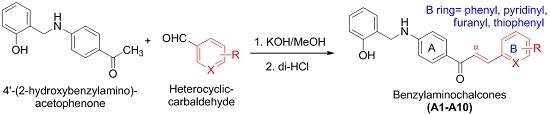
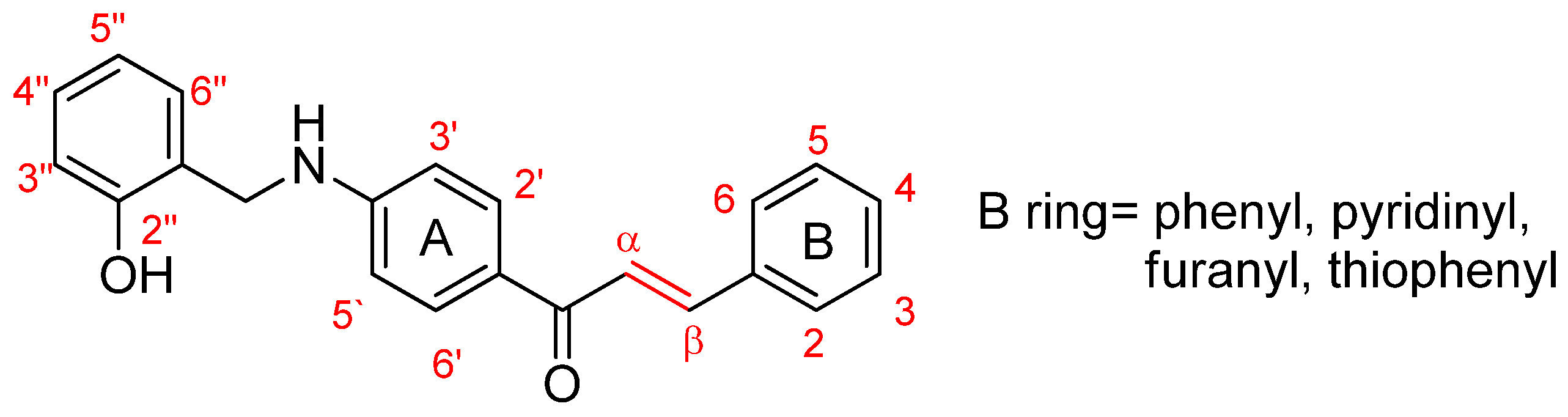
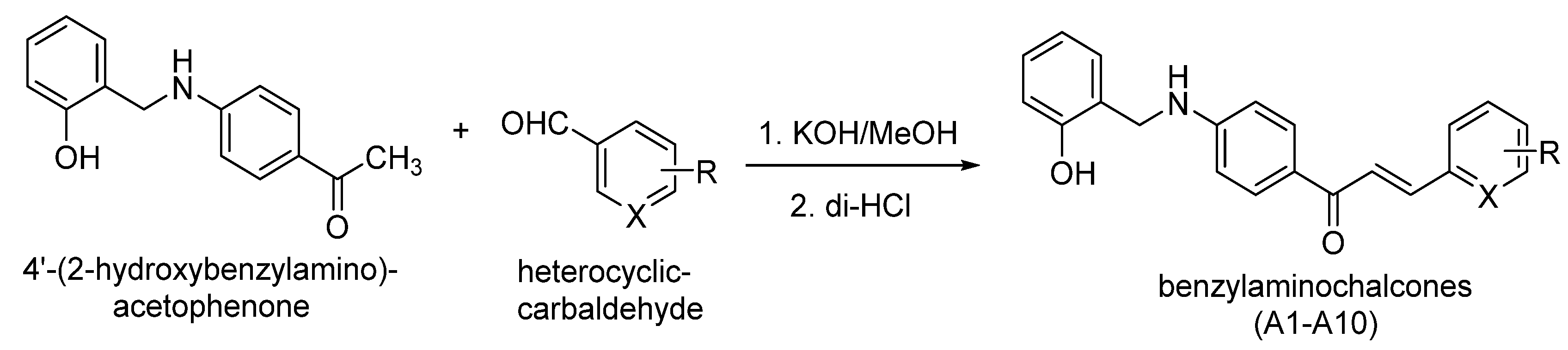
Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. General Procedures for the Preparation of Compounds A1–A10
2.4. In Vitro Inhibition Assay on AChE
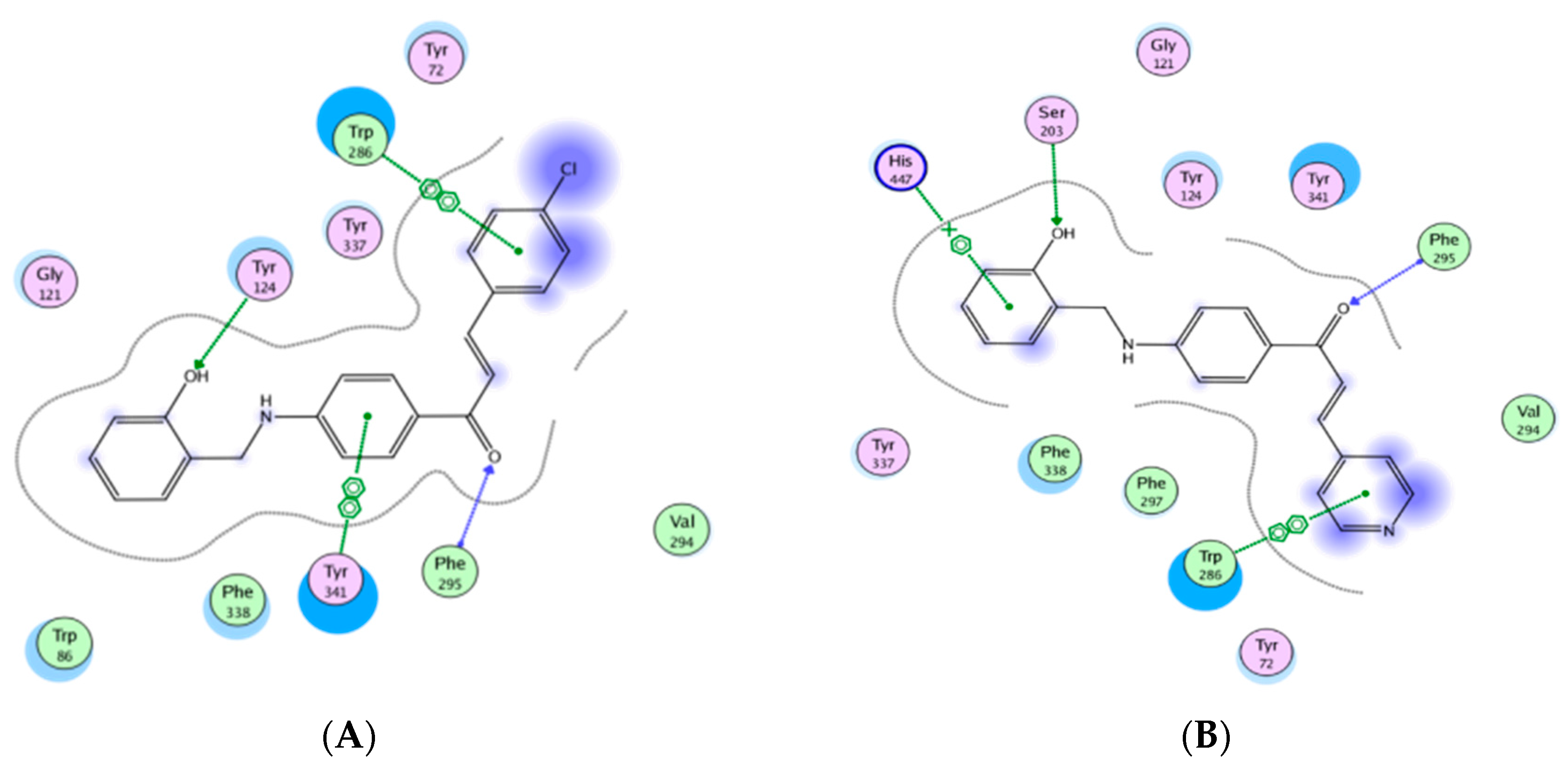
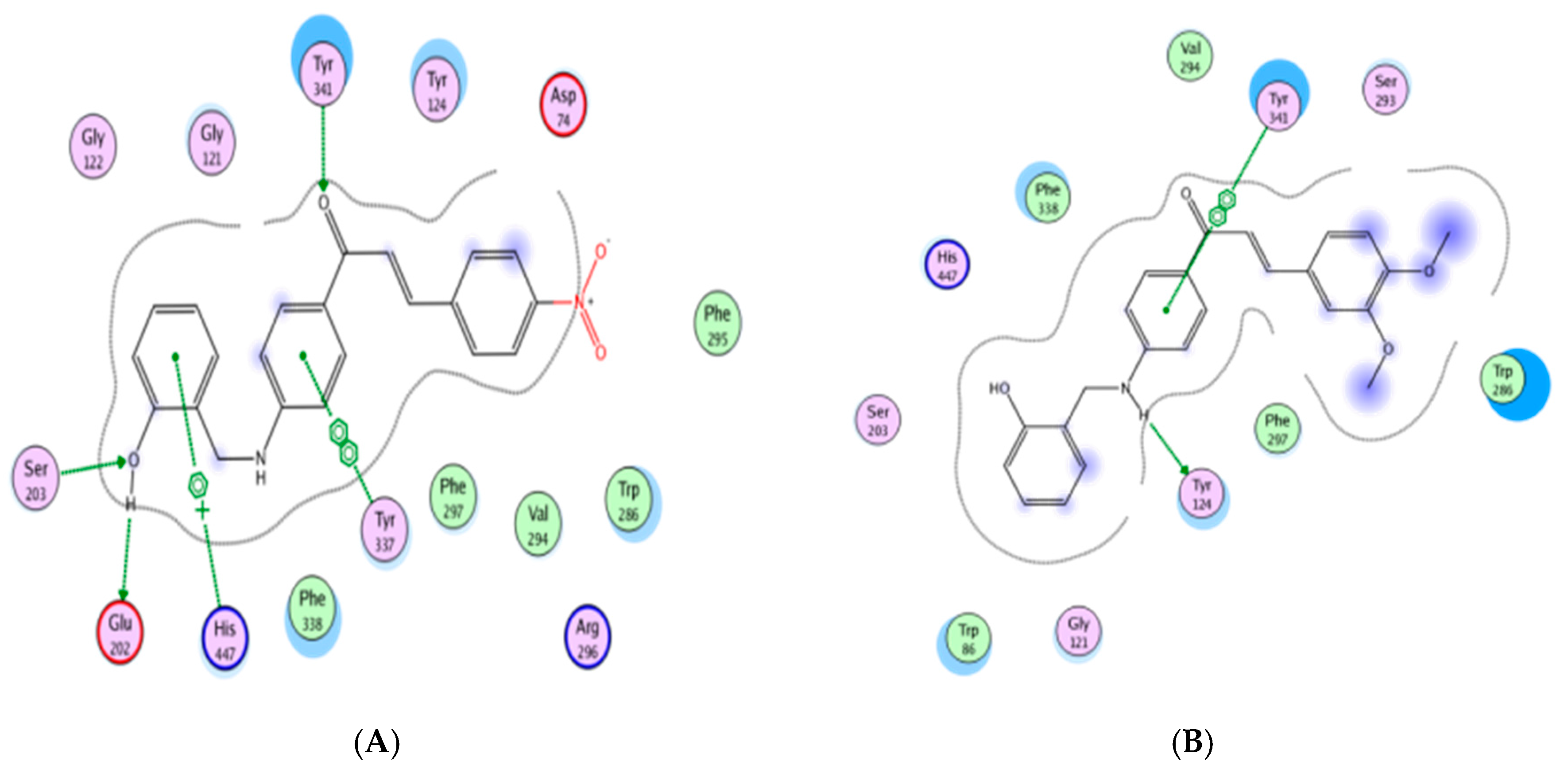
2.5. Molecular Modeling Study
3. Results and Discussion
3.1. Synthesis
3.1.1. (E)-3-(phenyl)-1-(4-((2-hydroxybenzyl)amino)phenyl)prop-2-ene-1-one (A1)
3.1.2. (E)-3-(2-chlorophenyl)-1-(4-((2-hydroxybenzyl)amino)phenyl)prop-2-ene-1-one (A2)
3.1.3. (E)-3-(4-chlorophenyl)-1-(4-((2-hydroxybenzyl)amino)phenyl)prop-2-ene-1-one (A3)
3.1.4. (E)-3-(4-nitrophenyl)-1-(4-((2-hydroxy-benzyl)amino)phenyl)prop-2-ene-1-one (A4)
3.1.5. (E)-3-(2,3-dimethoxyphenyl)-1-(4-((2-hydroxybenzyl)amino)phenyl)prop-2-ene-1-one (A5)
3.1.6. (E)-3-(3,4-dimethoxyphenyl)-1-(4-((2-hydroxybenzyl)amino)phenyl)prop-2-ene-1-one (A6)
3.1.7. (E)-3-(2,4-dimethoxyphenyl)-1-(4-((2-hydroxybenzyl)amino)phenyl)prop-2-ene-1-one (A7)
3.1.8. (E)-3-(pyridin-2-yl)-1-(4-((2-hydroxy-benzyl)amino)phenyl)prop-2-ene-1-one (A8)
3.1.9. (E)-3-(pyridin-4-yl)-1-(4-((2-hydroxy-benzyl)amino)phenyl)prop-2-ene-1-one (A9)
3.1.10. (E)-3-(furan-2-yl)-1-(4-((2-hydroxybenzyl)amino)phenyl)prop-2-ene-1-one (A10)
3.2. Anti-Acetylcholinesterase Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scarpini, E.; Scheltens, P.; Feldman, H. Treatment of Alzheimer's disease: Current status and new perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar] [CrossRef]
- Piazzi, L.; Rampa, A.; Bisi, A.; Gobbi, S.; Belluti, F.; Cavalli, A.; Bartolini, M.; Andrisano, V.; Valenti, P.; Recanatini, M. 3-(4-{[Benzyl(methyl)amino]methyl}phenyl)-6,7-dimethoxy-2H-2-chromenone (AP2238) Inhibits Both Acetylcholinesterase and Acetylcholinesterase-Induced β-Amyloid Aggregation: A Dual Function Lead for Alzheimer's Disease Therapy. J. Med. Chem. 2003, 46, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer's Disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Tumiatti, V.; Minarini, A.; Bolognesi, M.L.; Milelli, A.; Rosini, M.; Melchiorre, C. Tacrine Derivatives and Alzheimers Disease. Curr. Med. Chem. 2010, 17, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wang, H.; Xia, Z.; Lu, M.; Zhang, W.; Wang, X.; Fu, U.; Tang, Y.; Sheng, W.; Li, W.; et al. Bis-(−)-nor-meptazinols as Novel Nanomolar Cholinesterase Inhibitors with High Inhibitory Potency on Amyloid-β Aggregation. J. Med. Chem. 2008, 51, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–417. [Google Scholar] [CrossRef]
- Ritchie, C.W.; Ames, D.; Clayton, T.; Lai, R. Metaanalysis of Randomized Trials of the Efficacy and Safety of Donepezil, Galantamine, and Rivastigmine for the Treatment of Alzheimer Disease. Am. J. Geriat. Psychiat. 2004, 4, 358–369. [Google Scholar] [CrossRef]
- Harel, M.; Sonoda, L.K.; Silman, I.; Sussman, I.; Rosenberry, T.L. Crystal Structure of Thioflavin T Bound to the Peripheral Site of Torpedo californica Acetylcholinesterase Reveals How Thioflavin T Acts as a Sensitive Fluorescent Reporter of Ligand Binding to the Acylation Site. J. Am. Chem. Soc. 2008, 130, 7856–7861. [Google Scholar] [CrossRef] [PubMed]
- Butini, S.; Campiani, G.; Borriello, M.; Gemma, S.; Panico, A.; Persico, M.; Catalanotti, B.; Ros, S.; Brindisi, M.; Agnusdei, M.; et al. Exploiting Protein Fluctuations at the Active-Site Gorge of Human Cholinesterases: Further Optimization of the Design Strategy to Develop Extremely Potent Inhibitors. J. Med. Chem. 2008, 51, 3154–3170. [Google Scholar] [CrossRef] [PubMed]
- Bourne, Y.; Taylor, P.; Radic, Z.; Marchot, P. Structural insights into ligand interactions at the acetylcholinesterase peripheral anionic site. EMBO J. 2003, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reyes, A.E.; Chacon, M.A.; Dinamarca, M.C.; Cerpa, W.; Morgan, C.; Inestrosa, N.C. Acetylcholinesterase-Aβ Complexes Are More Toxic than Aβ Fibrils in Rat Hippocampus. Am. J. Pathol. 2004, 164, 2163–2174. [Google Scholar] [CrossRef]
- Ozturan, O.E.; Tan, O.U.; Ozadali, K.; Kucukkilinc, T.; Balkan, A.; Ucar, G. Synthesis, molecular modeling and evaluation of novel N′-2-(4-benzylpiperidin-/piperazin-1-yl)acylhydrazone derivatives as dual inhibitors for cholinesterases and Aβ aggregation. Bioorg. Med. Chem. Lett. 2013, 23, 440–443. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A Structural Motif of Acetylcholinesterase that Promotes Amyloid β-Peptide Fibril Formation. Biochem. 2001, 40, 10447–10457. [Google Scholar] [CrossRef]
- Munoz-Ruiz, P.; Rubio, L.; Garcia-Palomero, E.; Dorronsoro, I.; del Monte-Millan, M.; Valenzuela, R.; Usan, P.; de Austria, C.; Bartolini, M.; Andrisano, V.; et al. Design, Synthesis, and Biological Evaluation of Dual Binding Site Acetylcholinesterase Inhibitors: New Disease-Modifying Agents for Alzheimer's Disease. J. Med. Chem. 2005, 48, 7223–7233. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, M.; Rusak, G.; Domacinovic, B.J.; Sinko, G.; Jelic, D.; Antolovic, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Pueyo, I.; Calvo, M.I. Flavonoids as Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2011, 34, 5289–5302. [Google Scholar] [CrossRef]
- Kim, H.; Park, B.S.; Lee, K.G.; Choi, C.Y.; Jang, S.S.; Kim, Y.H.; Lee, S.E. Effects of Naturally Occurring Compounds on Fibril Formation and Oxidative Stress of β-Amyloid. J. Agric. Food Chem. 2005, 53, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Fan, P.; Perez, R.G. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-β-induced neuronal cell death. Bioorg. Med. Chem. 2011, 19, 4021–4027. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Park, H.M.; Hyung, S.J.; DeToma, A.S.; Kim, C.; Ruotolo, B.T.; Lim, M.H. Exploring the reactivity of flavonoid compounds with metal-associated amyloid-β species. Dalton Trans. 2012, 21, 6558–6566. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.J.; Kim, D.W.; Park, K.H. Inhibitory Evaluation of Sulfonamide Chalcones on β–Secretase and Acylcholinesterase. Molecules 2012, 18, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.B.; March, J. Advanced Organic Chemistry, 5th ed.; Wiley Interscience: New York, NY, USA, 2001; pp. 1218–1223. [Google Scholar]
- Cho, T.B.; Kang, S.K. Clean and simple chemoselective reduction of imines to amines using boric acid-activated sodium borohydride under solvent-free conditions. Synlett 2004, 9, 1484–1488. [Google Scholar] [CrossRef]
- Do, T.-H.; Nguyen, D.-M.; Truong, V.-D.; Do, T.-H.-T.; Le, M.-T.; Pham, T.-Q.; Thai, K.-M.; Tran, T.-D. Synthesis and Selective Cytotoxic Activities on Rhabdomyosarcoma and Noncancerous Cells of Some Heterocyclic Chalcones. Molecules 2016, 21, 329. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-D.; Do, T.-H.; Tran, N.-C.; Ngo, T.-D.; Huynh, T.-N.-P.; Tran, C.-D.; Thai, K.-M. Synthesis and anti Methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg. Med. Chem. Lett. 2012, 22, 4555–4560. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.-D.; Nguyen, T.-T.-N.; Do, T.-H.; Huynh, T.-N.-P.; Tran, C.-D.; Thai, K.-M. Synthesis and Antibacterial Activity of Some Heterocyclic Chalcone Analogues Alone and in Combination with Antibiotics. Molecules 2012, 17, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- MOE. 2008.10 edition. Chemical Computing Group Inc., 1010 Sherbrooke St. W, Suite 910, Montreal, Quebec, Canada H3A 2R7. Available online: http://www.chemcomp.com/ (accessed on 3 January 2015).
- LeadIT. 2.0.2 edition. BioSolveIT GmbH, An der Ziegelei 79, 53757 St. Augustin, Germany. Available online: http://www.biosolveit.de/ (accessed on 3 January 2015).
- Sybyl. Available online: http://www.tripos.com (accessed on 3 January 2015).
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.L.; Sussman, J.L. Acetylcholinesterase: From 3D Structure to Function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank. Available online: http://www.pdb.org/pdb/explore/explore.do?structureId=1dx6 (accessed on 3 January 2015).
- ChemBioDrawUltra. 12.0 edition. PerkinElmer, CambridgeSoft. Available online: http://www.cambridgesoft.com/ (accessed on 3 January 2015).
- Thai, K.-M.; Le, D.-P.; Tran, N.-V.-K.; Nguyen, T.-T.-H.; Tran, T.-D.; Le, M.-T. Computational assay of Zanamivir binding affinity with original and mutant influenza neuraminidase 9 using molecular docking. J. Theor. Biol. 2015, 385, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Thai, K.-M.; Ngo, T.-D.; Phan, T.-V.; Tran, T.-D.; Nguyen, N.-V.; Nguyen, T.-H.; Le, M.-T. Virtual screening for novel Staphylococcus Aureus NorA efflux pump inhibitors from natural products. Med. Chem. 2015, 11, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Thai, K.-M.; Huynh, N.-T.; Ngo, T.-D.; Mai, T.-T.; Nguyen, T.-H.; Tran, T.-D. Three- and four-class classification models for P-glycoprotein inhibitors using counter-propagation neural networks. SAR QSAR Environ. Res. 2015, 26, 139–163. [Google Scholar] [CrossRef] [PubMed]
| Samples | Compositions | ||||
|---|---|---|---|---|---|
| ATCI | DTNB | Tris-buffer pH8 | Chalcon | AChE | |
| Control blank sample with enzyme | + | + | + | - | + |
| Blank sample | + | + | + | - | - |
| Test sample | + | + | + | + | + |
| Blank test sample | + | + | + | + | - |
| Compound | Ar | IC50 (µM) | Docking Score (kJ/mol) |
|---|---|---|---|
| A1 | 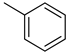 | >100 | −18.23 |
| A2 |  | >100 | −20.34 |
| A3 |  | 23.71 | −20.55 |
| A4 | 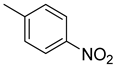 | 31.57 | −21.89 |
| A5 | 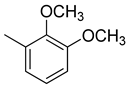 | >100 | −20.56 |
| A6 | 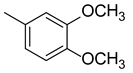 | 23.02 | −19.32 |
| A7 | 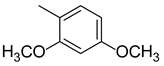 | >100 | −18.34 |
| A8 |  | >100 | −20.07 |
| A9 |  | 38.97 | −21.42 |
| A10 | 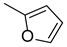 | 89.19 | −21.22 |
| Galantamine | - | 0.8 | −23.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.-D.; Nguyen, T.-C.-V.; Nguyen, N.-S.; Nguyen, D.-M.; Nguyen, T.-T.-H.; Le, M.-T.; Thai, K.-M. Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Appl. Sci. 2016, 6, 198. https://doi.org/10.3390/app6070198
Tran T-D, Nguyen T-C-V, Nguyen N-S, Nguyen D-M, Nguyen T-T-H, Le M-T, Thai K-M. Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Applied Sciences. 2016; 6(7):198. https://doi.org/10.3390/app6070198
Chicago/Turabian StyleTran, Thanh-Dao, Thi-Cam-Vi Nguyen, Ngoc-Son Nguyen, Dai-Minh Nguyen, Thi-Thu-Ha Nguyen, Minh-Tri Le, and Khac-Minh Thai. 2016. "Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors" Applied Sciences 6, no. 7: 198. https://doi.org/10.3390/app6070198
APA StyleTran, T.-D., Nguyen, T.-C.-V., Nguyen, N.-S., Nguyen, D.-M., Nguyen, T.-T.-H., Le, M.-T., & Thai, K.-M. (2016). Synthesis of Novel Chalcones as Acetylcholinesterase Inhibitors. Applied Sciences, 6(7), 198. https://doi.org/10.3390/app6070198