Inorganic Salt Hydrate for Thermal Energy Storage
Abstract
:1. Introduction
2. Properties of Inorganic Salt Hydrated PCMs
3. Supercooling of Salt Hydrates
4. Phase Separation of Salt Hydrates
5. Application
5.1. Salt Hydrates for Hot Water Tanks
5.2. Salt Hydrates for Wallboards
5.3. Salt Hydrates for Refrigeration System
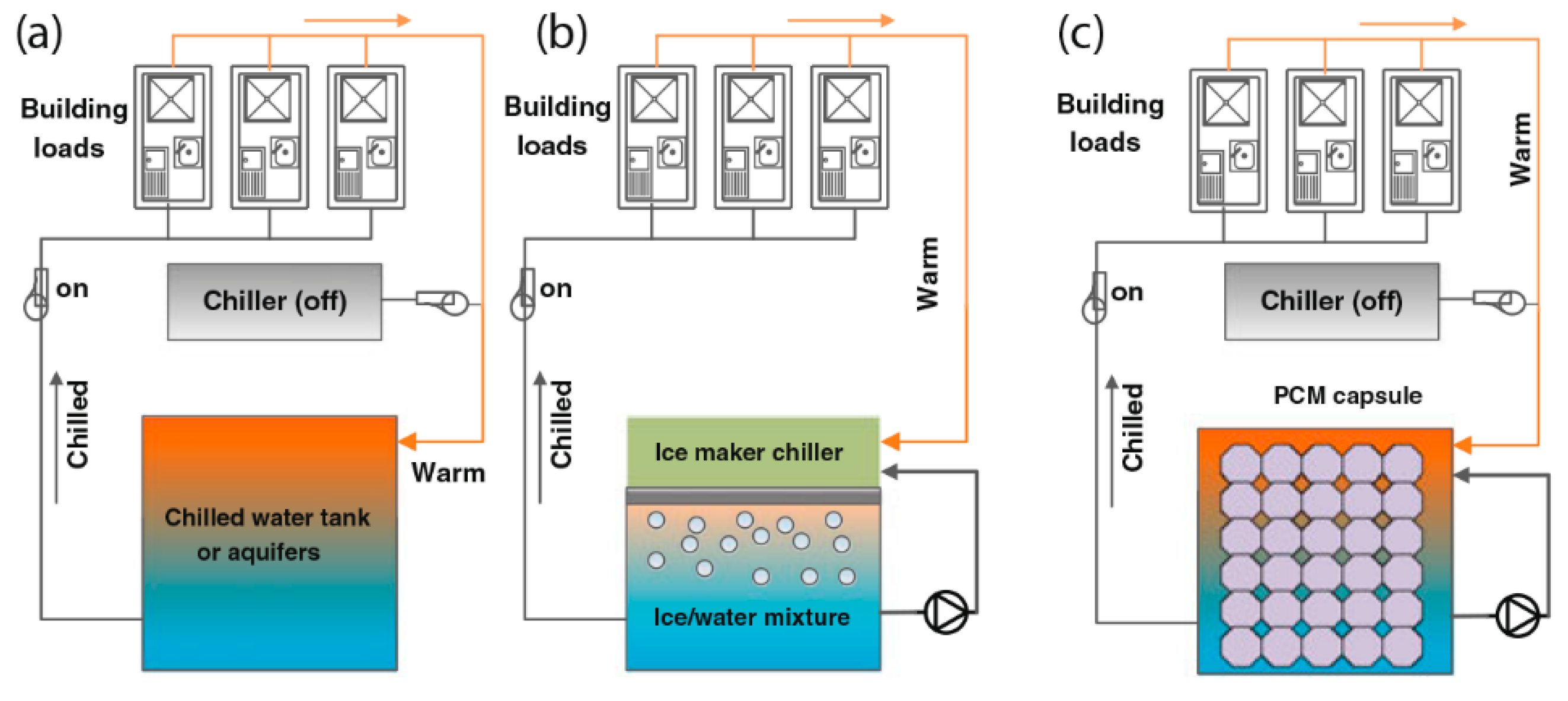
5.4. Salt Hydrates for Air Conditioning System
- (1)
- Transfer the electricity peak and time of power consumption
- (2)
- Capacity and power of refrigeration equipment can be reduced by 30~50%
- (3)
- Improve efficiency of operation and utilization
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cabeza, L.F.; Castell, A.; Barreneche, C.D.; De Gracia, A.; Fernández, A. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Li, T.X.; Wu, D.L.; He, F.; Wang, R.Z. Experimental investigation on copper foam/hydrated salt composite phase change material for thermal energy storage. Int. J. Heat Mass Transf. 2017, 115, 148–157. [Google Scholar] [CrossRef]
- Da Cunha, J.P.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Castellani, B.; Morini, E.; Filipponi, M.; Nicolini, A.; Palombo, M.; Cotana, F.; Rossi, F. Clathrate hydrates for thermal energy storage in buildings: Overview of proper hydrate-forming compounds. Sustainability 2014, 6, 6815–6829. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Salt hydrates as latent heat storage materials: Thermophysical properties and costs. Sol. Energy Mater. Sol. Cells 2016, 145, 255–286. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, X.; Ma, Z. A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl. Energy 2016, 165, 472–510. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change nanomaterials for thermal energy storage. In Nanotechnology for Energy Sustainability; Wiley: Hoboken, NJ, USA, 2017; pp. 459–484. [Google Scholar]
- Wang, T.; Wang, S.; Luo, R.; Zhu, C.; Akiyama, T.; Zhang, Z. Microencapsulation of phase change materials with binary cores and calcium carbonate shell for thermal energy storage. Appl. Energy 2016, 171, 113–119. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martínez, M.; Cabeza, L.F.; Fernández, A.I. Types, methods, techniques, and applications for microencapsulated phase change materials (MPCM): A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Aguayo, M.; Das, S.; Maroli, A.; Kabay, N.; Mertens, J.C.; Rajan, S.D.; Sant, G.; Chawla, N.; Neithalath, N. The influence of microencapsulated phase change material (PCM) characteristics on the microstructure and strength of cementitious composites: Experiments and finite element simulations. Cem. Concr. Compos. 2016, 73, 29–41. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Hussain, S.I.; Dinesh, R. Roseline, A. Enhanced thermal performance and study the influence of sub cooling on activated carbon dispersed eutectic PCM for cold storage applications. Energy Build. 2017, 143, 17–24. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, Z.; Ghafoor, A. A review of performance enhancement of PCM based latent heat storage system within the context of materials, thermal stability and compatibility. Energy Convers. Manag. 2016, 115, 132–158. [Google Scholar] [CrossRef]
- Lorente, S.; Bejan, A.; Niu, J.L. Construal design of latent thermal energy storage with vertical spiral heaters. Int. J. Heat Mass Transf. 2015, 81, 283–288. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Li, X.; Zhou, Y.; Sun, Q.; Yun, Q. The preparation, characterization and modification of a new phase change material: CaCl2·6H2O–MgCl2·6H2O eutectic hydrate salt. Sol. Energy Mater. Sol. Cells 2014, 126, 51–55. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Preparation and thermal properties of Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrate salt as a novel phase change material for energy storage. Appl. Therm. Eng. 2017, 112, 606–609. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Use of nano-α-Al2O3 to improve binary eutectic hydrated salt as phase change material. Sol. Energy Mater. Sol. Cells 2017, 160, 18–25. [Google Scholar] [CrossRef]
- Zalba, B.; Marı́n, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Yinping, Z.; Yi, J. A simple method, the T-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201–205. [Google Scholar] [CrossRef]
- Safari, A.; Saidur, R.; Sulaiman, F.; Xu, Y.; Dong, J. A review on supercooling of phase change materials in thermal energy storage systems. Renew. Sustain. Energy Rev. 2016, 70, 905–919. [Google Scholar] [CrossRef]
- Feng, G.; Xu, X.; He, N.; Li, H.; Huang, K. Testing research of energy storage system during Na2SO4·10H2O phase change. Mater. Res. Innov. 2015, 19, 972–977. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Sun, R.; Lai, M.; Du, R.; Zhang, Z. In the anti-supercooling effect of surface-modified nano-scaled SiO2 in hydrated salts phase transition system. J. Phys. Conf. Ser. 2009, 188. [Google Scholar] [CrossRef]
- Sutjahja, I.M.; Rahayu, S.; Kurniati, N.; Pallitine, I.D.; Kurnia, D. The role of chemical additives to the phase change process of CaCl2·6H2O to optimize its performance as latent heat energy storage system. J. Phys. Conf. Ser. 2016, 739. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Nian, H.; Ren, X.; Dong, O.; Hai, C.; Shen, Y.; Zeng, J. Phase change behavior of latent heat storage media based on calcium chloride hexahydrate composites containing strontium chloride hexahydrate and oxidation expandable graphite. Appl. Therm. Eng. 2016, 102, 38–44. [Google Scholar] [CrossRef]
- Palittin, I.D.; Kurniati, N.; Sutjahja, I.; Kurnia, D. Sonocrystallization technique to optimizing the crystallization process of PCM CaCl2·6H2O. Adv. Mater. Res. 2015, 1112, 559–562. [Google Scholar] [CrossRef]
- Miyasaka, E.; Takai, M.; Hidaka, H.; Kakimoto, Y.; Hirasawa, I. Effect of ultrasonic irradiation on nucleation phenomena in a Na2HPO4·12H2O melt being used as a heat storage material. Ultrason. Sonochem. 2006, 13, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Nian, H.; Zhang, X.; Dong, O.; Ren, X.; Zeng, J.; Hai, C.; Shen, Y. Advanced nanocomposite phase change material based on calcium chloride hexahydrate with aluminum oxide nanoparticles for thermal energy storage. Energy Fuels 2017, 31, 6560–6567. [Google Scholar] [CrossRef]
- Mao, J.; Hou, P.; Liu, R.; Chen, F.; Dong, X. Preparation and thermal properties of SAT-CMC-DSP/EG composite as phase change material. Appl. Therm. Eng. 2017, 119, 585–592. [Google Scholar] [CrossRef]
- Mao, J.; Li, J.; Peng, G.; Li, J. A selection and optimization experimental study of additives to thermal energy storage material sodium acetate trihydrate. In Proceedings of the International Conference on Energy and Environment Technology, Guilin, China, 16–18 October 2009. [Google Scholar]
- Mao, J.; Dong, X.; Hou, P.; Lian, H. Preparation research of novel composite phase change materials based on sodium acetate trihydrate. Appl. Therm. Eng. 2017, 118, 817–825. [Google Scholar] [CrossRef]
- Ramirez, B.M.L.G.; Glorieux, C.; Martinez, E.S.M.; Cuautle, J.J.A.F. Tuning of thermal properties of sodium acetate trihydrate by blending with polymer and silver nanoparticles. Appl. Therm. Eng. 2014, 62, 838–844. [Google Scholar] [CrossRef]
- Choi, J.C.; Sang, D.K. Heat-transfer characteristics of a latent heat storage system using MgCl2·6H2O. Energy 1992, 17, 1153–1164. [Google Scholar] [CrossRef]
- Pilar, R.; Svoboda, L.; Honcova, P.; Oravova, L. Study of magnesium chloride hexahydrate as heat storage material. Thermochim. Acta 2012, 546, 81–86. [Google Scholar] [CrossRef]
- Ushak, S.; Gutierrez, A.; Barreneche, C.; Fernandez, A.I.; Grágeda, M.; Cabeza, L.F. Reduction of the subcooling of bischofite with the use of nucleatings agents. Sol. Energy Mater. Sol. Cells 2016, 157, 1011–1018. [Google Scholar] [CrossRef]
- Sun, D.; Sung, W.P.; Chen, R. Studies on magnesium chloride hexahydrate as phase change materials. Appl. Mech. Mater. 2011, 71, 2598–2601. [Google Scholar]
- Honcova, P.; Pilar, R.; Danielik, V.; Soska, P.; Sadovska, G.; Honc, D. Suppressing supercooling in magnesium nitrate hexahydrate and evaluating corrosion of aluminium alloy container for latent heat storage application. J. Therm. Anal. Calorim. 2017, 129, 1573–1581. [Google Scholar] [CrossRef]
- Ushak, S.; Suárez, M.; Véliz, S.; Fernández, A.G.; Flores, E.; Galleguillos, H.R. Characterization of calcium chloride tetrahydrate as a phase change material and thermodynamic analysis of the results. Renew. Energy 2016, 95, 213–224. [Google Scholar] [CrossRef]
- Tang, Y.R.; Gao, D.L.; Guo, Y.F.; Wang, S.Q.; Deng, T.L. In Supercooling and phase separation of inorganic salt hydrates as PCMs. Appl. Mech. Mater. 2011, 71, 2602–2605. [Google Scholar] [CrossRef]
- Dannemand, M.; Johansen, J.B.; Furbo, S. Solidification behavior and thermal conductivity of bulk sodium acetate trihydrate composites with thickening agents and graphite. Sol. Energy Mater. Sol. Cells 2016, 145, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.K.; Park, M.; Kim, H.-Y.; Park, S.-J. Thermal property and latent heat energy storage behavior of sodium acetate trihydrate composites containing expanded graphite and carboxymethyl cellulose for phase change materials. Appl. Therm. Eng. 2015, 75, 978–983. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Chen, P.; Rojas, R.; Hajian, A.; Berglund, L. Cellulose nanofibers enable paraffin encapsulation and the formation of stable thermal regulation nanocomposites. Nano Energy 2017, 34, 541–548. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Z.; Yu, X.; Li, B. Preparation and thermal energy storage of carboxymethyl cellulose-modified nanocapsules. BioEnergy Res. 2013, 6, 1135–1141. [Google Scholar] [CrossRef]
- Jin, X.; Medina, M.A.; Zhang, X.; Zhang, S. Phase-change characteristic analysis of partially melted sodium acetate trihydrate using DSC. Int. J. Thermophys. 2014, 35, 45–52. [Google Scholar] [CrossRef]
- Ryu, H.W.; Woo, S.W.; Shin, D.K. Prevention of supercooling and stabilization of inorganic salt hydrates as latent heat storage materials. Sol. Energy Mater. Sol. Cells 1992, 27, 161–172. [Google Scholar]
- Gutierrez, A.; Ushak, S.; Galleguillos, H.; Fernandez, A.; Cabeza, L.F.; Grágeda, M. Use of polyethylene glycol for the improvement of the cycling stability of bischofite as thermal energy storage material. Appl. Energy 2015, 154, 616–621. [Google Scholar] [CrossRef]
- Kazemi, Z.; Mortazavi, S.M. A new method of application of hydrated salts on textiles to achieve thermoregulating properties. Thermochim. Acta 2014, 589, 56–62. [Google Scholar] [CrossRef]
- Duan, Z.-J.; Zhang, H.-Z.; Sun, L.-X.; Cao, Z.; Xu, F.; Zou, Y.-J.; Chu, H.-L.; Qiu, S.-J.; Xiang, C.-L.; Zhou, H.-Y. CaCl2·6H2O/expanded graphite composite as form-stable phase change materials for thermal energy storage. J. Therm. Anal. Calorim. 2013, 115, 111–117. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, D.; Lv, H.; Zhang, Y.; Wu, F.; Shen, D.; Fu, P. Mixed mill-heating fabrication and thermal energy storage of diatomite/paraffin phase change composite incorporated gypsum-based materials. Appl. Therm. Eng. 2017, 118, 703–713. [Google Scholar] [CrossRef]
- Wi, S.; Jeong, S.-G.; Chang, S.J.; Lee, J.; Kim, S. Energy-efficient heat storage using gypsum board with fatty acid ester as layered phase change material. Energy Technol. 2017, 5, 1392–1398. [Google Scholar] [CrossRef]
- Lasfargues, M.; Bell, A.; Ding, Y. In Situ production of titanium dioxide nanoparticles in molten salt phase for thermal energy storage and heat-transfer fluid applications. J. Nanopart. Res. 2016, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Deng, Y.; Guan, W.; Wang, X.; Qian, T. Preparation of paraffin/porous TiO2 foams with enhanced thermal conductivity as PCM, by covering the TiO2 surface with a carbon layer. Appl. Energy 2016, 171, 37–45. [Google Scholar] [CrossRef]
- Tiagi, V.; Kaushik, S.C. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar]
- Liu, F.; Wang, J.; Qian, X. Integrating phase change materials into concrete through microencapsulation using cenospheres. Cem. Concr. Compos. 2017, 80, 317–325. [Google Scholar] [CrossRef]
- Ram, M.K.; Myers, P.D.; Jotshi, C.; Goswami, D.Y.; Stefanakos, E.K.; Arvanitis, K.D.; Papanicolaou, E.; Belessiotis, V. Microencapsulated dimethyl terephthalate phase change material for heat transfer fluid performance enhancement. Int. J. Energy Res. 2017, 41, 252–262. [Google Scholar] [CrossRef]
- Streufert, J.R. Encapsulation of Shock-Sensitive Materials and Their Implementation into Matrices. Master’s Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 30 April 2015. [Google Scholar]
- Huang, J.; Wang, T.; Zhu, P.; Xiao, J. Preparation, characterization, and thermal properties of the microencapsulation of a hydrated salt as phase change energy storage materials. Thermochim. Acta 2013, 557, 1–6. [Google Scholar] [CrossRef]
- Korhammer, K.; Druske, M.-M.; Fopah-Lele, A.; Rammelberg, H.U.; Wegscheider, N.; Opel, O.; Osterland, T.; Ruck, W. Sorption and thermal characterization of composite materials based on chlorides for thermal energy storage. Appl. Energy 2016, 162, 1462–1472. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, Z.; Gao, X.; Fang, X.; Fang, Y.; Zhang, Z. Investigations on the thermal stability, long-term reliability of LiNO3/KCl—Expanded graphite composite as industrial waste heat storage material and its corrosion properties with metals. Appl. Energy 2017, 188, 521–528. [Google Scholar] [CrossRef]
- Cheng, F.; Wen, R.; Huang, Z.; Fang, M.; Liu, Y.G.; Wu, X.; Min, X. Preparation and analysis of lightweight wall material with expanded graphite (EG)/paraffin composites for solar energy storage. Appl. Therm. Eng. 2017, 120, 107–114. [Google Scholar] [CrossRef]
- Xu, T.; Li, Y.; Chen, J.; Liu, J. Preparation and thermal energy storage properties of lino 3-kcl-nano 3/expanded graphite composite phase change material. Sol. Energy Mater. Sol. Cells 2017, 169, 215–221. [Google Scholar] [CrossRef]
- Huang, X.; Alva, G.; Liu, L.; Fang, G. Preparation, characterization and thermal properties of fatty acid eutectics/bentonite/expanded graphite composites as novel form–stable thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 166, 157–166. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. Hydrated salts/expanded graphite composite with high thermal conductivity as a shape-stabilized phase change material for thermal energy storage. Energy Convers. Manag. 2015, 101, 164–171. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Li, S. Graphene oxide modified hydrate salt hydrogels: Form-stable phase change materials for smart thermal management. J. Mater. Chem. A 2016, 4, 18134–18143. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, H.; Xia, Y.; Zou, Y.; Xiang, C.; Chu, H.; Qiu, S.; Xu, F.; Sun, L. Preparation and thermophysical properties of a novel form-stable CaCl2·6H2O/sepiolite composite phase change material for latent heat storage. J. Therm. Anal. Calorim. 2017, 20, 1–7. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. Preparation and characterization of hydrated salts/silica composite as shape-stabilized phase change material via sol–gel process. Thermochim. Acta 2014, 591, 10–15. [Google Scholar] [CrossRef]
- Solé, A.; Miró, L.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Corrosion of metals and salt hydrates used for thermochemical energy storage. Renew. Energy 2015, 75, 519–523. [Google Scholar] [CrossRef]
- Padovan, R.; Manzan, M. Genetic optimization of a PCM enhanced storage tank for Solar Domestic Hot Water Systems. Sol. Energy 2014, 103, 563–573. [Google Scholar]
- Kara, Y.A. Diurnal performance analysis of phase change material walls. Appl. Therm. Eng. 2016, 102, 1–8. [Google Scholar] [CrossRef]
- Kleiner, F.; Posern, K.; Osburg, A. Thermal conductivity of selected salt hydrates for thermochemical solar heat storage applications measured by the light flash method. Appl. Therm. Eng. 2017, 113, 1189–1193. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, J.M.; Nsofor, E.C. Solidification enhancement of PCM in a triplex-tube thermal energy storage system with nanoparticles and fins. Appl. Energy 2018, 211, 975–986. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Ibáñez, M.; Solé, C.; Roca, J.; Nogués, M. Experimentation with a water tank including a PCM module. Sol. Energy Mater. Sol. Cells 2006, 90, 1273–1282. [Google Scholar] [CrossRef]
- Porteiro, J.; Míguez, J.L.; Crespo, B.; de Lara, J.; Pousada, J.M. On the behavior of different PCMs in a hot water storage tank against thermal demands. Materials 2016, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Akeiber, H.; Nejat, P.; Majid, M.Z.A.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R.; Zaki, S.A. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Copiello, S. Building energy efficiency: A research branch made of paradoxes. Renew. Sustain. Energy Rev. 2017, 69, 1064–1076. [Google Scholar] [CrossRef]
- Navarro, L.; de Gracia, A.; Niall, D.; Castell, A.; Browne, M.; McCormack, S.J.; Griffiths, P.; Cabeza, L.F. Thermal energy storage in building integrated thermal systems: A review. Part 2. Integration as passive system. Renew. Energy 2016, 85, 1334–1356. [Google Scholar] [CrossRef]
- Brancato, V.; Frazzica, A.; Sapienza, A.; Freni, A. Identification and characterization of promising phase change materials for solar cooling applications. Sol. Energy Mater. Sol. Cells 2017, 160, 225–232. [Google Scholar] [CrossRef]
- Leang, E.; Tittelein, P.; Zalewski, L.; Lassue, S. Numerical study of a composite Trombe solar wall integrating microencapsulated PCM. Energy Procedia 2017, 122, 1009–1014. [Google Scholar] [CrossRef]
- Hadjieva, M.; Stoykov, R.; Filipova, T. Composite salt-hydrate concrete system for building energy storage. Renew. Energy 2000, 19, 111–115. [Google Scholar] [CrossRef]
- Ye, R.; Lin, W.; Yuan, K.; Fang, X.; Zhang, Z. Experimental and numerical investigations on the thermal performance of building plane containing CaCl2·6H2O/expanded graphite composite phase change material. Appl. Energy 2017, 193, 325–335. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Ye, R.; Fang, X.; Zhang, Z. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation. Renew. Energy 2017, 114, 733–743. [Google Scholar] [CrossRef]
- Shportko, K.; Kremers, S.; Woda, M.; Lencer, D.; Robertson, J.; Wuttig, M. Resonant bonding in crystalline phase-change materials. Nat. Mater. 2008, 7, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Laing, D.; Tamme, R. Characterization of sodium nitrate as phase change material. Int. J. Thermophys. 2012, 33, 91–104. [Google Scholar] [CrossRef]
- Gin, B.; Farid, M.M. The use of PCM panels to improve storage condition of frozen food. J. Food Eng. 2010, 100, 372–376. [Google Scholar] [CrossRef]
- Oró, E.; Miró, L.; Farid, M.M.; Cabeza, L.F. Improving thermal performance of freezers using phase change materials. Int. J. Refrig. 2012, 35, 984–991. [Google Scholar]
- Mo, S.; Chen, Y.; Jia, L.; Luo, X. Reduction of supercooling of water by TiO2 nanoparticles as observed using differential scanning calorimeter. J. Exp. Nanosci. 2013, 8, 533–539. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hwang, K.S.; Jang, S.P.; Lee, B.H.; Kim, J.H.; Choi, S.U.; Choi, C.J. Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int. J. Heat Mass Transf. 2008, 51, 2651–2656. [Google Scholar] [CrossRef]
- Mehling, H.; Cabeza, L.F. Heat and Cold Storage with PCM; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1107–1111. [Google Scholar]
- Altohamy, A.A.; Abd Rabbo, M.F.; Sakr, R.Y.; Attia, A.A.A. Effect of water based Al2O3 nanoparticle PCM on cool storage performance. Appl. Therm. Eng. 2015, 84, 331–338. [Google Scholar] [CrossRef]
- Hui, L.; Edem, N.K.; Nolwenn, L.P. Evaluation of a seasonal storage system of solar energy for house heating using different absorption couples. Energy Convers. Manag. 2011, 52, 2427–2436. [Google Scholar] [CrossRef]




| Salt Hydrates | Melting Temperature (°C) | Heat of Fusion (J/g) | Density (Solid) (103 kg/m3) | Thermal Conductivity (Solid) (W/mK) | Specific Heat (Solid) (J/g·°C) | Reference |
|---|---|---|---|---|---|---|
| LiClO3·3H2O | 8 | 253 | [13] | |||
| KF·4H2O | 19 | 231 | 1.45 | 1.84 | [5,11] | |
| Mn(NO3)2·6H2O | 25.8 | 125.9 | 1.60 | [13] | ||
| CaCl2·6H2O | 28 | 174 | 1.80 | 1.088 | 1.42 | [1,13] |
| LiNO3·3Н2О | 30 | 256 | [13] | |||
| Na2SO4·10H2O | 32.4 | 248 | 1.49 | [14] | ||
| Na2CO3·10H2O | 33 | 247 | 1.88 | [14] | ||
| CaBr2·4H2O | 34 | 115.5 | 1.52 | [19] | ||
| LiBr2·2H2O | 34 | 124 | [19] | |||
| Na2HPO4·12H2O | 35–44 | 280 | 0.514 | 1.7 | [5,11] | |
| Zn(NO3)2·6H2O | 36 | 149.6 | 1.94 | 1.34 | [19] | |
| KF·2H2O | 42 | 162 | [14,19] | |||
| MgI2·2H2O | 42 | 133 | [5,11] | |||
| Ca(NO3)2·4H2O | 42.4 | 1.46 | [14] | |||
| Fe(NO3)2·9H2O | 47 | 155 | [14] | |||
| Na2SiO3·4H2O | 48 | 168 | [14] | |||
| K2HPO4·7H2O | 48 | 99 | [14] | |||
| MgSO4·7H2O | 48.5 | 202 | [1] | |||
| Na2S2O3·5H2O | 49 | 220 | 1.75 | 1.46 | [1] | |
| Ca(NO3)2·3H2O | 51 | 104 | 1.46 | [14] | ||
| FeCl3·2H2O | 56 | 90 | [1] | |||
| Ni(NO3)2·6H2O | 57 | 169 | [1] | |||
| CH3COONa·3H2O | 58 | 226–264 | 1.45 | 1.97 | [1,14] | |
| MgCl2·4H2O | 58 | 178 | [14] | |||
| Na3PO4·12H2O | 65–69 | 190 | [14] | |||
| LiCH3COO·2H2O | 70 | 150 | [14] | |||
| Na2P2O7·12H2O | 70 | 184 | [14] | |||
| Ba(OH)2·8H2O | 78 | 266 | [14] | |||
| Al(NO3)2·8H2O | 89 | 1.17 | [1] | |||
| Al(NO3)2·8H2O | 89.3 | 150 | [1] | |||
| Mg(NO3)·6Н2О | 89.9 | 163 | 1.636 | 1.81 | 0.669 | [1] |
| NH4Al(SO4)2·12H2O | 95 | 269 | 1.65 | 1.71 | [1] | |
| Al2(SO3)2·12H2O | 112 | [1] | ||||
| MgCl2·6Н2О | 117 | 167 | 1.56 | 1.59 | [14] |
| Eutectic Mixtures | Melting Temperature (°C) | Heat of Fusion (J/g) | Reference |
|---|---|---|---|
| 55% CaCl2·6H2O + 45% CaBr2·6H2O | 14.7 | 140 | [1] |
| 75% CaCl2·6H2O + 25% MgCl2·6H2O | 21.4 | 102.3 | [16] |
| 66.6% CaCl2·6H2O + 33.3% MgCl2·6H2O | 25 | 127 | [1] |
| 40% Na2CO3·10H2O + 60% Na2HPO4·12H2O | 27.3 | 220.2 | [17] |
| 47% Ca(NO3)2·10H2O + 33% Mg(NO3)2·10H2O | 30 | 136 | [1] |
| 25% Na2SO4·10H2O + 75% Na2HPO4·12H2O | 31.2 | 262.3 | [18] |
| 58.7% Mg(NO3)2·6H2O + 41.3% MgCl2·6H2O | 59 | 132.2 | [1,13] |
| 50% Mg(NO3)26H2O + 50% MgCl2·6H2O | 58–59 | 132 | [1] |
| 80% Mg(NO3)2·6H2O + 20% MgCl2·9H2O | 60 | 150 | [1] |
| 53% Mg(NO3)2·6H2O + 47% AL(NO3)2·9H2O | 66 | 168 | [13] |
| 14% LiNO3·3H2O + 86% Mg(NO3)2·6H2O | 72 | 180 | [1] |
| Salt Solution | Melting Temperature (°C) | Heat of Fusion (J/g) | Reference |
|---|---|---|---|
| 30.5% Al(NO3)3/H2O | −30.6 | 131.5 | [88,89] |
| 32.3% NH4F/H2O | −28.1 | 199.1 | [88] |
| 21.5% KF/H2O | −21.6 | 225.2 | [88] |
| 22.4% NaCl/H2O | −21.2 | 222 | [89] |
| 21.5% NH4Cl/H2O | −16 | 289 | [89] |
| 39.7% (NH4)SO4/H2O | −18.5 | 269 | [88] |
| 36.8% K2HPO4/H2O | −13.5 | 189 | [88] |
| 22.1% BaCl2/H2O | −7.7 | 102 | [89] |
| 27.2% ZnSO4/H2O | −6.5 | 208 | [89] |
| 18.63% MgSO4/H2O | −4.8 | 84.96 | [89] |
| 3.9% NaF/H2O | −3.5 | 309.2 | [89] |
| 5.9% Na2CO3/H2O | −2.1 | 281 | [88] |
| 6.49% K2SO4/H2O | −1.55 | 268.8 | [89] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic Salt Hydrate for Thermal Energy Storage. Appl. Sci. 2017, 7, 1317. https://doi.org/10.3390/app7121317
Xie N, Huang Z, Luo Z, Gao X, Fang Y, Zhang Z. Inorganic Salt Hydrate for Thermal Energy Storage. Applied Sciences. 2017; 7(12):1317. https://doi.org/10.3390/app7121317
Chicago/Turabian StyleXie, Ning, Zhaowen Huang, Zigeng Luo, Xuenong Gao, Yutang Fang, and Zhengguo Zhang. 2017. "Inorganic Salt Hydrate for Thermal Energy Storage" Applied Sciences 7, no. 12: 1317. https://doi.org/10.3390/app7121317





