The Effect of Laser Pulse Widths on Laser—Ag Nanoparticle Interaction: Femto- to Nanosecond Lasers
Abstract
:1. Introduction
2. Background
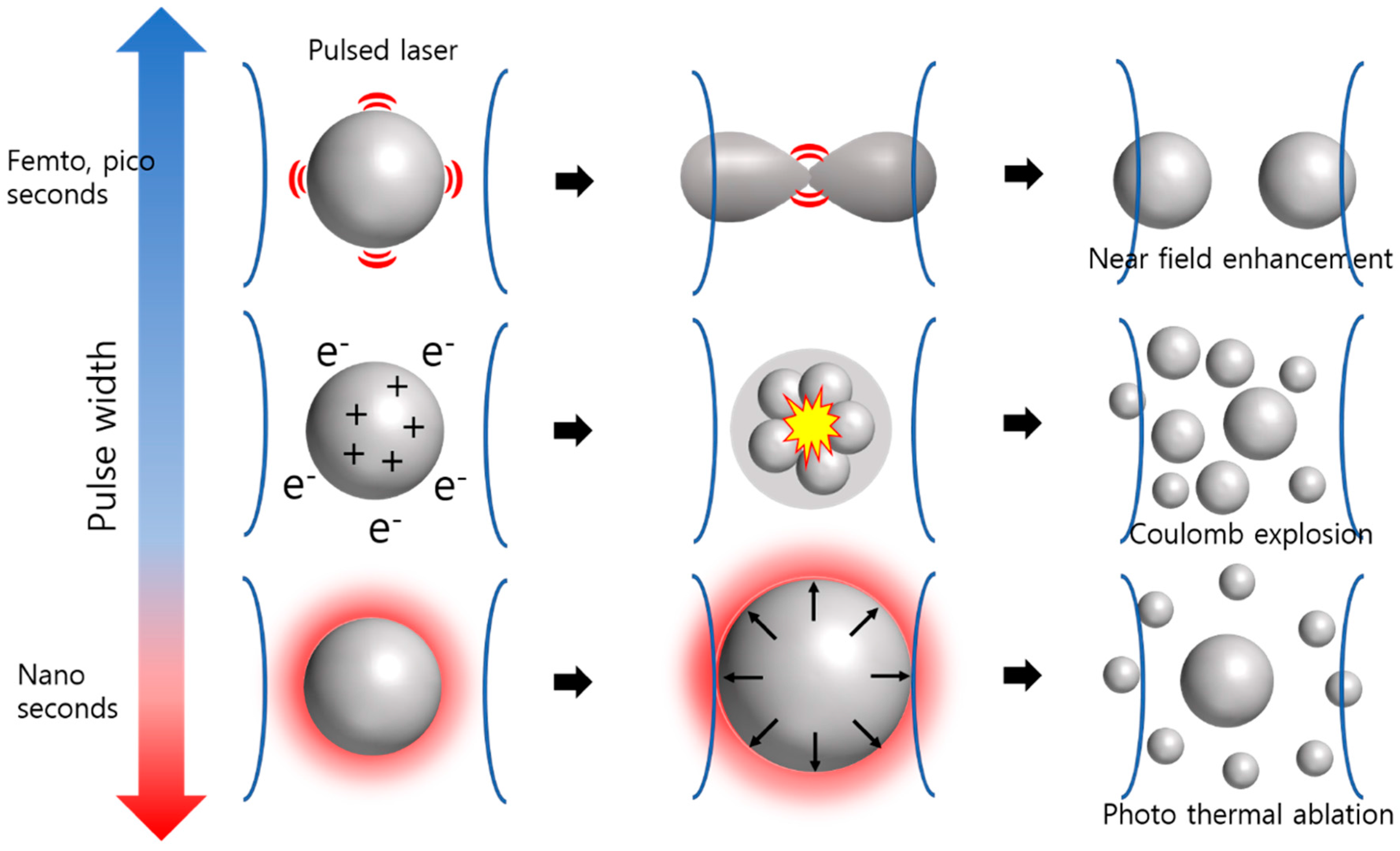
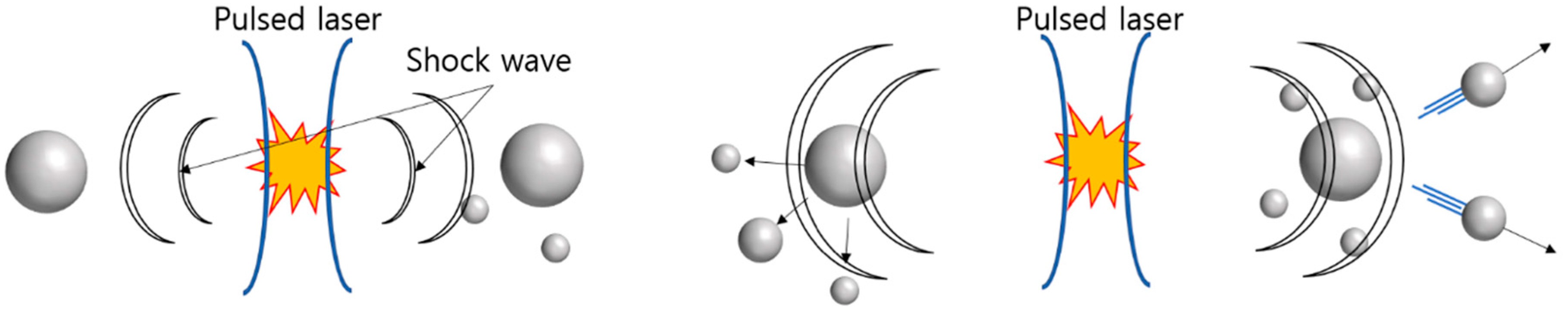
2.1. Effect of Laser Pulse Width
2.2. Simulation of Laser Fragmentation in the Nanosecond Region Using the Wave-Optic Theory
3. Numerical Simulation and Experimental Setup
3.1. Numerical Analysis
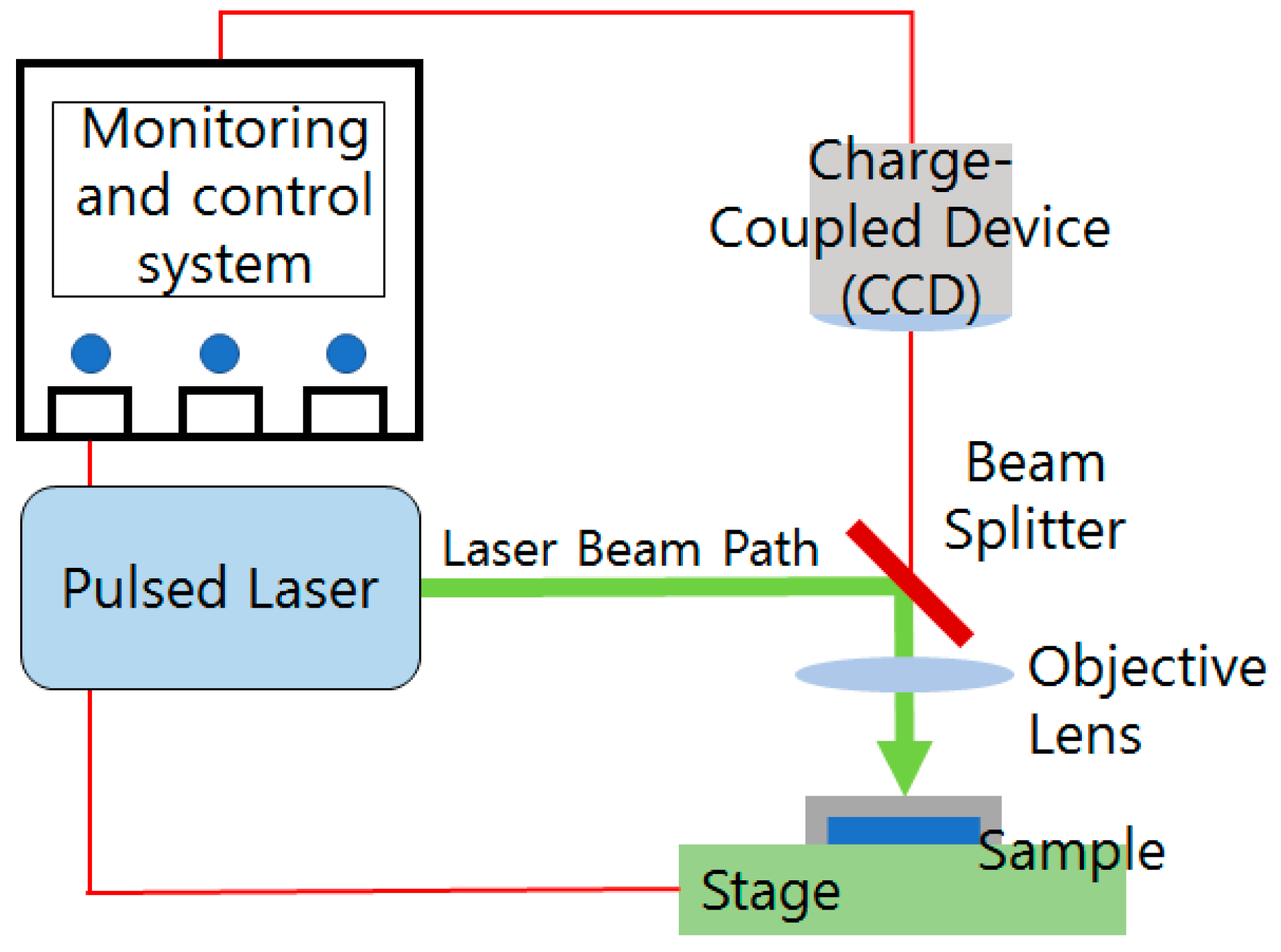
3.2. Setup for Laser Fragmentation
4. Results and Discussion
4.1. Simulation of Nanoparticle Temperature Distribution According to the Laser Pulse Width
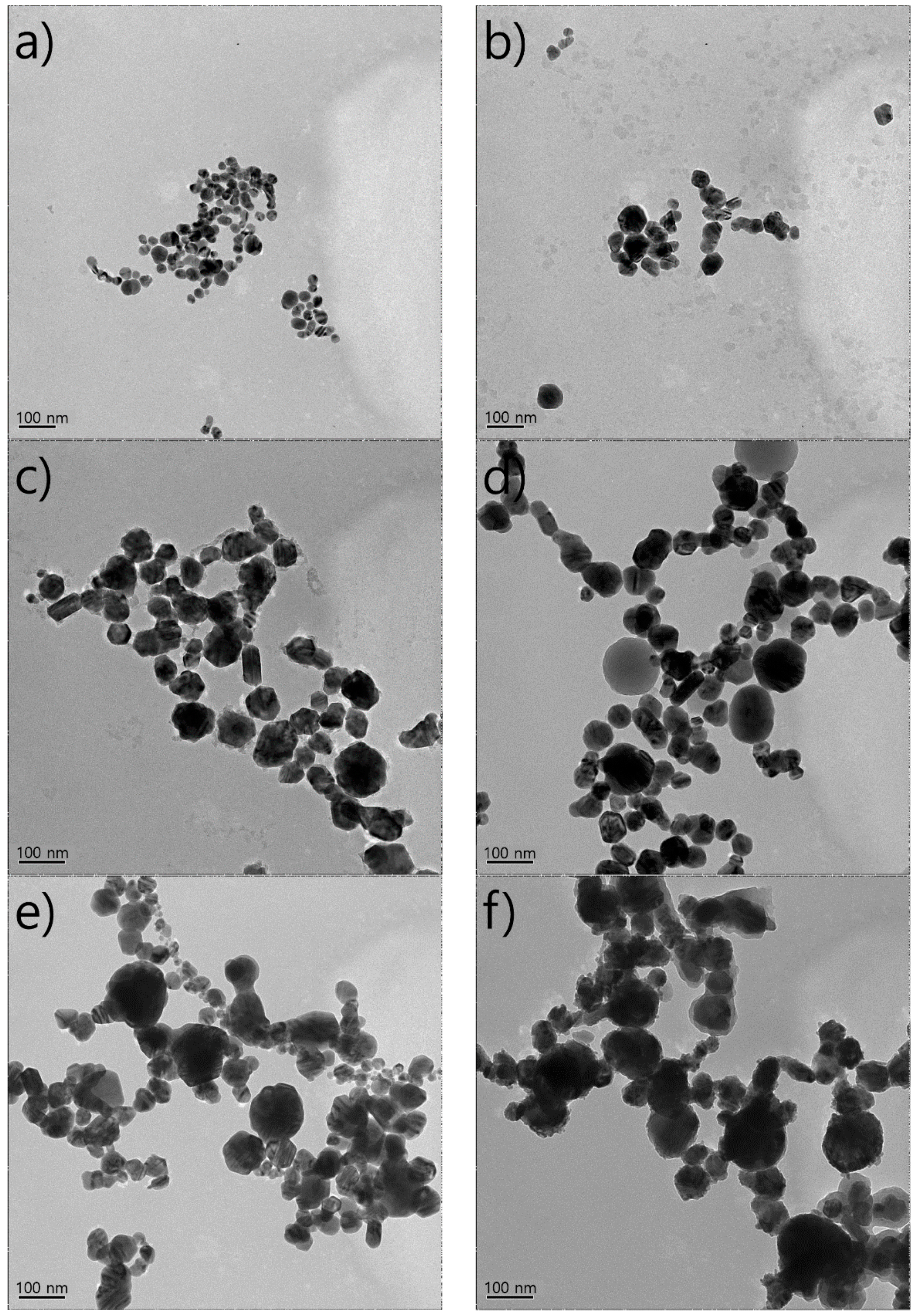
4.2. Nanoparticle Size Distribution
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yguerabide, J.; Ygerabide, E.E. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. Anal. Biochem. 1998, 262, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, B.; Wang, Y.; Guan, S. Preparation of spherical metal–organic frameworks encapsulating Ag nanoparticles and study on its antibacterial activity. Mater. Sci. Eng. C 2017, 80, 698–707. [Google Scholar] [CrossRef]
- Chatterjee, S.; Shariff, S.M.; Majumdar, J.D.; Choudhury, A.R. Development of nano-structured Al2O3-TiB2-TiN coatings by combined SHS and laser surface alloying. Int. J. Adv. Manuf. Technol. 2008, 38, 938–943. [Google Scholar] [CrossRef]
- Raj, D.R.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for the detection of cysteine using diosmin capped silver nanoparticles. Sens. Actuators A Phys. 2017, 253, 41–48. [Google Scholar] [CrossRef]
- Middha, M.; Kumar, R.; Raina, K.K. Effects of chirality on optical and electro-optic behavior of nematic liquid crystals doped with functionalized silver nanoparticles. J. Mol. Liq. 2016, 219, 631–636. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Takeuchi, S.; Mitsunobu, S.; Ok, Y.S. Chemical speciation of silver (Ag) in soils under aerobic and anaerobic conditions: Ag nanoparticles vs. ionic Ag. J. Hazard. Mater. 2017, 322, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Ueno, K.; Misawa, H. Plasmonic antenna effects on photochemical reactions. Acc. Chem. Res. 2011, 44, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Gratzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Mayer, A.C.; Scully, S.R.; Hardin, B.E.; Rowell, M.W.; McGehee, M.D. Polymer-based solar cells. Mater. Today 2007, 10, 28–33. [Google Scholar] [CrossRef]
- Kamat, P.V. Quantum dot solar cells. Semiconductor nanocrystals as light harvesters. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- Nikolay, T.; Larina, L.; Shevaleevskiy, O.; Ahn, B.T. Electronic structure study of lightly Nb-doped TiO2 electrode for dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 1480–1486. [Google Scholar] [CrossRef]
- Jiang, C.; Leung, C.W.; Pong, P.W.T. Self-assembled thin films of Fe3O4-Ag composite nanoparticles for spintronic applications. Appl. Surf. Sci. 2017, 419, 692–696. [Google Scholar] [CrossRef]
- He, W.; Ye, C. Flexible Transparent Conductive Films on the Basis of Ag Nanowires: Design and Applications: A Review. J. Mater. Sci. Technol. 2015, 31, 581–588. [Google Scholar] [CrossRef]
- Oh, H.; Lee, M. Laser-direct fabrication of invisible Ag nanowire electrode pattern on flexible plastic substrate. Thin Solid Films 2017, 636, 375–383. [Google Scholar] [CrossRef]
- Koleva, M.E.; Nedyalkov, N.N.; Fukata, N.; Jevasuwan, W.; Karashanova, D. Laser-assisted approach for synthesis of plasmonic Ag/ZnO nanostructures. Superlattices Microstruct. 2017, 109, 886–896. [Google Scholar] [CrossRef]
- Moura, C.G.; Pereira, R.S.F.; Andritschky, M.; Lopes, A.L.B.; Silva, F.S. Effects of laser fluence and liquid media on preparation of small Ag nanoparticles by laser ablation in liquid Original research article. Opt. Laser Technol. 2017, 97, 20–28. [Google Scholar] [CrossRef]
- Vella, P.C.; Dimov, S.S.; Brousseau, E.; Whiteside, B.R. A new process chain for producing bulk metallic glass replication masters with micro-and nano-scale features. Int. J. Adv. Manuf. Technol. 2015, 76, 523–543. [Google Scholar] [CrossRef]
- Kuo, C.G.; Chao, C.G. A novel method of centrifugal processing for the synthesis of lead–bismuth eutectic alloy nanospheres and nanowires. Int. J. Adv. Manuf. Technol. 2007, 32, 468–472. [Google Scholar] [CrossRef]
- Chang, H.; Jwo, C.S.; Fan, P.S.; Pai, S.H. Process optimization and material properties for nanofluid manufacturing. Int. J. Adv. Manuf. Technol. 2007, 34, 300–306. [Google Scholar] [CrossRef]
- Kawasaki, M.; Nishimura, N. 1064-nm laser fragmentation of thin Au and Ag flakes in acetone for highly productive pathway to stable metal nanoparticles. Appl. Surf. Sci. 2006, 253, 2208–2216. [Google Scholar] [CrossRef]
- Serkov, A.A.; Kuzmin, P.G.; Shafeev, G.A. Laser-induced agglomeration of gold and silver nanoparticles dispersed in liquid. Chem. Phys. Lett. 2016, 647, 68–72. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Carraro, F.; Cattaruzza, E. Formation of alloy nanoparticles by laser ablation of Au/Fe multilayer films in liquid environment. J. Colloid Interface Sci. 2017, 489, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska-Warkocka, Z.; Pyatenko, A.; Koshizaki, N.; Kawaguchi, K. Synthesis of various 3D porous gold-based alloy nanostructures with branched shapes. J. Colloid Interface Sci. 2016, 483, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Tomko, J.; O’Malley, S.M.; Trout, C.; Naddeo, J.J.; Bubb, D.M. Cavitation bubble dynamics and nanoparticle size distributions in laser ablation in liquids. Colloid Surf. A 2017, 522, 368–372. [Google Scholar] [CrossRef]
- Tanabe, R.; Nguyen, T.T.P.; Sugiura, T.; Ito, Y. Bubble dynamics in metal nanoparticle formation by laser ablation in liquid studied through high-speed laser stroboscopic videography. Appl. Surf. Sci. 2015, 351, 327–331. [Google Scholar] [CrossRef]
- Takeda, Y.; Mafuné, F. Formation of wide bandgap cerium oxide nanoparticles by laser ablation in aqueous solution. Chem. Phys. Lett. 2014, 599, 110–115. [Google Scholar] [CrossRef]
- Maximova, K.; Aristov, A.; Sentis, M.; Kabashin, A.V. Size-controllable synthesis of bare gold nanoparticles by femtosecond laser fragmentation in water. Nanotechnology 2015, 26, 065601. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V.; Flumiani, M.; Hartland, G.V. Picosecond Dynamics of Silver Nanoclusters. Photoejection of Electrons and Fragmentation. J. Phys. Chem. B 1998, 102, 3123–3128. [Google Scholar] [CrossRef]
- Kohsakowski, S.; Santagata, A.; Dell’Aglio, M.; de Giacomo, A.; Barcikowski, S.; Wagener, P.; Gökce, B. High productive and continuous nanoparticle fabrication by laser ablation of a wire-target in a liquid jet. Appl. Surf. Sci. 2017, 403, 487–499. [Google Scholar] [CrossRef]
- Leitz, K.H.; Redlingshöfer, B.; Reg, Y.; Otto, A.; Schmidt, M. Metal Ablation with Short and Ultrashort Laser Pulses. Phys. Procedia 2011, 12, 230–238. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Laser Photothermal Melting and Fragmentation of Gold Nanorods: Energy and Laser Pulse-Width Dependence. J. Phys. Chem. A 1999, 103, 1165–1170. [Google Scholar] [CrossRef]
- Inasawa, S.; Sugiyama, M.; Noda, S.; Yamaguchi, Y. Spectroscopic Study of Laser-Induced Phase Transition of Gold Nanoparticles on Nanosecond Time Scales and Longer. J. Phys. Chem. B 2006, 110, 3114–3119. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Furube, A.; Okamoto, T.; Hashimoto, S. Femtosecond Laser-Induced Size Reduction of Aqueous Gold Nanoparticles: In Situ and Pump−Probe Spectroscopy Investigations Revealing Coulomb Explosion. J. Phys. Chem. C 2011, 115, 8503–8512. [Google Scholar] [CrossRef]
- Bae, C.H.; Nam, S.H.; Park, S.M. Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl. Surf. Sci. 2002, 197, 628–634. [Google Scholar] [CrossRef]
- Peng, Z.; Spliethoff, B.; Tesche, B.; Walther, T.; Kleinermanns, K. Laser-Assisted Synthesis of Au-Ag Alloy Nanoparticles in Solution. J. Phys. Chem. B 2006, 110, 2549–2554. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Noack, J.; Nahen, K.; Theisen, D.; Busch, S.; Parlitz, U.; Hammer, D.X.; Noojin, G.D.; Rockwell, B.A.; Birngruber, R. Energy balance of optical breakdown in water at nanosecond to femtosecond time scales. Appl. Phys. B 1999, 68, 271–280. [Google Scholar] [CrossRef]
- Reich, S.; Schönfeld, P.; Wagener, P.; Letzel, A.; Ibrahimkutty, S.; Gökce, B.; Barcikowski, S.; Menzel, A.; Rolo, T.D.S.; Plech, A. Pulsed laser ablation in liquids: Impact of the bubble dynamics on particle formation. J. Colloid Interface Sci. 2017, 489, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Inasawa, S.; Sugiyama, M.; Yamaguchi, Y. Laser-Induced Shape Transformation of Gold Nanoparticles below the Melting Point: The Effect of Surface Melting. J. Phys. Chem. B 2005, 109, 3104–3111. [Google Scholar] [CrossRef] [PubMed]
- Mafuné, F.; Okamoto, T.; Ito, M. Surfactant-free small Ni nanoparticles trapped on silica nanoparticles prepared by pulsed laser ablation in liquid. Chem. Phys. Lett. 2014, 591, 193–196. [Google Scholar] [CrossRef]
- Neuenschwander, B.; Jaeggi, B.; Schmid, M. From fs to sub-ns: Dependence of the material removal rate on the pulse duration for metals. Phys. Procedia 2013, 41, 794–801. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Logunov, S.L.; El-Sayed, M.A. Picosecond Dynamics of Colloidal Gold Nanoparticles. J. Phys. Chem. 1996, 100, 8053–8056. [Google Scholar] [CrossRef]
- Eesley, G.L. Observation of nonequilibrium electron heating in copper. Phys. Rev. Lett. 1983, 517, 2140–2143. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Femtosecond transient-absorption dynamics of colloidal gold nanorods: Shape independence of the electron-phonon relaxation time. Phys. Rev. B 2000, 61, 6086. [Google Scholar] [CrossRef]
- Besner, S.; Kabashin, A.V.; Meunier, M. Fragmentation of colloidal nanoparticles by femtosecond laser-induced supercontinuum generation. Appl. Phys. Lett. 2006, 89, 233122. [Google Scholar] [CrossRef]
- Werner, D.; Hashimoto, S. Improved Working Model for Interpreting the Excitation Wavelength- and Fluence-Dependent Response in Pulsed Laser-Induced Size Reduction of Aqueous Gold Nanoparticles. J. Phys. Chem. C 2011, 115, 5063–5072. [Google Scholar] [CrossRef]
- Plech, A.; Kotaidis, V.; Lorenc, M.; Boneberg, J. Femtosecond laser near-field ablation from gold nanoparticles. Nat. Phys. 2006, 2, 44–47. [Google Scholar] [CrossRef]
- Ueno, K.; Juodkazis, S.; Shibuya, T.; Yokota, Y.; Mizeikis, V.; Sasaki, K.; Misawa, H. Nanoparticle Plasmon-Assisted Two-Photon Polymerization Induced by Incoherent Excitation Source. J. Am. Chem. Soc. 2008, 130, 6928–6929. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Hashimoto, S.; Uwada, T. Remarkable Photothermal Effect of Interband Excitation on Nanosecond Laser-Induced Reshaping and Size Reduction of Pseudospherical Gold Nanoparticles in Aqueous Solution. Langmuir 2010, 26, 9956–9963. [Google Scholar] [CrossRef] [PubMed]
- Zedan, A.F.; Moussa, S.; Terner, J.; Atkinson, G.; El-Shall, M.S. Ultrasmall gold nanoparticles anchored to graphene and enhanced photothermal effects by laser irradiation of gold nanostructures in graphene oxide solutions. ACS Nano 2013, 7, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, B.; Jaeggi, B.; Schmid, M.; Hennig, G. Surface structuring with ultra-short laser pulses: Basics, limitations and needs for high throughput. Phys. Procedia 2014, 56, 1047–1058. [Google Scholar] [CrossRef]
- Kawasaki, M.; Masuda, K. Laser Fragmentation of Water-Suspended Gold Flakes via Spherical Submicroparticles to Fine Nanoparticles. J. Phys. Chem. B 2005, 109, 9379–9388. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W. Laser Beam Scattering Analysis inside Porous Materials by FEM. In Future Information Technology, Application, and Service; Park, J., Leung, V., Wang, C.L., Shon, T., Eds.; Lecture Notes in Electrical Engineering; Springer: Dordrecht, The Netherlands, 2012; Volume 164, pp. 603–614. ISBN 978-94-007-4515-5. [Google Scholar]

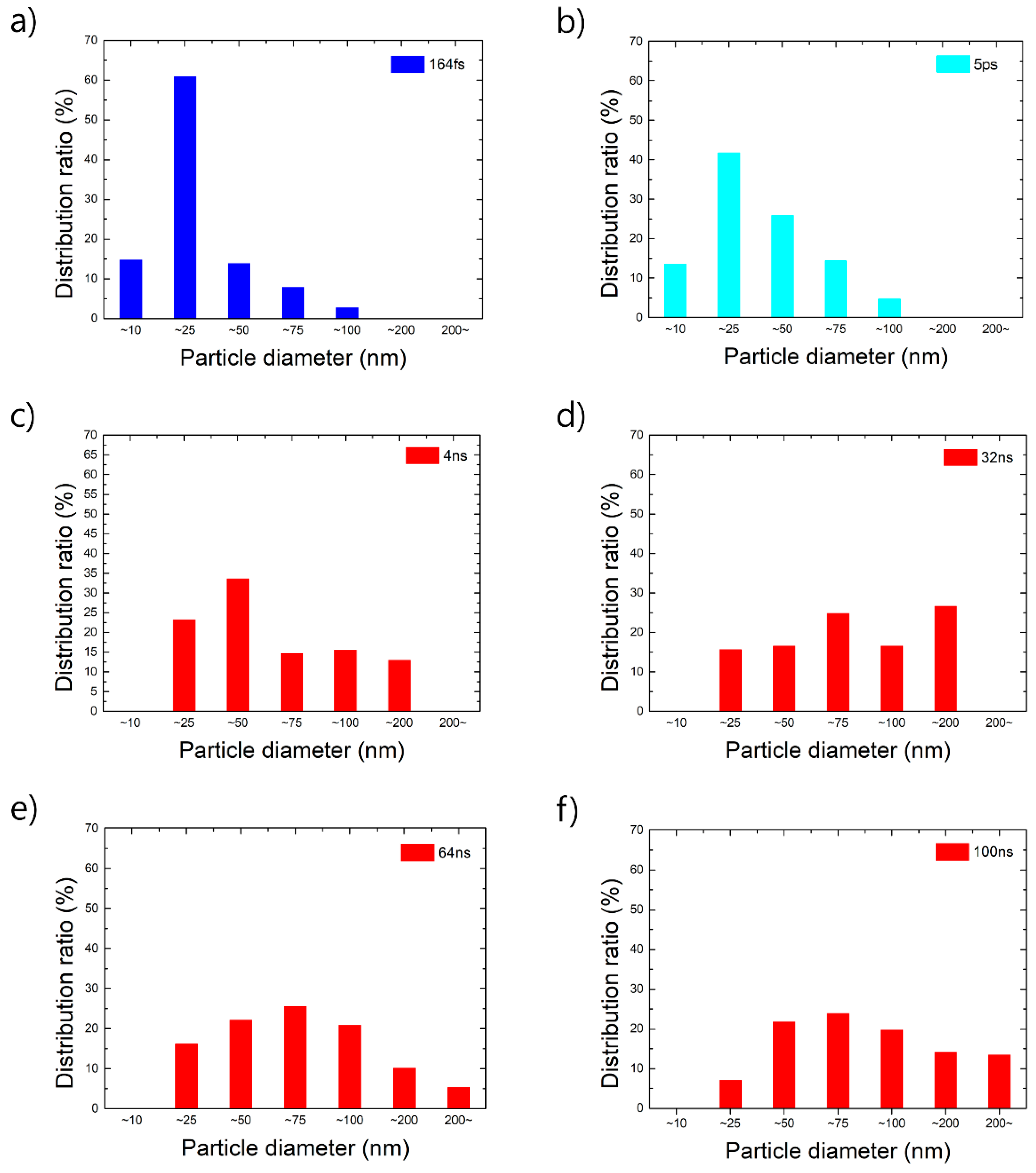
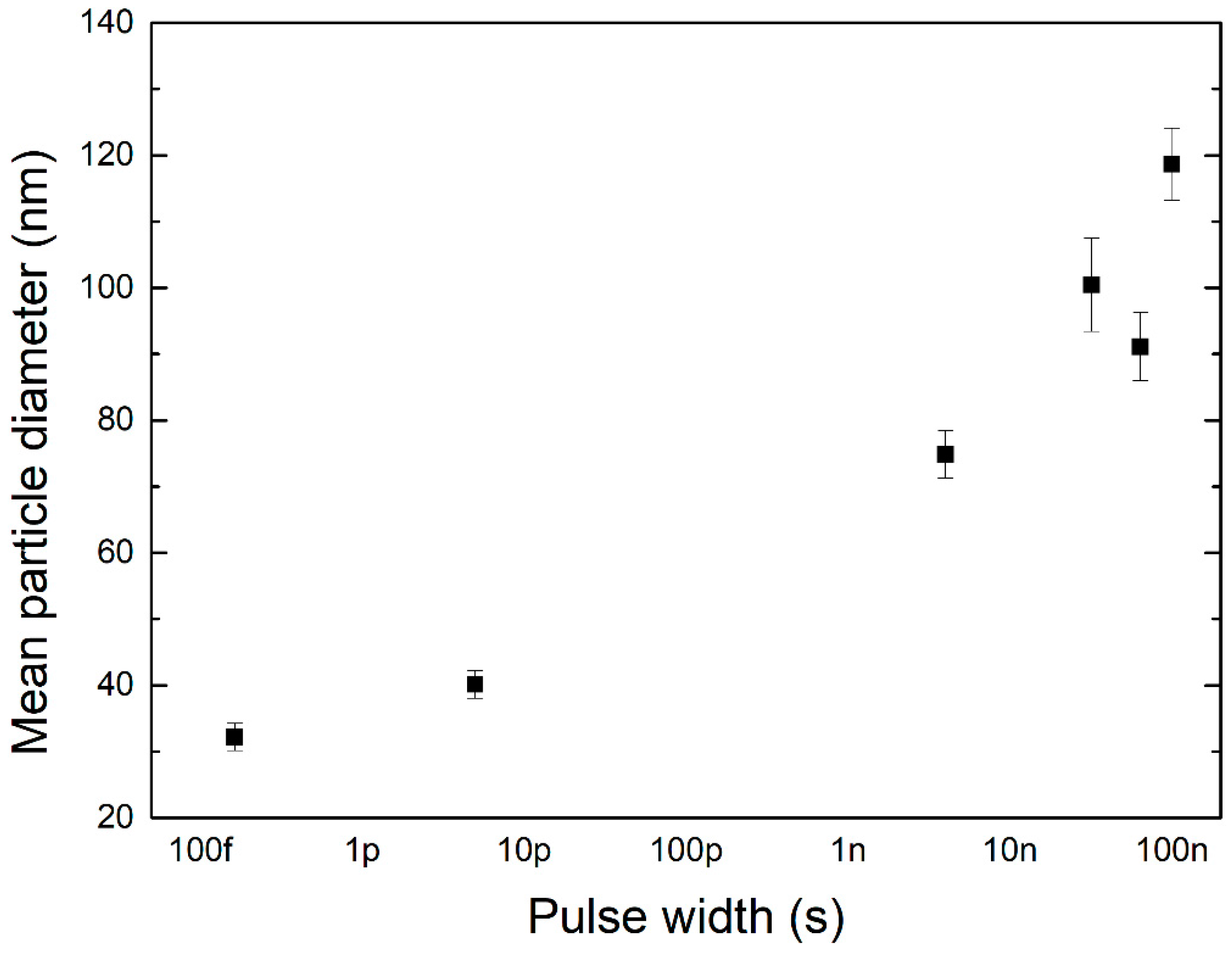
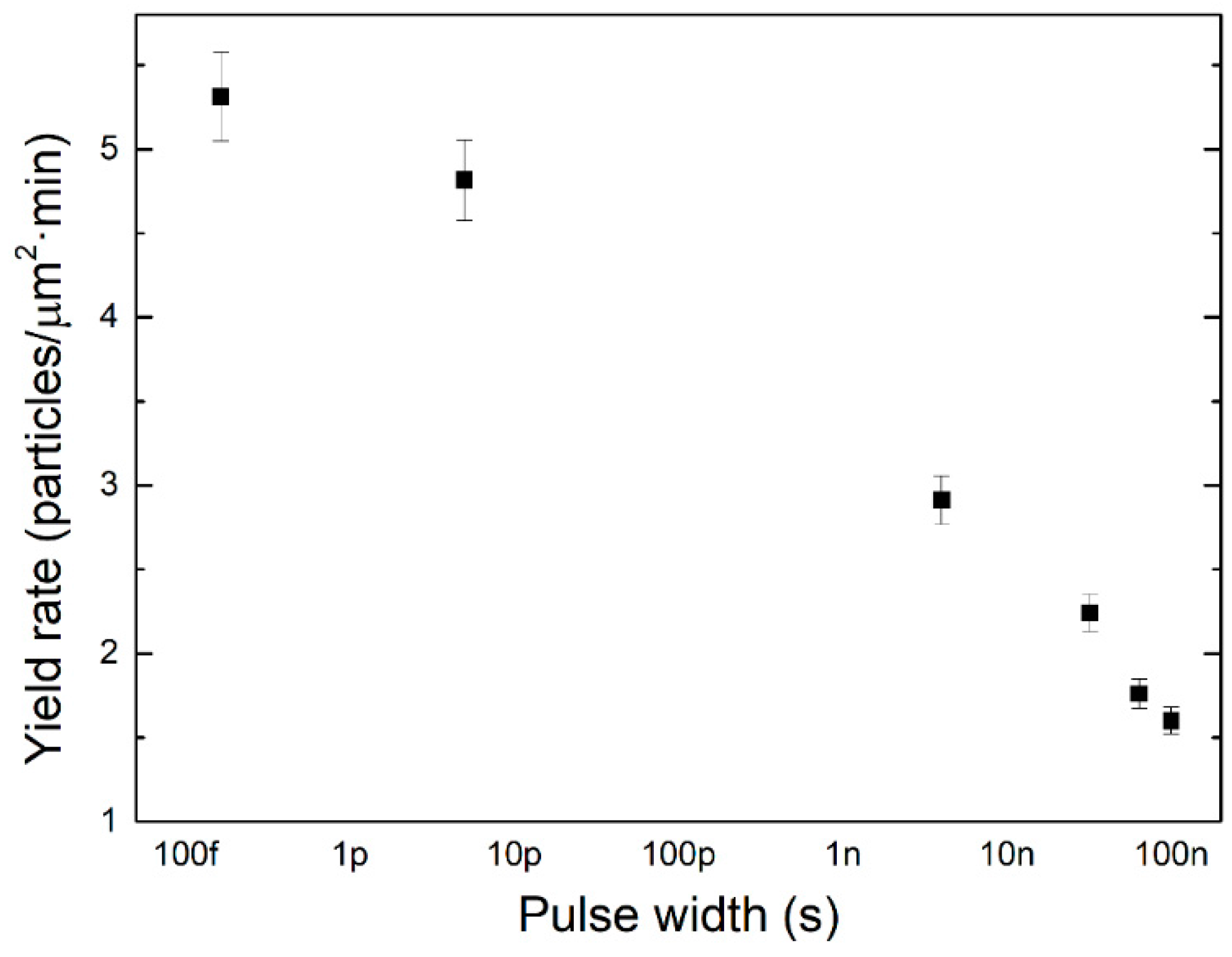
| Material | Wavelength | Pulse Width | Fluence or Pulse Energy | Particle Size | Reference |
|---|---|---|---|---|---|
| Au | 400 and 532 nm | 150 fs | 7.3 and 3.6 mJ/cm2 | ~60 nm | [33] |
| Au | 1025 nm | 450 fs | 10–130 μJ | 7–50 nm | [27] |
| Ag | 355 and 532 nm | 6 ns and 18 ps | 10 and 2–3 mJ | 5–60 nm | [28] |
| Au | 355 nm | 30 ps | 6.3 and 17 mJ/cm2 | NA | [32] |
| Au/Ag | 532 and 1064 nm | ~5 ns | 35 mJ | ~10 nm or less | [20] |
| Ag | 355 and 1064 nm | 7 and 5 ns | NA | ~26 nm | [34] |
| Au–Ag alloy | 355 and 532 nm | 6 ns | 150 and 130 mJ | ~10 nm | [35] |
| Pulse Width | Femtosecond Regime | Picosecond Regime | Nanosecond Regime |
|---|---|---|---|
| Fluence (mJ/cm2) | 0.13 | 0.13 | 0.10 |
| Repetition rate (kHz) | 200 | 200 | 500 |
| Delay time (μs) | 5 | 5 | 2 |
| Pulse duration | 164 fs | 5 ps | 4, 32, 64, and 100 ns |
| Wavelength (nm) | 1070 | 1070 | 1070 |
| Process time (min) | 10 | 10 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, J.-W.; Yoon, S.; Choi, H.W.; Kim, J.; Farson, D.; Cho, S.-H. The Effect of Laser Pulse Widths on Laser—Ag Nanoparticle Interaction: Femto- to Nanosecond Lasers. Appl. Sci. 2018, 8, 112. https://doi.org/10.3390/app8010112
Jeon J-W, Yoon S, Choi HW, Kim J, Farson D, Cho S-H. The Effect of Laser Pulse Widths on Laser—Ag Nanoparticle Interaction: Femto- to Nanosecond Lasers. Applied Sciences. 2018; 8(1):112. https://doi.org/10.3390/app8010112
Chicago/Turabian StyleJeon, Jin-Woo, Sangwoo Yoon, Hae Woon Choi, Joohan Kim, Dave Farson, and Sung-Hak Cho. 2018. "The Effect of Laser Pulse Widths on Laser—Ag Nanoparticle Interaction: Femto- to Nanosecond Lasers" Applied Sciences 8, no. 1: 112. https://doi.org/10.3390/app8010112







