong, Y.; Shang, G.; Ke, F.; Gao, H.; et al. Removal of fluoride from drinking water using tea waste loaded with Al/Fe oxides: A novel, safe and efficient biosorbent. Appl. Surf. Sci. 2015, 328, 34–44. [Google Scholar] [CrossRef]
- Abdelwahab, O.; El Sikaily, A.; Khaled, A.; ElNemr, A. Mass-transfer processes of chromium (VI) adsorption onto guava seeds. Chem. Ecol. 2007, 23, 73–85. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Stolle, A.; Stelter, M. Sustainable synthesis of high-surface-area graphite oxide via dry ball milling. ACS Sustain. Chem. Eng. 2018, 6, 6358–6369. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M.; Radwan, A. Optimization of Cadmium (CD2+) removal from aqueous solutions by novel biosorbent. Int. J. Phytorem. 2016, 18, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Guerrero, A.; Alfaro-Cuevas-Villanueva, R.; Rutiaga-Quiñones, J.G.; Cortés-Martínez, R. Fluoride removal by aluminum-modified pine sawdust: Effect of competitive ions. Ecol. Eng. 2016, 94, 365–379. [Google Scholar] [CrossRef]
- Nadeem, R.; Manzoor, Q.; Iqbal, M.; Nisar, J. Biosorption of Pb (II) onto immobilized and native Mangifera indica waste biomass. J. Ind. Eng. Chem. 2016, 35, 185–194. [Google Scholar] [CrossRef]
- Guaya, D.; Valderrama, C.; Farran, A.; Armijos, C.; Cortina, J.L. Simultaneous phosphate and ammonium removal from aqueous solution by a hydrated aluminum oxide modified natural zeolite. Chem. Eng. J. 2015, 271, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Badawi, M.A.; Negm, N.A.; Kana, M.A.; Hefni, H.H.; Moneem, M.A. Adsorption of aluminum and lead from wastewater by chitosan-tannic acid modified biopolymers: Isotherms, kinetics, thermodynamics and process mechanism. Int. J. Biol. Macromol. 2017, 99, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Halladj, R. Adsorptive removal of fluoride ions from aqueous solution by using sonochemically synthesized nanomagnesia/alumina adsorbents: An experimental and modeling study. J. Taiwan Inst. Chem. Eng. 2014, 45, 2518–2525. [Google Scholar] [CrossRef]
- Peng, C.; Xi, J.; Chen, G.; Feng, Z.; Ke, F.; Ning, J.; Li, D.; Ho, C.T.; Wan, X. Highly selective defluoridation of brick tea infusion by tea waste supported aluminum oxides. J. Sci. Food Agric. 2017, 97, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Edenzon, K.; Fernandez, L.; Razmpour, S.; Woodburn, J.; Cebe, P. Nanocomposites of poly (vinylidene fluoride) with multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2010, 115, 3238–3248. [Google Scholar] [CrossRef]
- Goldberg, S.; Johnston, C.T. Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J. Colloid Interface Sci. 2001, 234, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Tossell, J.A. Theoretical studies on arsenic oxide and hydroxide species in minerals and in aqueous solution. Geochim. Cosmochim. Acta 1997, 61, 1613–1623. [Google Scholar] [CrossRef]
- Tang, W.; Su, Y.; Li, Q.; Gao, S.; Shang, J.K. Superparamagnetic magnesium ferrite nanoadsorbent for effective arsenic (III, V) removal and easy magnetic separation. Water Res. 2013, 47, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Ouma, I.L.; Naidoo, E.B.; Ofomaja, A.E. Thermodynamic, kinetic and spectroscopic investigation of arsenite adsorption mechanism on pine cone-magnetite composite. J. Environ. Chem. Eng. 2018, 6, 5409–5419. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Fawzy, M. Bio-based methods for wastewater treatment: Green sorbents. In Phytoremediation; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Lagergren, S. Zur theorie der sogenannten adsorption gelosterstoffe. Kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G.; Wase, D.A.J.; Forster, C.F. Study of the sorption of divalent metal ions on to peat. Adsorp. Sci. Technol. 2000, 18, 639–650. [Google Scholar] [CrossRef]
- Low, M.J.D. Kinetics of chemisorption of gases on solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Bibi, S.; Farooqi, A.; Hussain, K.; Haider, N. Evaluation of industrial based adsorbents for simultaneous removal of arsenic and fluoride from drinking water. J. Clean. Prod. 2015, 87, 882–896. [Google Scholar] [CrossRef]
- Ramos-Vargas, S.; Cortés-Martínez, R. Remoción simultánea de arsénico y fluoruros de soluciones acuosas mediante semillas de guayaba modificadas. In VIII Congreso Internacional de Ingeniería Bioquímica; XIX Congreso Nacional de Ingeniería Bioquímica: Mazatlán, Mexico, 2014. (In Spanish) [Google Scholar]
- Rathore, V.K.; Dohare, D.K.; Mondal, P. Competitive adsorption between arsenic and fluoride from binary mixture on chemically treated laterite. J. Environ. Chem. Eng. 2016, 4, 2417–2430. [Google Scholar] [CrossRef]
- Qiao, J.; Cui, Z.; Sun, Y.; Hu, Q.; Guan, X. Simultaneous removal of arsenate and fluoride from water by Al-Fe (hydr) oxides. Front. Environ. Sci. Eng. 2014, 8, 169–179. [Google Scholar] [CrossRef]
- Nordin, J.P.; Sullivan, D.J.; Phillips, B.L.; Casey, W.H. Mechanisms for fluoride-promoted dissolution of bayerite [β-Al(OH)3(s)] and boehmite [γ-AlOOH]: 19F-NMR spectroscopy and aqueous surface chemistry. Geochim. Cosmochim. Acta 1999, 63, 3513–3524. [Google Scholar] [CrossRef]
- George, S.; Pandit, P.; Gupta, A.B. Residual aluminium in water defluoridated using activated alumina adsorption–Modeling and simulation studies. Water Res. 2010, 44, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, T.; Lei, C.; Zhang, F. Evaluation of Al-based nanoparticle-impregnated sawdust as an adsorbent from byproduct for the removal of arsenic (V) from aqueous solutions. Environ. Prog. Sustain. Energy 2017, 36, 1314–1322. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Vázquez Mejía, G.; Martínez-Miranda, V.; Fall, C.; Linares-Hernández, I.; Solache-Ríos, M. Comparison of Fe–Al-modified natural materials by an electrochemical method and chemical precipitation for the adsorption of F− and As (V). Environ. Technol. 2016, 37, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Brahman, K.D.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Arain, S.S.; Saraj, S.; Arain, M.B.; Arain, S.A. Biosorptive removal of inorganic arsenic species and fluoride from aqueous medium by the stem of Tecomella undulate. Chemosphere 2016, 150, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Sharif, F.; Bashir, S.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; et al. Arsenic removal by natural and chemically modified water melon rind in aqueous solutions and groundwater. Sci. Total Environ. 2018, 645, 1444–1455. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Xu, X.; Phan, T.; Hu, X.; Nie, G. High adsorption performance for As (III) and As (V) onto novel aluminum-enriched biochar derived from abandoned Tetra Paks. Chemosphere 2018, 208, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Ravulapalli, S.; Kunta, R. Defluoridation studies using active carbon derived from the barks of Ficus racemosa plant. J. Fluor. Chem. 2017, 193, 58–66. [Google Scholar] [CrossRef]
- Mohammad, A.; Majumder, C.B. Removal of fluoride from synthetic waste water by using “bio-adsorbents”. Int. J. Res. Eng. Technol. 2014, 3, 776–785. [Google Scholar]





| Model | Kinetic Parameters | Correlation Coefficient (R) |
|---|---|---|
| pseudo-first-order (Lagergren) | KL (min−1) = 0.0231 | 0.9719 |
| qe (mg/g) = 0.0813 | ||
| pseudo-second-order | K2 (g/mg min) = 0.0993 | 0.9554 |
| qe (mg/g) = 0.0813 | ||
| Elovich | a (mg/g min) = 0.0003 | 0.7531 |
| b (g/mg) = 1.0162 |
| Model | Kinetic Parameters | Correlation Coefficient (R) |
|---|---|---|
| Lagergren | KL (min−1) = 0.0235 | 0.9218 |
| qe (mg/g) = 834.26 | ||
| pseudo-second-order | K2 (g/mg min) = 5 × 10−5 | 0.8850 |
| qe (mg/g) = 712.5 | ||
| Elovich | a (mg/g min) = 5.3 × 10−9 | 0.7003 |
| b (g/mg) = 0.0420 |
| Adsorbent | Adsorbate | qmax (mg/g) | Solution pH | Reference |
|---|---|---|---|---|
| Al-GS | As(V) | 4 | 6 | This work |
| Fe-Al modified zeolite | As(V) | 3.31 | 2 | [49] |
| Fe-Al modified pozzolan | As(V) | 3.35 | 2 | [49] |
| Stem of Tecomella undulata | As(V) | 0.159 | 7 | [50] |
| Watermelon rind | As(V) | 3.29 | 5.3 | [51] |
| Aluminum-enriched biochar from Tetra Paks | As(V) | 33.2 | 3 | [52] |
| Al-GS | F− | 0.3445 | 6 | This work |
| Fe-Al modified zeolite | F− | 0.866 | 2 | [49] |
| Fe-Al modified pozzolan | F− | 0.834 | 2 | [49] |
| Stem of Tecomella undulata | F− | 6.16 | 7 | [50] |
| Active carbon of Ficus racemosa | F− | 1.65 | 7 | [53] |
| Sweet lemon peel powder | F− | 0.744 | 4 | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Vargas, S.; Alfaro-Cuevas-Villanueva, R.; Huirache-Acuña, R.; Cortés-Martínez, R. Removal of Fluoride and Arsenate from Aqueous Solutions by Aluminum-Modified Guava Seeds. Appl. Sci. 2018, 8, 1807. https://doi.org/10.3390/app8101807
Ramos-Vargas S, Alfaro-Cuevas-Villanueva R, Huirache-Acuña R, Cortés-Martínez R. Removal of Fluoride and Arsenate from Aqueous Solutions by Aluminum-Modified Guava Seeds. Applied Sciences. 2018; 8(10):1807. https://doi.org/10.3390/app8101807
Chicago/Turabian StyleRamos-Vargas, Sarai, Ruth Alfaro-Cuevas-Villanueva, Rafael Huirache-Acuña, and Raúl Cortés-Martínez. 2018. "Removal of Fluoride and Arsenate from Aqueous Solutions by Aluminum-Modified Guava Seeds" Applied Sciences 8, no. 10: 1807. https://doi.org/10.3390/app8101807






