Improving Performance of Cold-Chain Insulated Container with Phase Change Material: An Experimental Investigation
Abstract
:1. Introduction
2. Materials and Methods
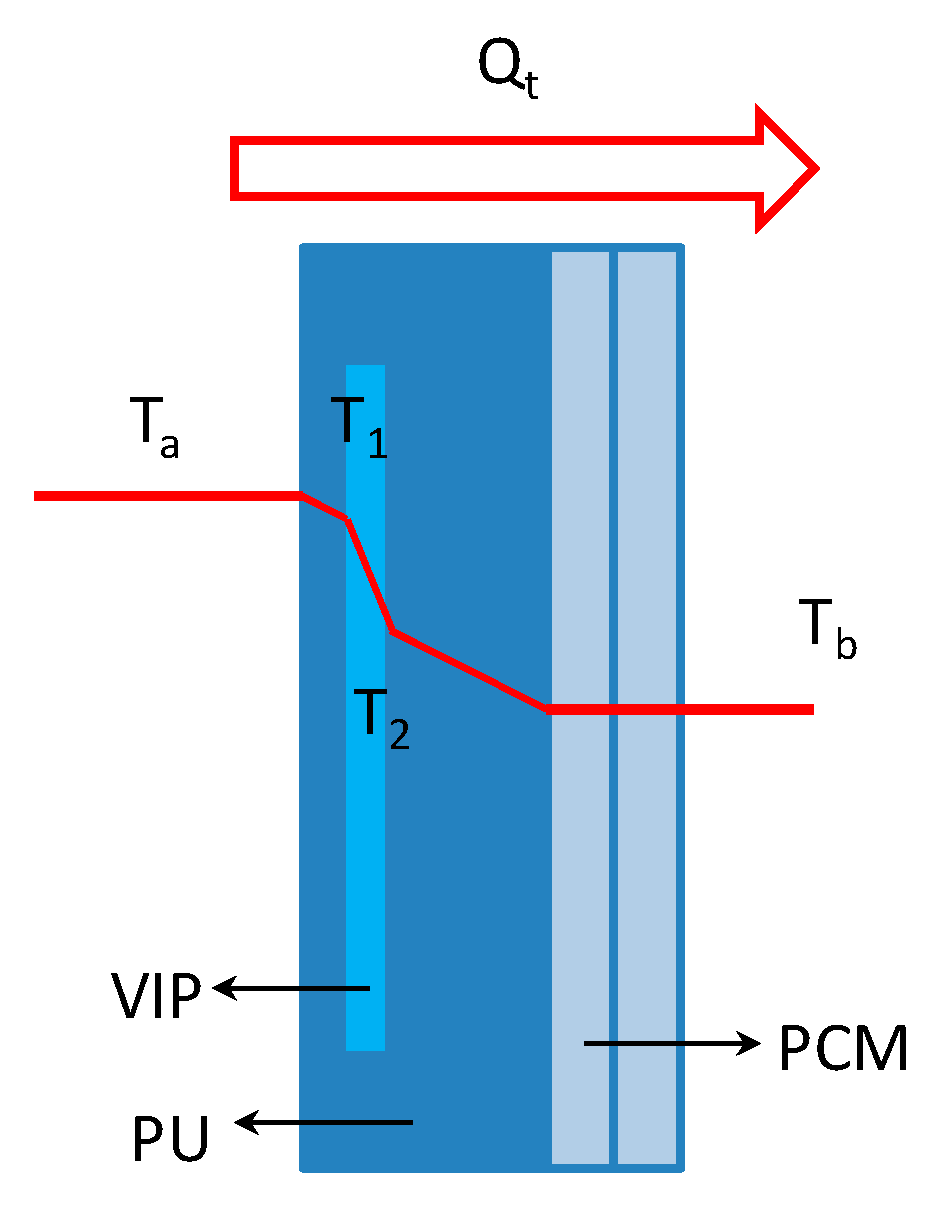
2.1. Configuration of Cold-Chain Insulated Container
2.2. Heat Transfer Calculation
- (1)
- the ambient temperature and the air temperature inside the container distribute homogeneously; and,
- (2)
- the PCM’s temperature keeps constant and distributes homogeneously during the phase transition process.
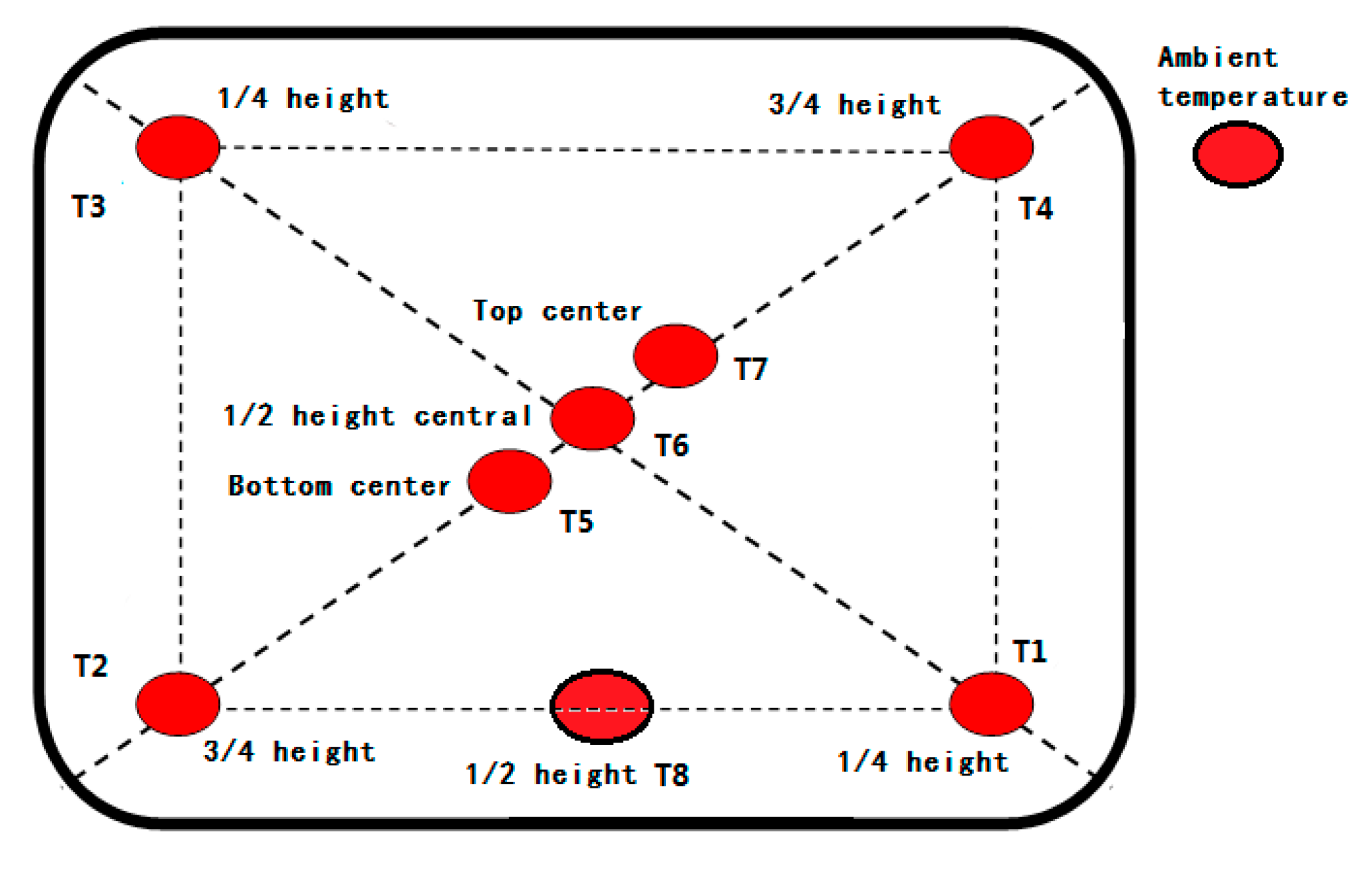
2.3. Experimental Method and Procedure
- (1)
- under extremely high temperature condition, the ambient temperature is set at 35 ± 2 °C;
- (2)
- under extremely low temperature condition, the ambient temperature is set at −20 ± 2 °C; and,
- (3)
- under alternating temperature condition, the ambient temperature is set at 35 ± 2 °C for the first half of the time and −20 ± 2 °C for the second half of the time.
2.3.1. Preconditioning for the Use at Extremely High Temperature
- (1)
- place twelve PCM panels in a −20 °C freezer or below for a minimum of 48 h to ensure PCM was fully frozen;
- (2)
- place all frozen water panels in a 4~5 °C cooler for 60 h until the surface temperature reaches 0 °C, or place all frozen OP5E panels in a 4~5 °C cooler for 60 h until the surface temperature reaches 5 °C;
- (3)
- insert the PCM panels and temperature sensors into the container;
- (4)
- place the container and a temperature sensor into the temperature-controlled chamber; and,
- (5)
- set the chamber temperature at 35 °C and start the test.
2.3.2. Preconditioning for the Use at Extremely Low Temperature
- (1)
- place twelve PCM panels and the container in a 5~6 °C cooler for 8 h;
- (2)
- insert the PCM panels and temperature sensors into the container; and,
- (3)
- place the container and a temperature sensor into the temperature-controlled chamber.
- (4)
- set the chamber temperature at −20 °C and start the test.
2.3.3. Preconditioning for the Use at Alternating Temperature
- (1)
- place six PCM panels in a −20 °C freezer or below for 30 h;
- (2)
- place six PCM panels in a 5~6 °C cooler for 8 h;
- (3)
- insert the six frozen PCM panels into the container near to the drug or food packing and the other six cooled panels near to the insulator base.
- (4)
- insert the temperature sensors into the container; and,
- (5)
- place the container and a temperature sensor into the temperature-controlled chamber.
- (6)
- set the chamber temperature at 35 °C for the first half of the time and then −20 °C for the next half of the time and start the test.
3. Results and Discussion
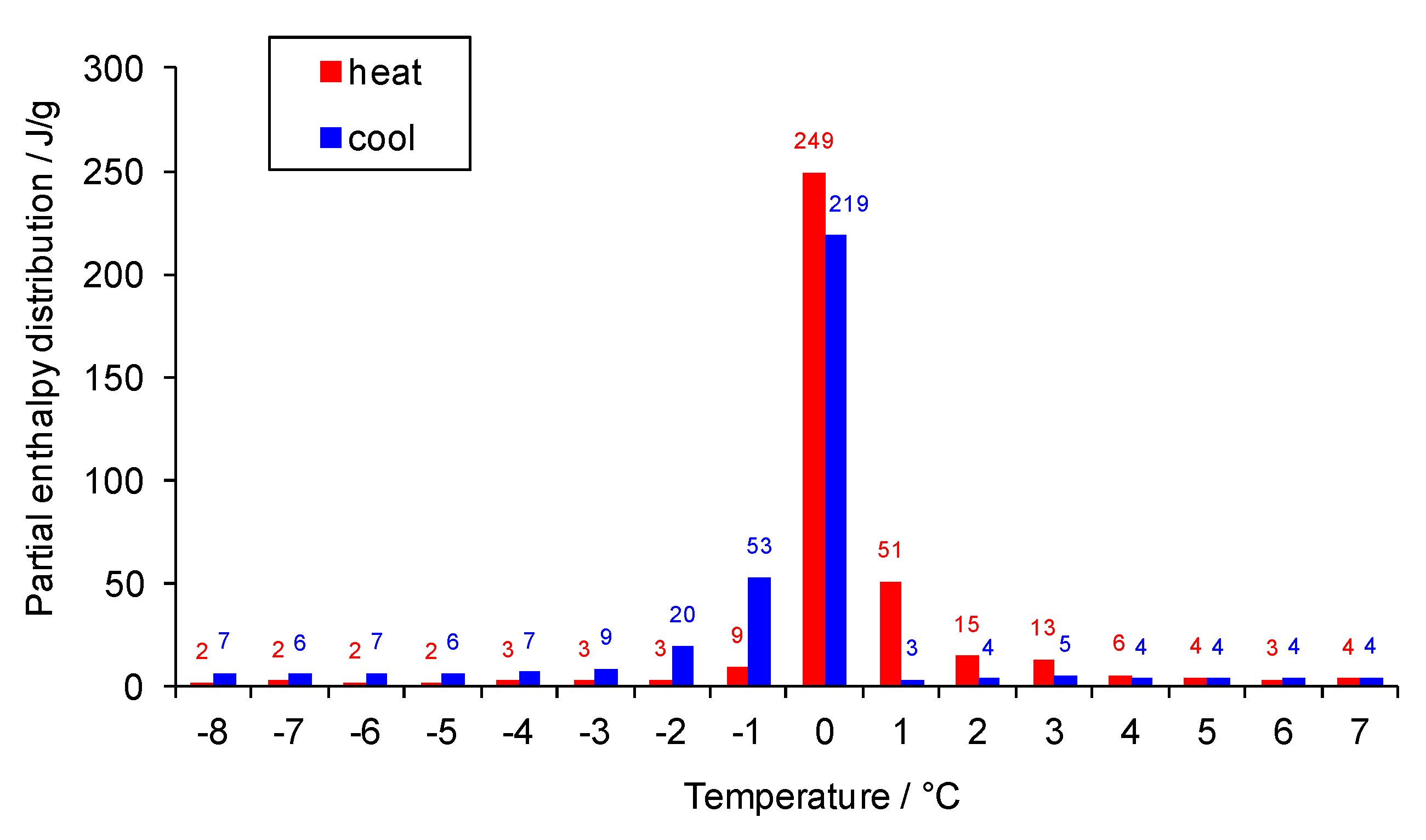
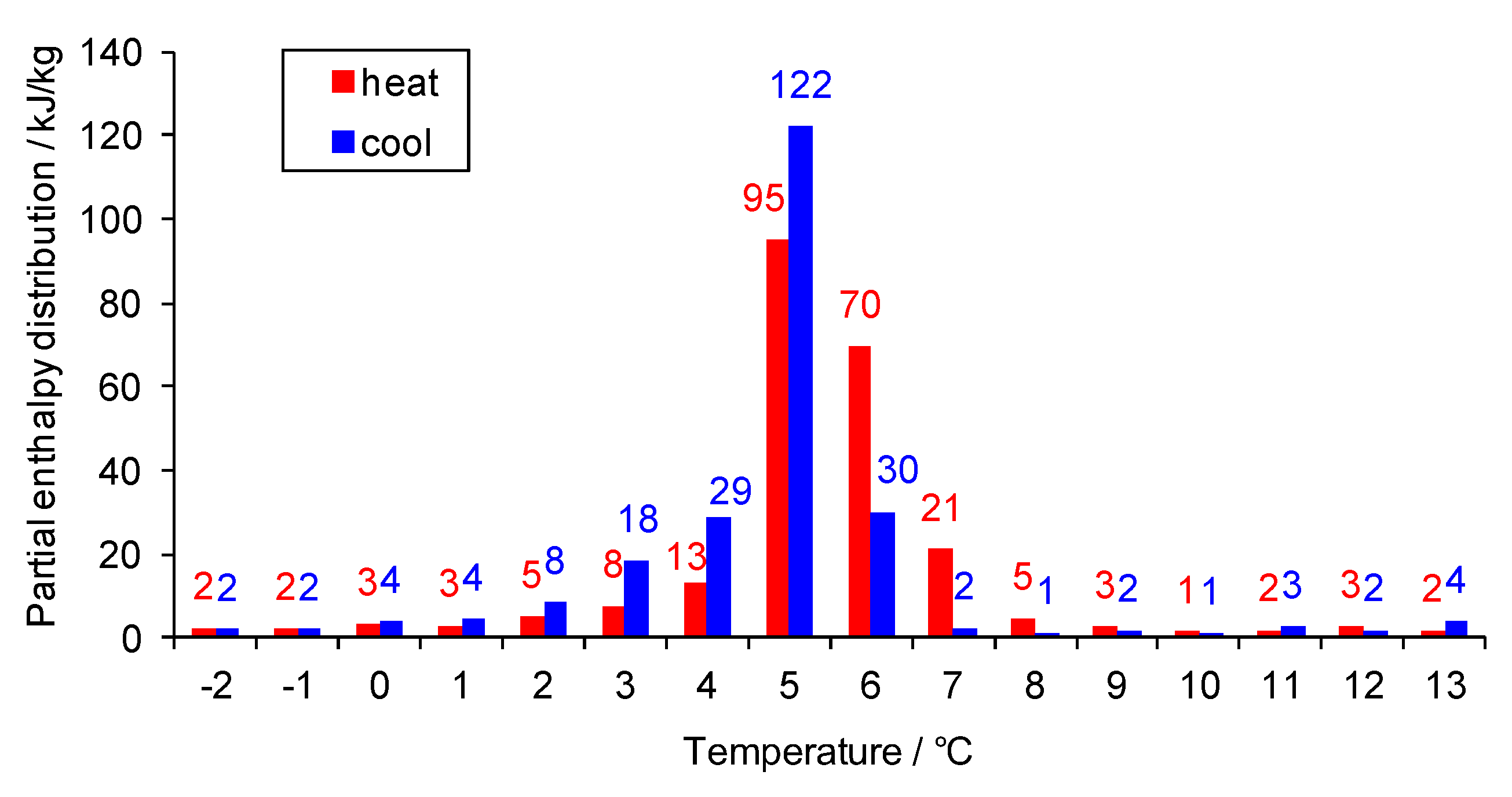
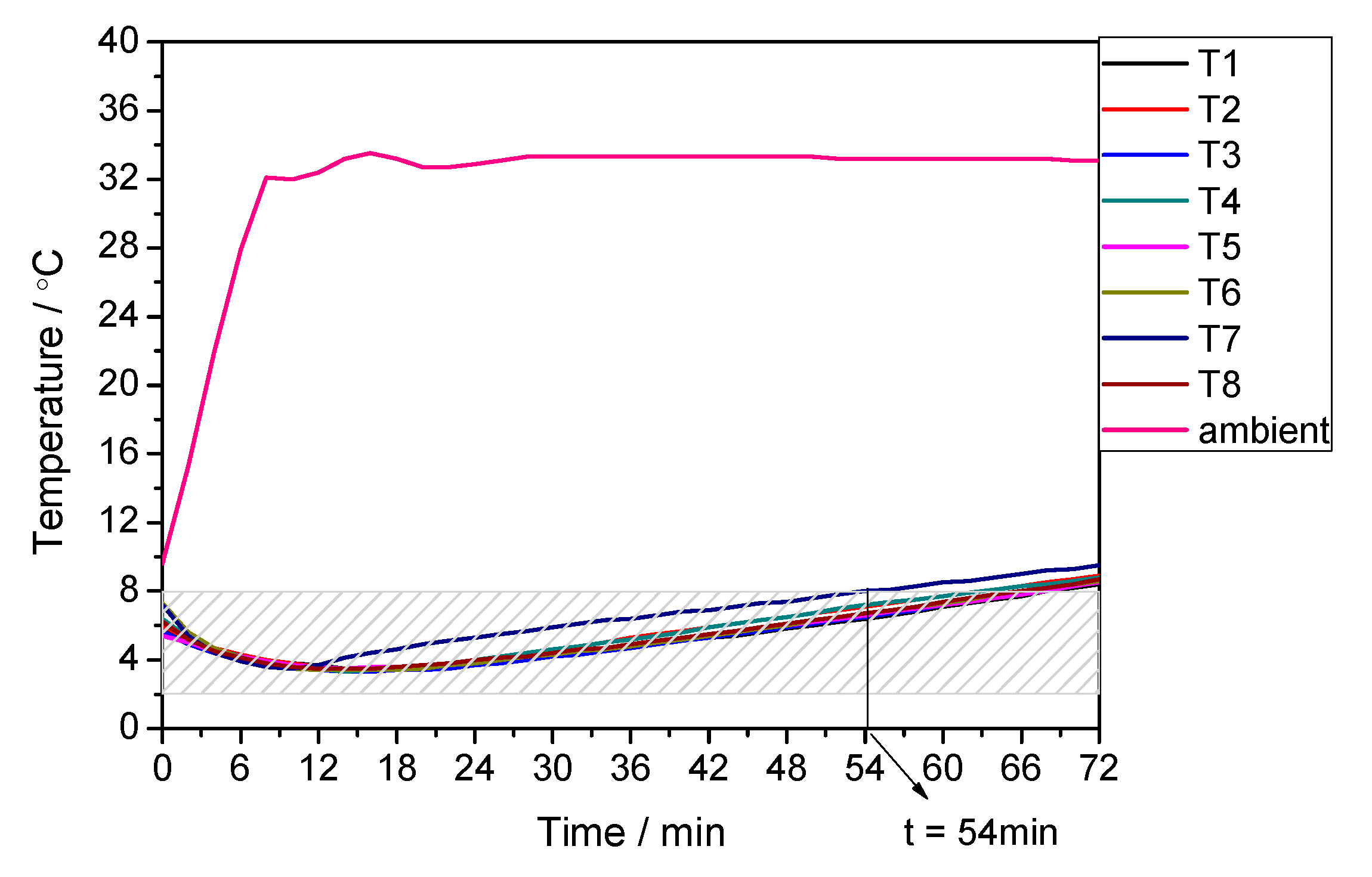
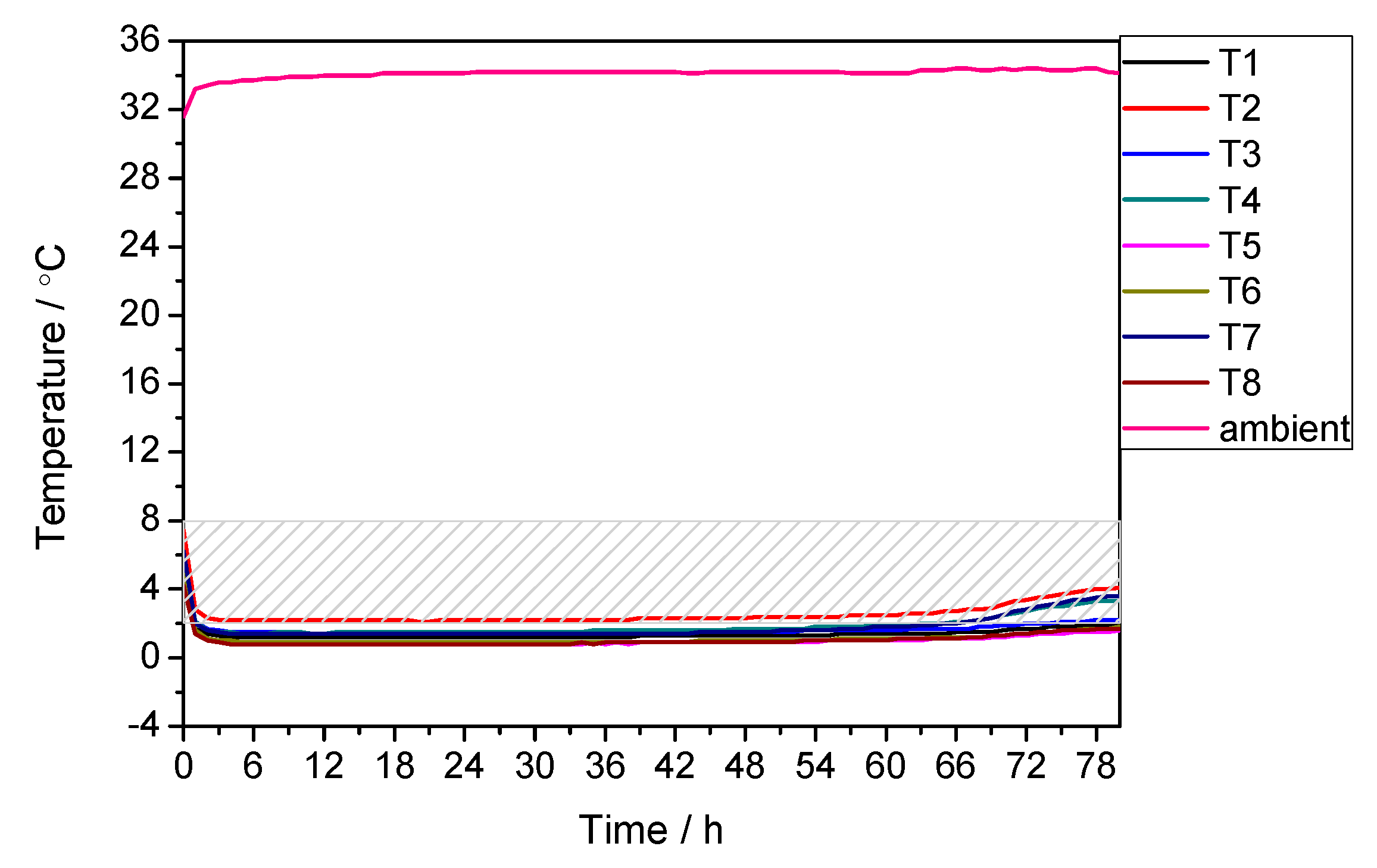
3.1. Performance under Extremely High Temperature Condition
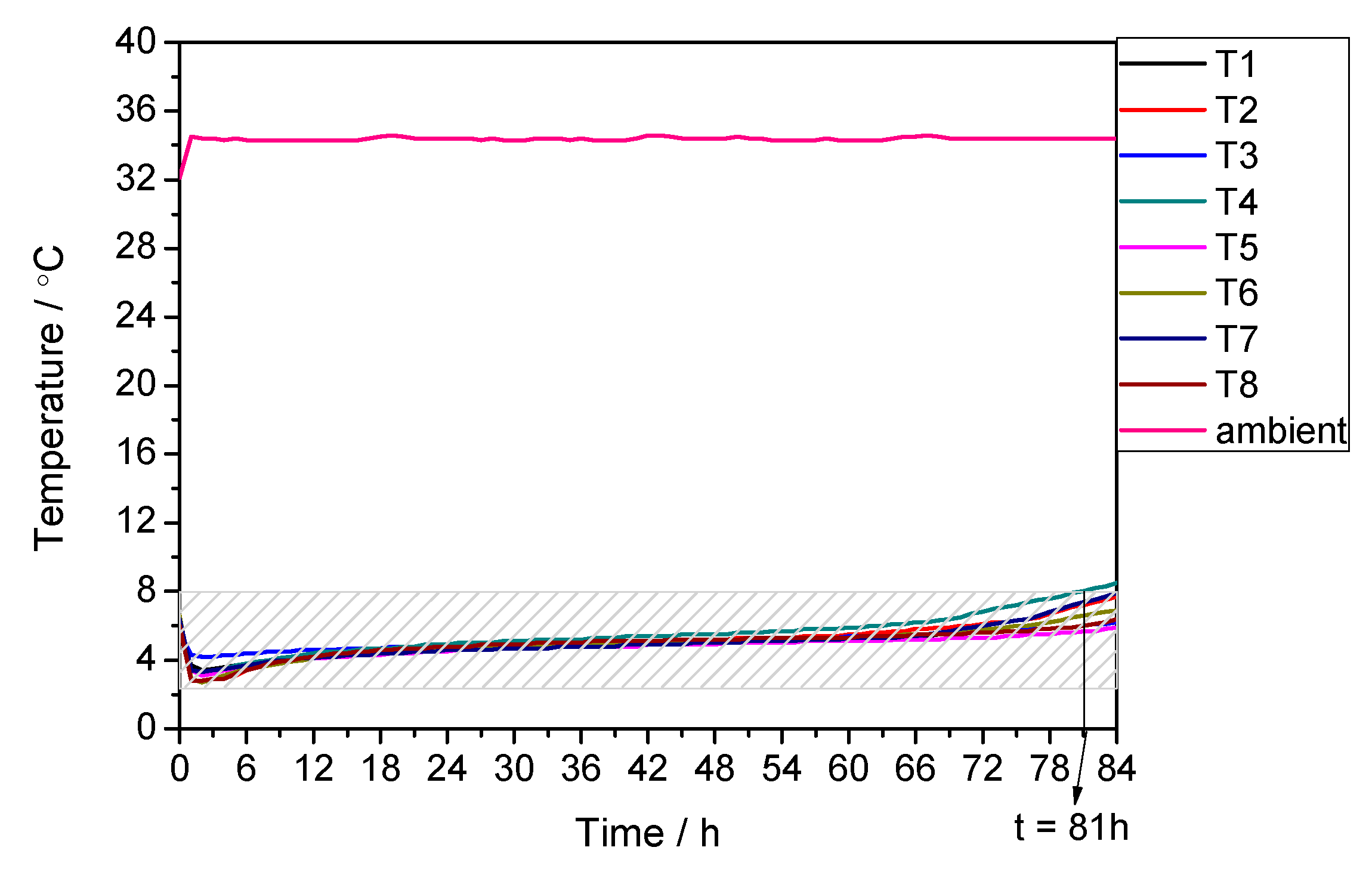
3.2. Performance under Extremely Low Temperature Condition
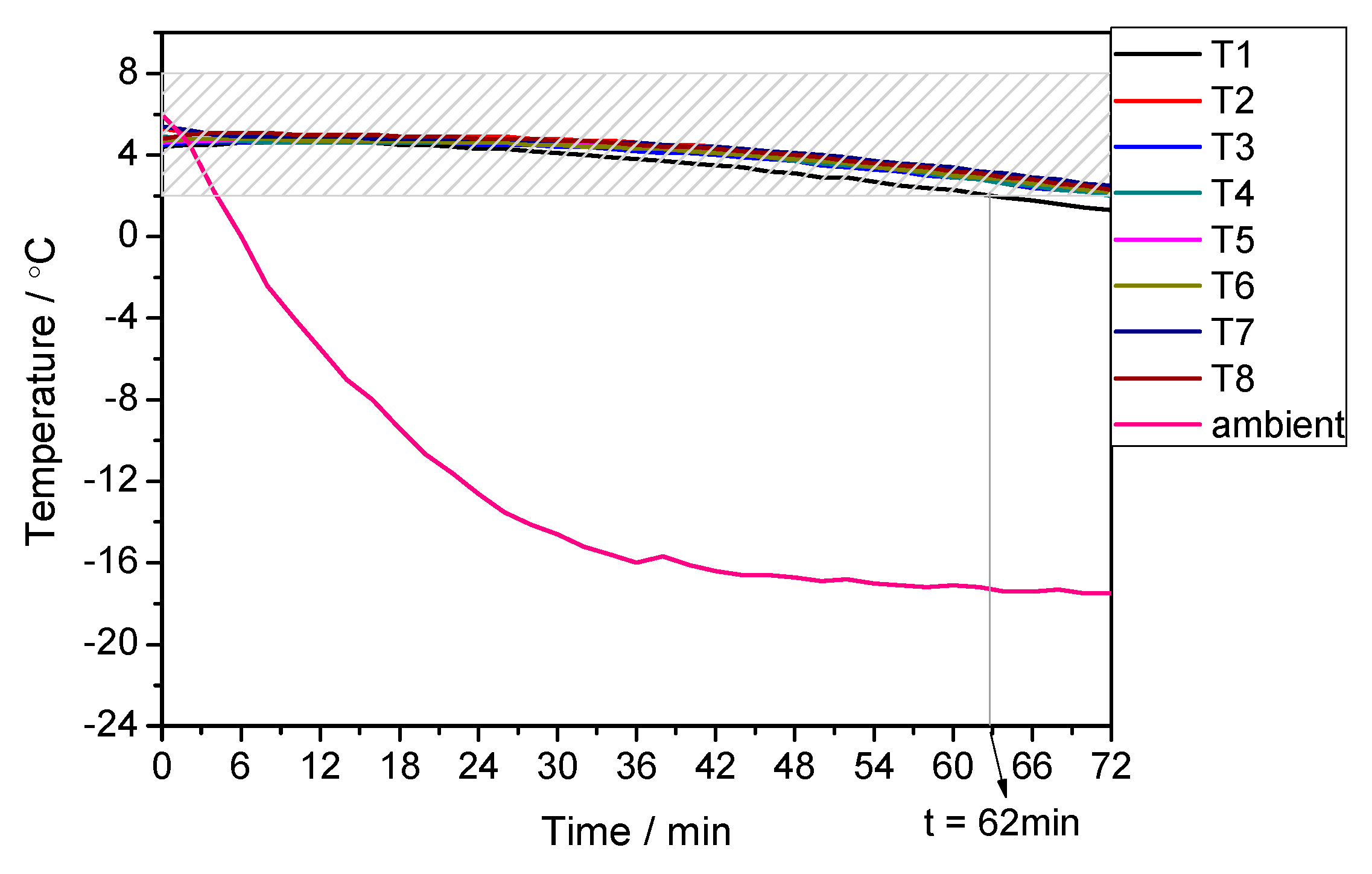
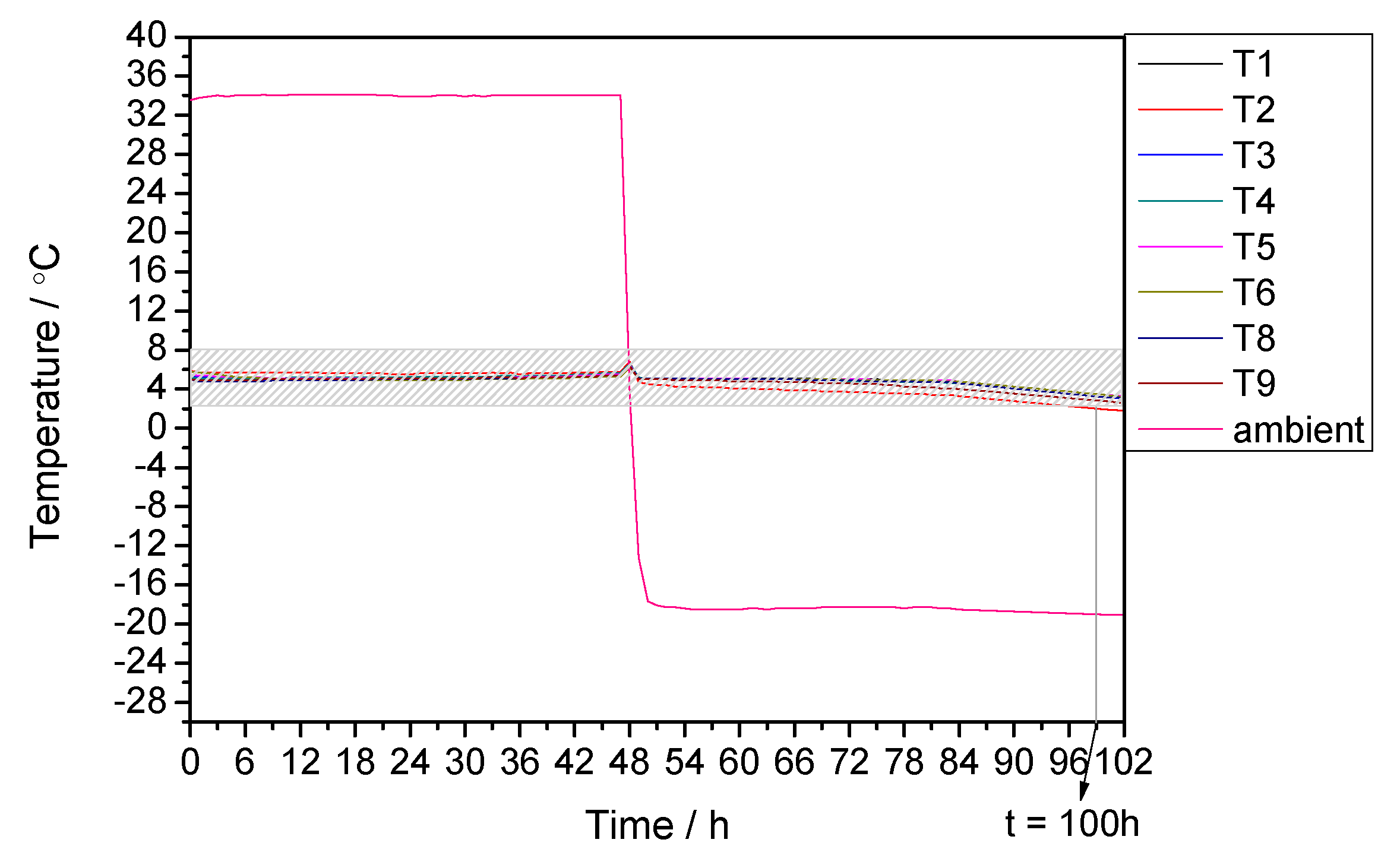
3.3. Performance under Alternating Temperature Condition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
| F | average heat transfer area of the container, m2 |
| Fa | external heat transfer area of the container, m2 |
| Fb | internal heat transfer area of the container, m2 |
| Δh | melting/freezing enthalpy of PCM, kJ/kg |
| ΔH | heat capacity of PCM, kJ |
| K | overall heat transfer coefficient, W/(m2·K) |
| m | total mass of PCM, kg |
| q | total heat flow, W |
| q1 | heat flow rate, conduction and convection, W |
| q2 | heat flow rate, air leakage, W |
| t | temperature-controlled time, h |
| Ta | ambient temperature, °C |
| Tb | air temperature inside the container, °C |
| α1 | air heat transfer coefficient outside, W/(m2·K) |
| α2 | air heat transfer coefficient inside the container, W/(m2·K) |
| additional thermal load factor | |
| λ1 | thermal conductivity of PU, W/(m·K) |
| λ2 | thermal conductivity of VIP, W/(m·K) |
| λ3 | thermal conductivity of PCM, W/(m·K) |
| δ1 | thickness of PU, m |
| δ2 | thickness of VIP, m |
| δ3 | thickness of PCM, m |
References
- Gac, A. Refrigerated transport: What’s new? Int. J. Refrig. 2002, 25, 501–503. [Google Scholar]
- Evans, A.; Hammond, E.C.; Gigiel, A.J.; Fostera, A.M.; Reinholdt, L.; Fikiin, K.; Zilio, C. Assessment of methods to reduce the energy consumption of food cold stores. Appl. Therm. Eng. 2014, 62, 697–705. [Google Scholar] [CrossRef]
- Ahmed, A.; Meade, O.; Medina, M.A. Reducing heat transfer across the insulated walls of refrigerated truck trailer by application of phase change materials. Energy Convers. Manag. 2010, 51, 383–392. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Development of a novel refrigeration system for refrigerated trucks incorporating phase change material. Appl. Energy 2012, 92, 336–342. [Google Scholar] [CrossRef]
- Fioretti, R.; Principi, P.; Copertaro, B. A refrigerated container envelope with a PCM (Phase Change Material) layer: Experimental and theoretical investigation in a representative town in Central Italy. Energy Convers. Manag. 2016, 122, 131–141. [Google Scholar] [CrossRef]
- Copertaro, B.; Principi, P.; Fioretti, R. Thermal performance analysis of PCM in refrigerated container envelops in the Italian context—Numerical modeling and validation. Appl. Therm. Eng. 2016, 105, 873–881. [Google Scholar] [CrossRef]
- EMBALL’ISO Company. Available online: http://www.emballiso.com/en/products-packaging-isothermal/long-duration-small-packaging/vype-new-generation-packaging-isothermal (accessed on 26 October 2017).
- Ren, J.S.; Li, A.M. Characteristics of cool storage plate refrigerated truck and calculation of cooling capacity. Spec. Purp. Veh. 2010, 8, 47–49. [Google Scholar]




| Parameter | Water | OP5E |
|---|---|---|
| Specific heat capacity/kJ/(kg·K) | 4.2 | 2.0 |
| Solid density (−15 °C)/kg/L | 0.996 | 0.88 |
| Liquid density (20 °C)/kg/L | 0.998 | 0.76 |
| Thermal conductivity liquid/W/(m·K) | 0.56 | 0.2 |
| Volume expansion/% | 0.3 | 13 |
| Total mass used in the container (kg) | 10.87 | 8.28 |
| Ambient Temperature (°C) | Maximum or Minimum Temperature Allowed in the Container (°C) | Temperature-Controlled Time (h) | Deviation (%) | |
|---|---|---|---|---|
| Experimental | Theoretical | |||
| 35 | 8 | 81 | 88 | −7.95 |
| −20 | 2 | 102 | 108 | −5.56 |
| 35 °C for the first 48 h and −20 °C for rest time | 8 °C for the first 48 h and 2 °C for the rest time | 100 | 98 | +2.04 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Piontek, U. Improving Performance of Cold-Chain Insulated Container with Phase Change Material: An Experimental Investigation. Appl. Sci. 2017, 7, 1288. https://doi.org/10.3390/app7121288
Huang L, Piontek U. Improving Performance of Cold-Chain Insulated Container with Phase Change Material: An Experimental Investigation. Applied Sciences. 2017; 7(12):1288. https://doi.org/10.3390/app7121288
Chicago/Turabian StyleHuang, Li, and Udo Piontek. 2017. "Improving Performance of Cold-Chain Insulated Container with Phase Change Material: An Experimental Investigation" Applied Sciences 7, no. 12: 1288. https://doi.org/10.3390/app7121288




