Advanced Microbubbles as a Multifunctional Platform Combining Imaging and Therapy
Abstract
:1. Introduction
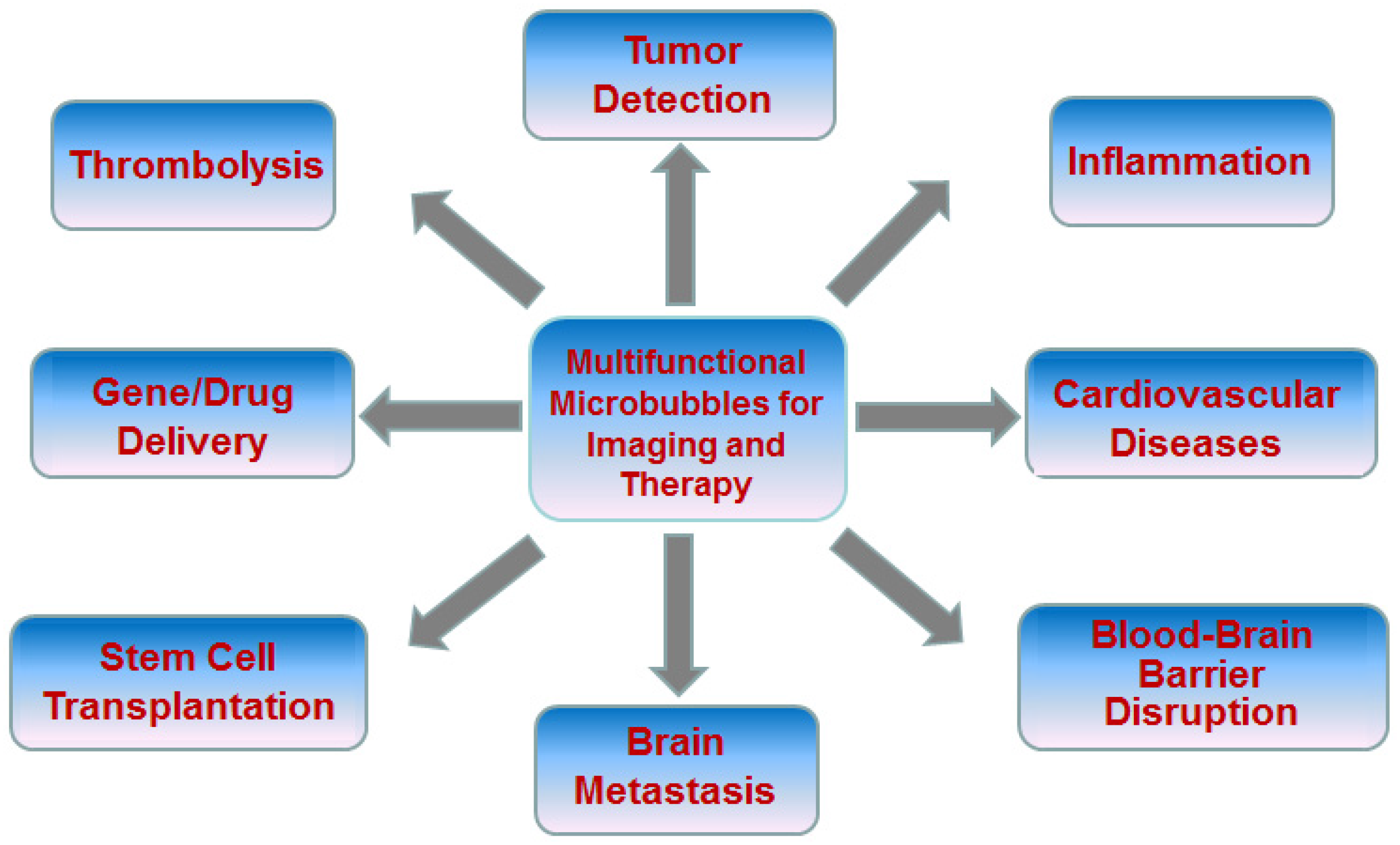
2. Advanced Microbubbles for Imaging and Therapy
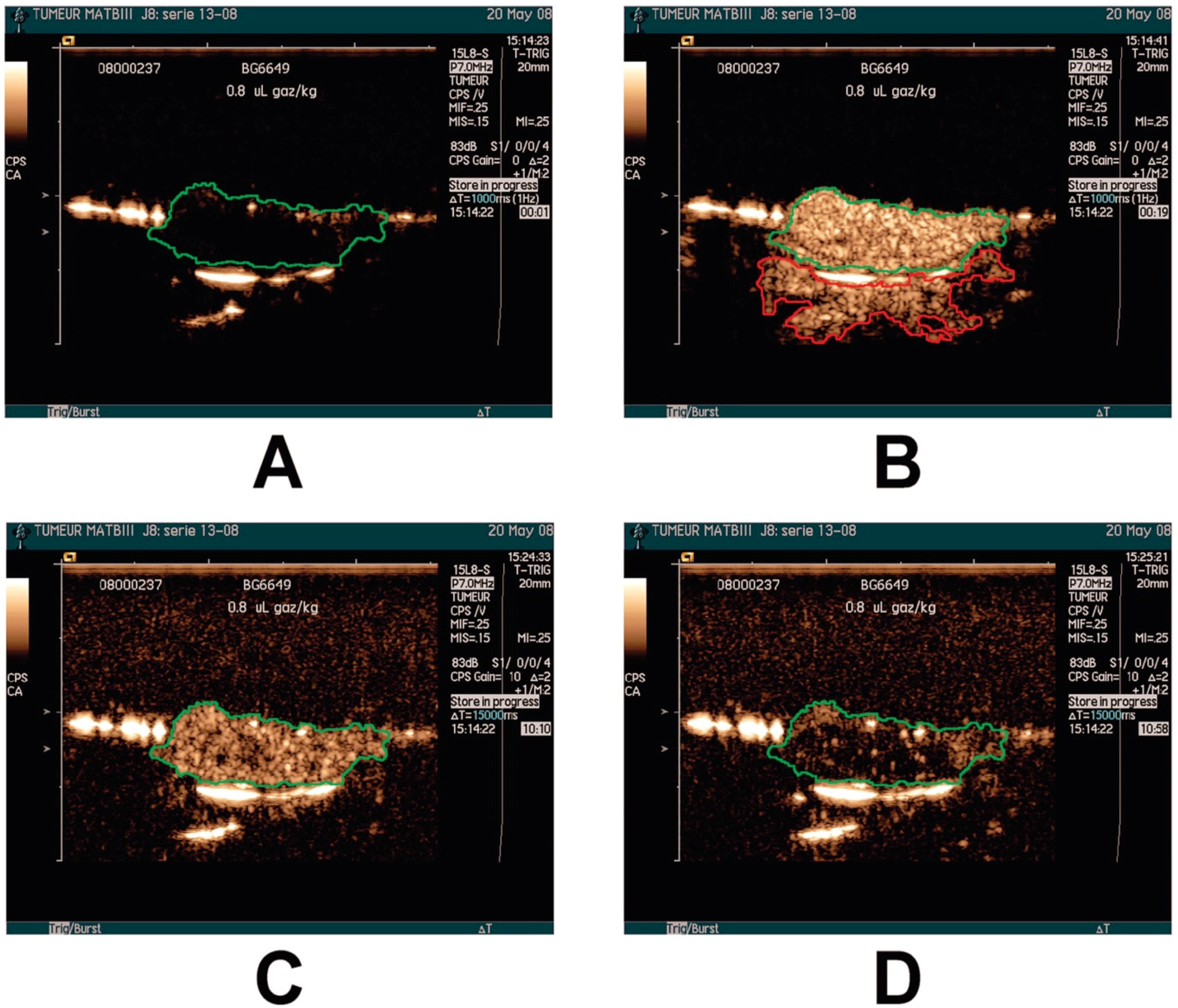
2.1. Tumor Detection
2.2. Inflammation
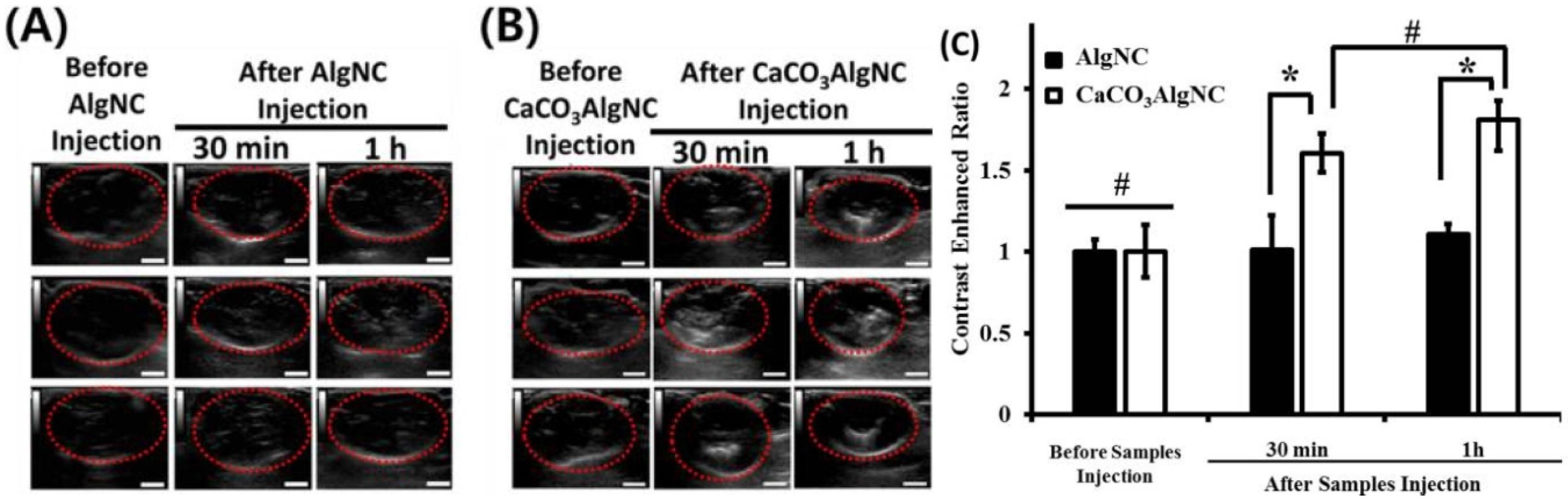
2.3. Cardiovascular Diseases
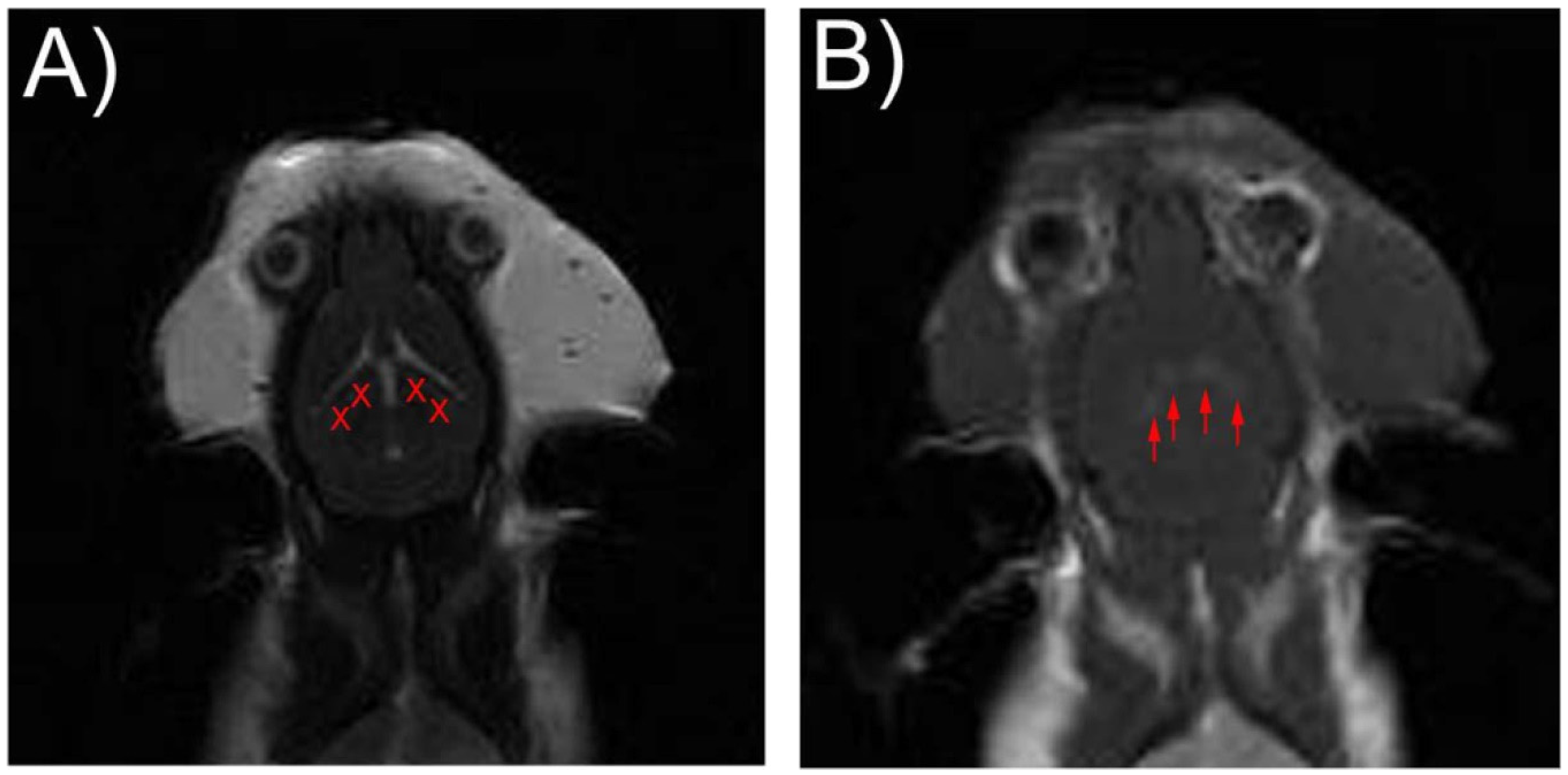
2.4. Blood-Brain Barrier Disruption
2.5. Thrombolysis
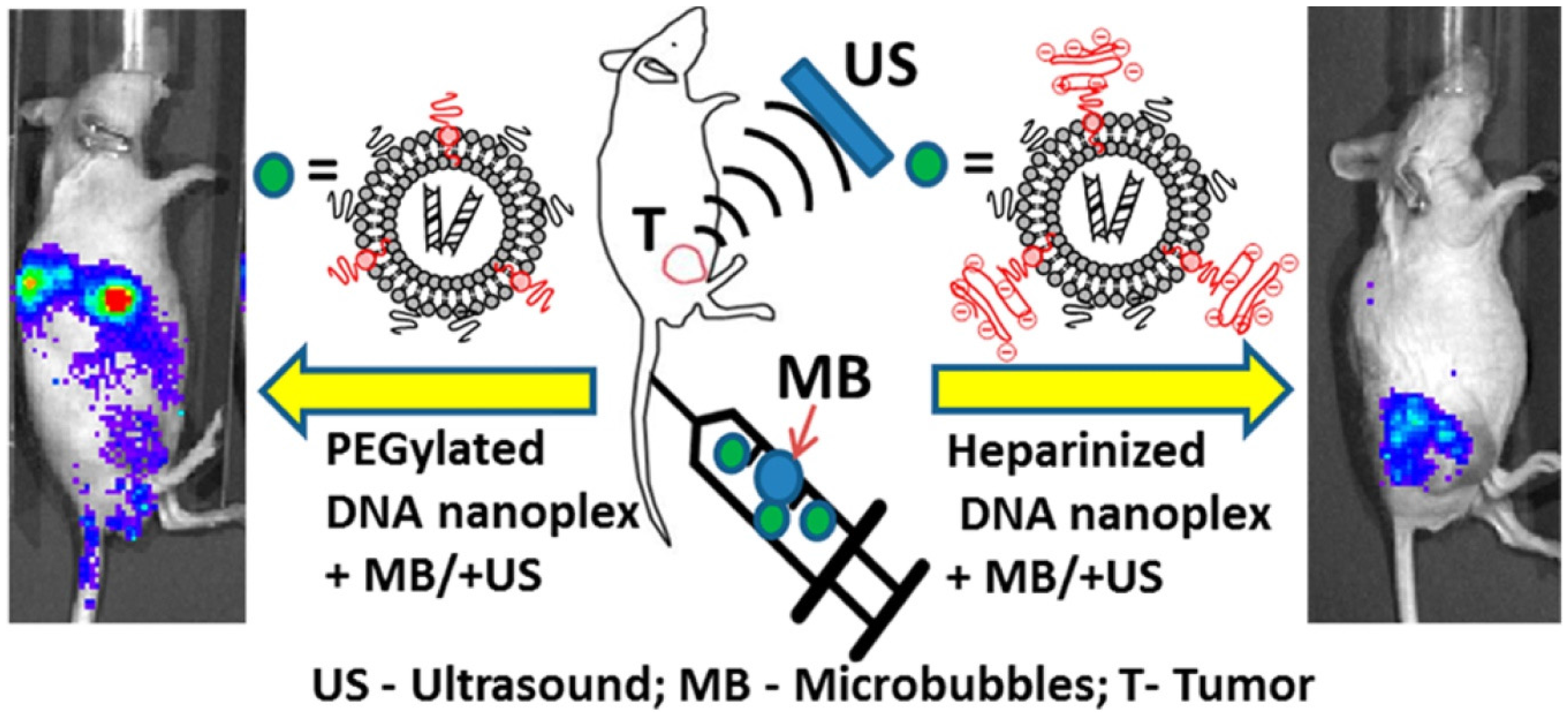
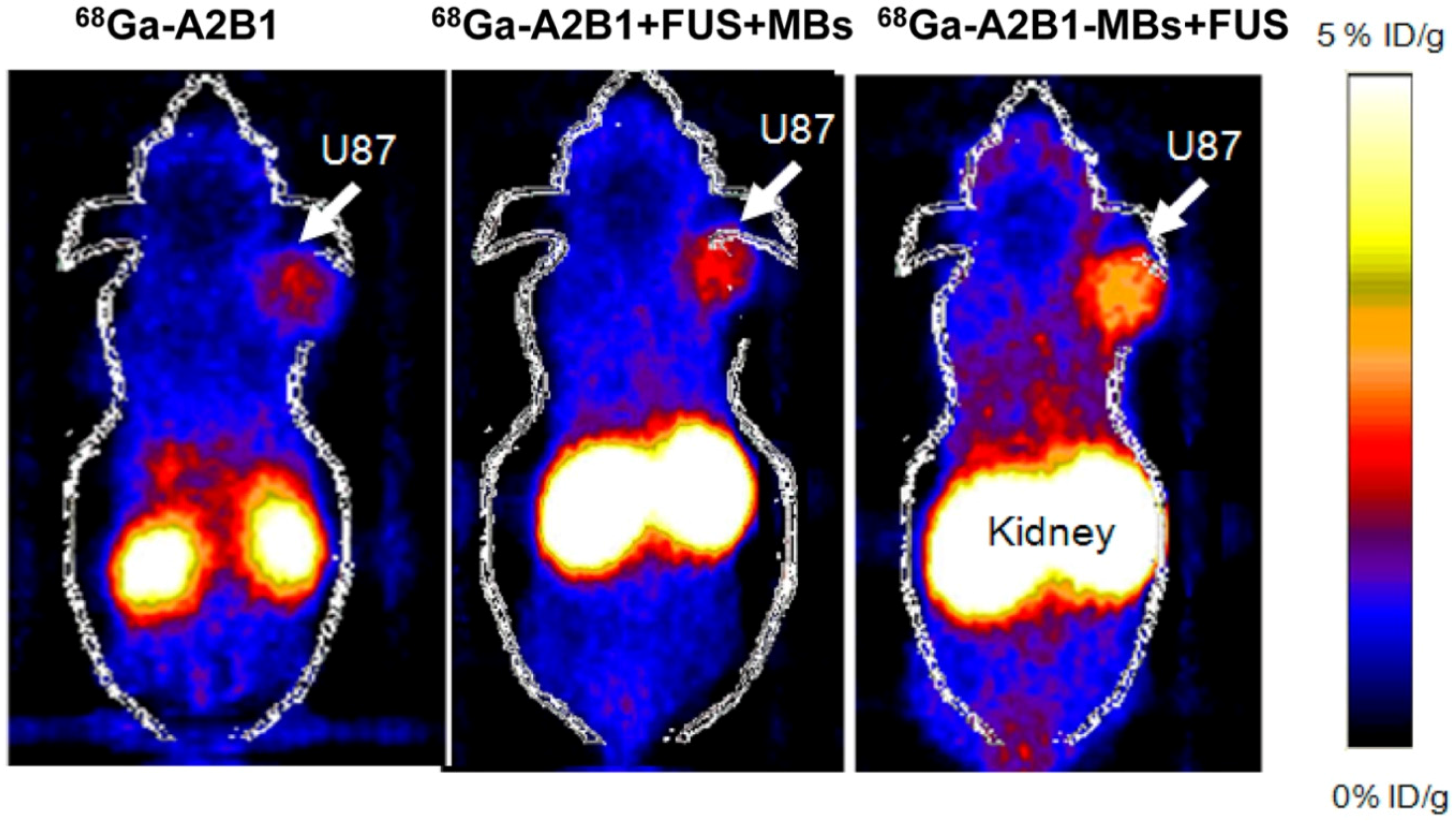
2.6. Drug/Gene Delivery
2.7. Stem Cell Transplantation
3. Challenges and Perspectives
Acknowledgments
Conflicts of Interest
References
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef] [PubMed]
- Gramiak, R.; Shah, P. Echocardiography of the aortic root. Investig. Radiol. 1968, 3, 356–366. [Google Scholar] [CrossRef]
- Christian, N.; Torben, L. International guidelines for contrast-enhanced ultrasonography: Ultrasound imaging in the new millennium. Ultrasonography 2016, 35, 89–103. [Google Scholar]
- Xie, F.; Lof, J.; Everbach, C.; He, A.; Bennett, R.M.; Matsunaga, T.; Johanning, J.; Porter, T.R. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. JACC Cardiovasc. Imaging 2009, 2, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Maruyama, T.; Matsumoto, Y. Use of a microbubble agent to increase the effects of high intensity focused ultrasound on liver tissue. Eur. Radiol. 2005, 15, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.; Misty, L.; Carol, H. Development of Therapeutic Microbubbles for Enhancing Ultrasound-Mediated Gene Delivery. J. Control. Release 2014, 182, 111–120. [Google Scholar]
- Lee, H.; Yoon, Y.; Bae, Y. Theragnostic ultrasound using microbubbles in the treatment of prostate cancer. Ultrasonography 2016, 35, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Blomley, M. Which US microbubble contrast agent is best for gene therapy? Radiology 2003, 229, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.S. Adherence of albunex to an apical left ventricular thrombus. J. Am. Soc. Echocardiogr. 1999, 12, 761–762. [Google Scholar] [CrossRef]
- Dolan, M.S.; Dent, J.; de Filippi, C.; Christopher, T.; Wible, J.H. Increasing the dose and rate of Albunex infusion leads to superior left ventricular contrast effect. J. Am. Soc. Echocardiogr. 1998, 11, 426–432. [Google Scholar] [CrossRef]
- Elhendy, A.; O’Leary, E.L.; Xie, F.; McGrain, A.C.; Anderson, J.R. Comparative accuracy of real-time myocardial contrast perfusion imaging and wall motion analysis during dobutamine stress echocardiography for the diagnosis of coronary artery disease. J. Am. Coll. Cardiol. 2004, 44, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Elhendy, A.; Tsutsui, J.M.; O’Leary, E.L.; Xie, F.; Porter, T.R. Noninvasive diagnosis of coronary artery bypass graft disease by dobutamine stress real-time myocardial contrast perfusion imaging. J. Am. Soc. Echocardiogr. 2006, 19, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Clevert, D.A.; Rupp, N. Contrast-enhanced ultrasound with Optison in percutaneous thermoablation of liver tumors. Rofo 2003, 175, 1403–1412. [Google Scholar] [PubMed]
- Moreno, R. Usefulness of Contrast Agents in the Diagnosis of Left Ventricular Pseudoaneurysm after Acute Myocardial Infarction. Eur. J. Echocardiogr. 2002, 3, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Drelich-Zbroja, A.; Jargiello, T.; Szymanska, A.; Krzyzanowski, W.; Szczerbo-Trojanowska, M. Can Levovist-enhanced Doppler ultrasound replace angiography in abdominal branches of the aorta imaging? Ultrasound Med. Biol. 2003, 29. [Google Scholar] [CrossRef]
- Von Herbay, A.; Haeussinger, D.; Gregor, M.; Vogt, C. Characterization and detection of hepatocellular carcinoma (HCC): Comparison of the ultrasound contrast agents SonoVue (BR1) and Levovist (SHU508A)--comparison of SonoVue and Levovist in HCC. Ultraschall Med. 2007, 28, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rubiera, M.; Ribo, M.; Delgado-Mederos, R.; Santamarina, E.; Maisterra, O. Do bubble characteristics affect recanalization in stroke patients treated with microbubble-enhanced sonothrombolysis? Ultrasound Med. Biol. 2008, 34, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; von Bardeleben, S.; Barletta, G.; Pasques, A.; Kasprzak, J. Comparison of two- and three-dimensional unenhanced and contrast-enhanced echocardiographies versus cineventriculography versus cardiac magnetic resonance for determination of left ventricular function. Am. J. Cardiol. 2014, 113, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.L.; Sun, S.G.; Yang, R.J.; Wang, J. A new method of measurement of cerebral circulation time: Contrast-enhanced ultrasonography in healthy adults and patients with intracranial shunts. Ultrasound Med. Biol. 2014, 40, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.B.; Yang, H.L.; Zhang, J.; Yin, J.K.; Yang, Y.L. The Optimized Fabrication of Nanobubbles as Ultrasound Contrast Agents for Tumor Imaging. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Petrone, G.; Ferrante, A.; Lauriola, L.; Vincenzoni, C. The role of contrast-enhanced ultrasound (CEUS) in visualizing atherosclerotic carotid plaque vulnerability: Which injection protocol? Which scanning technique? Eur. J. Radiol. 2015, 84, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mao, F.; Wang, W.P.; Ji, Z.B.; Fan, P.L. Value of Contrast-Enhanced Ultrasound in Guidance of Percutaneous Biopsy in Peripheral Pulmonary Lesions. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wan, C.; Du, L.; Li, F. Enhanced downregulation of transforming growth factorbeta2 in rat retinal pigment epithelium cells by adenoassociated virusmediated ribonucleic acid interference combined with ultrasound or microbubbles. Mol. Med. Rep. 2015, 11, 1099–1104. [Google Scholar] [PubMed]
- Lamanauskas, N.; Novell, A.; Escoffre, J.M.; Venslauskas, M.; Satkauskas, S. Bleomycin delivery into cancer cells in vitro with ultrasound and SonoVue(R) or BR14(R) microbubbles. J. Drug Target 2013, 21, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Nacu, A.; Kvistad, C.E.; Logallo, N.; Naess, H.; Waje-Andreassen, U. A pragmatic approach to sonothrombolysis in acute ischaemic stroke: The Norwegian randomised controlled sonothrombolysis in acute stroke study (NOR-SASS). BMC Neurol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, L.; Nanea, T. Assessment of myocardial perfusion using contrast echocardiography-Case report. J. Med. Life 2015, 8, 471–475. [Google Scholar] [PubMed]
- Porter, T.R.; Adolphson, M.; High, R.R.; Smith, L.M.; Olson, J. Rapid detection of coronary artery stenoses with real-time perfusion echocardiography during regadenoson stress. Circ. Cardiovasc. Imaging 2011, 4, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, K.; Umphrey, H.; Lockhart, M.; Robbin, M.; Forero-Torres, A. Ultrasound imaging of breast tumor perfusion and neovascular morphology. Ultrasound Med. Biol. 2015, 41, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.R.; Noble, M.L.; Sun, S.S.; Song, S.; Miao, C.H. Development of therapeutic microbubbles for enhancing ultrasound-mediated gene delivery. J. Control. Release 2014, 182, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Coussios, C.C.; Ammi, A.Y.; Mast, T.D.; de Courten-Myers, G.M. Ultrasound-enhanced thrombolysis using Definity as a cavitation nucleation agent. Ultrasound Med. Biol. 2008, 34, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Diagnostic Imaging of Hepatocellular Carcinoma: Recent Progress. Oncology 2011, 81, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Ito, T.; Takada, E.; Omoto, K.; Hirai, T.; Moriyasu, F. Effcacy of Sonazoid (Perflubutane) for Contrast-Enhanced Ultrasound in the Differentiation of Focal Breast Lesions: Phase 3 Multicenter Clinical Trial. Am. J. Roentgenol. 2014, 202, W400–W407. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Xu, X.; Chen, Y.; Deng, Z.; Liu, H. A Lipopeptide-Based alphavbeta(3) Integrin-Targeted Ultrasound Contrast Agent for Molecular Imaging of Tumor Angiogenesis. Ultrasound Med. Biol. 2015, 41, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.L.; Rosa, P.; Soletti, R.C.; Borges, H.L.; Machado, J.C. Contrast-Enhanced Images of Endoluminal Ultrasound Biomicroscopy from Mouse Colorectal Tumors Using VEGFR2-Targeted Contrast Agents. Ultrasound Med. Biol. 2013, 39, S15–S16. [Google Scholar] [CrossRef]
- Wei, S.; Fu, N.; Sun, Y.; Yang, Z.; Lei, L. Targeted contrast-enhanced ultrasound imaging of angiogenesis in an orthotopic mouse tumor model of renal carcinoma. Ultrasound Med. Biol. 2014, 40, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Marinelli, E.R.; Fan, H.; Nanjappan, P.; Song, B.; von Wronski, M.A.; Cherkaoui, S.; Tardy, I.; Pochon, S.; Schneider, M.; et al. A phospholipid-PEG2000 conjugate of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeting heterodimer peptide for contrast-enhanced ultrasound imaging of angiogenesis. Bioconjug. Chem. 2010, 21, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Sorace, A.G.; Saini, R.; Mahoney, M.; Hoyt, K. Molecular ultrasound imaging using a targeted contrast agent for assessing early tumor response to antiangiogenic therapy. J. Ultrasound Med. 2012, 31, 1543–1550. [Google Scholar] [PubMed]
- Bachawal, S.V.; Jensen, K.C.; Wilson, K.E.; Tian, L.; Lutz, A.M. Breast Cancer Detection by B7H3-Targeted Ultrasound Molecular Imaging. Cancer Res. 2015, 75, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, J.K.; Klauber-DeMore, N.; Streeter, J.; Samples, J.; Patterson, C. Ultrasound molecular imaging of secreted frizzled related protein-2 expression in murine angiosarcoma. PLoS ONE 2014, 9, e86642. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, X.; Kang, L.; Wei, H.; Xu, C. RGD-Targeted Ultrasound Contrast Agent for Longitudinal Assessment of Hep2 Tumor Angiogenesis In Vivo. PLoS ONE 2016, 11, e0149075. [Google Scholar]
- Mai, L.; Yao, A.; Li, J.; Wei, Q.; Yuchi, M. Cyanine5.5 conjugated nanobubbles as a tumor selective contrast agent for dual ultrasound-fluorescence imaging in a mouse model. PLoS ONE 2013, 8, e61224. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, J.H.; Kim, S.E.; Kang, S.S.; Tae, G. Nanosized Ultrasound Enhanced-Contrast Agent for in Vivo Tumor Imaging via Intravenous Injection. ACS Appl. Mater. Interfaces 2016, 8, 8409–8418. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cai, W.; Xu, L.; Lv, X.; Qiao, Y. Nanobubble-Affibody: Novel ultrasound contrast agents for targeted molecular ultrasound imaging of tumor. Biomaterials 2015, 37, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Jiang, J.; Han, B.; Zhang, S. Preparation and Characterization of Novel Perfluorooctyl Bromide Nanoparticle as Ultrasound Contrast Agent via Layer-by-Layer Self-Assembly for Folate-Receptor-Mediated Tumor Imaging. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Pysz, M.A.; Foygel, K.; Rosenberg, J.; Gambhir, S.S.; Schneider, M.; Willmann, J.K. Antiangiogenic cancer therapy: Monitoring with molecular US and a clinically translatable contrast agent (BR55). Radiology 2010, 256, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Pochon, S.; Tardy, I.; Bussat, P.; Bettinger, T.; Brochot, J.; von, W.M.; Passantino, L.; Schneider, M. BR55: A lipopeptide-based VEGFR2-targeted ultrasound contrast agent for molecular imaging ofangiogenesis. Investig. Radiol. 2010, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Bzyl, J.; Lederle, W.; Rix, A.; Grouls, C.; Tardy, I.; Pochon, S.; Siepmann, M.; Penzkofer, T.; Schneider, M.; Kiessling, F. Molecular and functional ultrasound imaging in differently aggressive breast cancer xenografts using two novel ultrasound contrast agents (BR55 and BR38). Eur. Radiol. 2011, 21, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Tardy, I.; Pochon, S.; Theraulaz, M.; Emmel, P.; Passantino, L.; Tranquart, F.; Schneider, M. Ultrasound molecular imaging of VEGFR2 in a rat prostate tumor model using BR55. Investig. Radiol. 2010, 45, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lyshchik, A.; Huamani, J.; Hallahan, D.E.; Fleischer, A.C. Relationship between retention of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeted ultrasonographic contrast agent and the level of VEGFR2 expression in an in vivo breast cancer model. J. Ultrasound Med. 2008, 27, 855–866. [Google Scholar] [PubMed]
- Korpanty, G.; Carbon, J.G.; Grayburn, P.A.; Fleming, J.B.; Brekken, R.A. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin. Cancer Res. 2007, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Paulmurugan, R.; Chen, K.; Gheysens, O.; Rodriguez-Porcel, M.; Lutz, A.M.; Chen, I.Y.; Chen, X.; Gambhir, S.S. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology 2008, 246, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, M.; Huppert, J.; Ladewig, G.; Hauff, P.; Reinhardt, M.; Mueller, M.M.; Woenne, E.C.; Jenne, J.W.; Maurer, M.; Kauffmann, G.W. Molecular profiling of angiogenesis with targeted ultrasound imaging: Early assessment of antiangiogenic therapy effects. Mol. Cancer Ther. 2008, 7, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Ellegala, D.B.; Leong-Poi, H.; Carpenter, J.E.; Klibanov, A.L.; Kaul, S.; Shaffrey, M.E.; Sklenar, J.; Lindner, J.R. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation 2003, 108, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Kimura, R.H.; Deshpande, N.; Lutz, A.M.; Cochran, J.R.; Gambhir, S.S. Targeted contrast-enhanced ultrasound imaging of tumor angiogenesis with contrast microbubbles conjugated to integrin-binding knottin peptides. J. Nucl. Med. 2010, 51, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, N.; Ren, Y.; Foygel, K.; Rosenberg, J.; Willmann, J.K. Tumor angiogenic marker expression levels during tumor growth: Longitudinal assessment with molecularly targeted microbubbles and US imaging. Radiology 2011, 258, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, M.; Peschke, P.; Huppert, J.; Hauff, P.; Reinhardt, M.; Maurer, M.; Karger, C.P.; Scholz, M.; Semmler, W.; Huber, P.E. Molecular ultrasound imaging of early vascular response in prostate tumors irradiated with carbon ions. Neoplasia 2009, 11, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; Lutz, A.M.; Paulmurugan, R.; Patel, M.R.; Chu, P.; Rosenberg, J.; Gambhir, S.S. Dual-targeted contrast agent for US assessment of tumor angiogenesis in vivo. Radiology 2008, 248, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.M.; Sorace, A.G.; Saini, R.; Umphrey, H.R.; Zinn, K.R.; Hoyt, K. A triple-targeted ultrasound contrast agent provides improved localization to tumor vasculature. J. Ultrasound Med. 2011, 30, 921–931. [Google Scholar] [PubMed]
- Zhang, H.; Tam, S.; Ingham, E.S.; Mahakian, L.M.; Lai, C.Y.; Tumbale, S.K.; Teesalu, T.; Hubbard, N.E.; Borowsky, A.D.; Ferrara, K.W. Ultrasound molecular imaging of tumor angiogenesis with a neuropilin-1-targeted microbubble. Biomaterials 2015, 56, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, T.; Bussat, P.; Tardy, I.; Pochon, S.; Hyvelin, J.M. Ultrasound molecular imaging contrast agent binding to both E- and P-selectin in different species. Investig. Radiol. 2012, 47, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Machtaler, S.; Bettinger, T.; Lutz, A.; Luong, R. Molecular imaging of inflammation in inflammatory bowel disease with a clinically translatable dual-selectin-targeted US contrast agent: Comparison with FDG PET/CT in a mouse model. Radiology 2013, 267, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Machtaler, S.; Knieling, F.; Luong, R.; Tian, L.; Willmann, J.K. Assessment of Inflammation in an Acute on Chronic Model of Inflammatory Bowel Disease with Ultrasound Molecular Imaging. Theranostics 2015, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, K.; Warram, J.M.; Wang, D.; Ratnayaka, S.; Traylor, A. Molecular Ultrasound Imaging of Tissue Inflammation Using an Animal Model of Acute Kidney Injury. Mol. Imaging Biol. 2015, 17, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Weller, G.E.; Lu, E.; Csikari, M.M.; Klibanov, A.L.; Fischer, D. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation 2003, 108, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Hagisawa, K.; Nishioka, T.; Suzuki, R.; Takizawa, T.; Maruyama, K. Enhancement of ultrasonic thrombus imaging using novel liposomal bubbles targeting activated platelet glycoprotein IIb/IIIa complex-in vitro and in vivo study. Int. J. Cardiol. 2011, 152, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liu, C.; Liao, Y.; Yang, L.; Huang, R. Ultrasound molecular imaging of arterial thrombi with novel microbubbles modified by cyclic RGD in vitro and in vivo. Thromb. Haemost. 2012, 107, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Hyvelin, J.M.; Tardy, I.; Bettinger, T.; von Wronski, M.; Costa, M. Ultrasound molecular imaging of transient acute myocardial ischemia with a clinically translatable P- and E-selectin targeted contrast agent: Correlation with the expression of selectins. Investig. Radiol. 2014, 49, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Hynynen, K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem. Neurosci. 2013, 4, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.J.; Shah, K.; Yeung, S.; Burgess, A.; Aubert, I. Focused Ultrasound-Induced Neurogenesis Requires an Increase in Blood-Brain Barrier Permeability. PLoS ONE 2016, 11, e0159892. [Google Scholar] [CrossRef] [PubMed]
- Kobus, T.; Zervantonakis, I.K.; Zhang, Y.; McDannold, N.J. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J. Control. Release 2016, 238, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Lelu, S.; Afadzi, M.; Berg, S.; Aslund, A.; Torp, S. Primary porcine brain endothelial cells as in vitro model to study effects of ultrasound on blood-brain barrier function. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2016. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Chang, E.L.; Ting, C.Y.; Lin, Y.C.; Liao, E.C. Folate-conjugated gene-carrying microbubbles with focused ultrasound for concurrent blood-brain barrier opening and local gene delivery. Biomaterials 2016, 106, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Samiotaki, G.; Acosta, C.; Wang, S.; Konofagou, E.E. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo. J. Cereb. Blood Flow Metab. 2015, 35, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsieh, H.Y.; Chen, C.M.; Wu, S.R.; Tsai, C.H. Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson’s disease mouse model. J. Control. Release 2016, 235, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Gotz, J. Scanning ultrasound removes amyloid-beta and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra233. [Google Scholar] [CrossRef] [PubMed]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; Park, J.; McDannold, N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J. Control. Release 2013, 169, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E. Can sound open the brain for therapies? Science 2015, 347, 1186–1187. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re342. [Google Scholar] [CrossRef] [PubMed]
- Trubestein, G.; Engel, C.; Etzel, F.; Sobbe, A.; Cremer, H. Thrombolysis by ultrasound. Clin. Sci. Mol. Med. Suppl. 1976, 3, 697s–698s. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Tachibana, S. Albumin Microbubble Echo-Contrast Material as an Enhancer for Ultrasound Accelerated Thrombolysis. Circulation 1995, 92, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Roessler, F.C.; Wang, Z.; Schumacher, S.; Ohlrich, M.; Kaps, M. In Vitro Examination of the Thrombolytic Efficacy of Desmoteplase and Therapeutic Ultrasound Compared with rt-PA. Ultrasound Med. Biol. 2015, 41, 3233–3240. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, N.; Tomkins, A.J.; Kampschulte, M.; Hyvelin, J.M.; Botteron, C. Sonothrombolysis with BR38 Microbubbles Improves Microvascular Patency in a Rat Model of Stroke. PLoS ONE 2016, 11, e0152898. [Google Scholar] [CrossRef] [PubMed]
- Petit, B.; Yan, F.; Bussat, P.; Bohren, Y.; Gaud, E. Fibrin degradation during sonothrombolysis- Effect of ultrasound, microbubbles and tissue plasminogen activator. J. Drug Deliv. Sci. Technol. 2015, 25, 29–35. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Huang, R.; Chen, G.; Zhong, L. Microbubble-Mediated Sonothrombolysis Improves Outcome after Thrombotic Microembolism-Induced Acute Ischemic Stroke. Stroke 2016, 47, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.M.; Huang, P.H.; Riedl, L.; Eisenhauer, A.C.; Sobieszczyk, P. Usefulness and safety of ultrasound-assisted catheter-directed thrombolysis for submassive pulmonary emboli. Am. J. Cardiol. 2015, 115, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Vancraeynest, D.; Havaux, X.; Pouleur, A.; Pasquet, A.; Gerber, B. Myocardial delivery of colloid nanoparticles using ultrasound-targeted microbubble destruction. Eur. Heart J. 2006, 27, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Z.; Lu, C.T.; Li, X.K.; Tang, Q.Q.; Tian, X.Q. Improving the cardio protective effect of aFGF in ischemic myocardium with ultrasound-mediated cavitation of heparin modified microbubbles: Preliminary experiment. J. Drug Target 2012, 20, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Li, S.H.; Wu, J.; Miyagi, Y.; Yau, T.M. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur. Heart J. 2011, 32, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.H.; Chung, H.Y.; Chen, W.S.; Yeh, M.K. Efficacy of Combined Ultrasound-and-Microbubbles-Mediated Diclofenac Gel Delivery to Enhance Transdermal Permeation in Adjuvant-Induced Rheumatoid Arthritis in the Rat. Ultrasound Med. Biol. 2016, 42, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Tang, Y.; Leng, Q.; Zhang, L.; Qiu, L. Targeted gene delivery to the synovial pannus in antigen-induced arthritis by ultrasound-targeted microbubble destruction in vivo. Ultrasonics 2016, 65, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, K.; Wang, J.; Bai, S.; Jiao, S. Design of simvastatin-loaded polymeric microbubbles as targeted ultrasound contrast agents for vascular imaging and drug delivery in the identification of atherosclerotic plaque. New J. Chem. 2016, 40, 1256–1262. [Google Scholar] [CrossRef]
- Chertok, B.; Langer, R.; Anderson, D.G. Spatial Control of Gene Expression by Nanocarriers Using Heparin Masking and Ultrasound-Targeted Microbubble Destruction. ACS Nano 2016, 10, 7267–7278. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Hsu, P.H.; Huang, C.W.; Hsieh, W.C.; Huang, F.T.; Chang, W.C.; Chiu, H.; Hsu, S.T.; Yen, T.C. Evaluation of prognostic integrin α2β1 PET tracer and concurrent targeting delivery using focused ultrasound for brain glioma detection. Mol. Pharm. 2014, 11, 3904–3914. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, L.; Zhong, L.; Kutty, S.; Wang, Y. Delivery of Hydrogen Sulfide by Ultrasound Targeted Microbubble Destruction Attenuates Myocardial Ischemia-reperfusion Injury. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Jiang, Y.; Luo, F.; Li, P. Effectiveness of localized ultrasound-targeted microbubble destruction with doxorubicin liposomes in H22 mouse hepatocellular carcinoma model. J. Drug Target 2015, 23, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Shu, S.; Wang, Z.; Luo, J.; Zhong, W. Enhanced homing of mesenchymal stem cells to the ischemic myocardium by ultrasound-targeted microbubble destruction. Ultrasonics 2012, 52, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Gao, Y.H.; Liu, Z.; Tan, K.B.; Hua, X. Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic ultrasound-mediated microbubble destruction improves cardiac function in myocardial infarction of New Zealand rabbits. Int. J. Cardiol. 2010, 138, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; You, X.; Xu, Q.; Gao, F.; Xie, X. Protein expression of mesenchymal stem cells after transfection of pcDNA3.1−-hVEGF165 by ultrasound-targeted microbubble destruction. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Q.; Zhuo, Z.; Wu, S.; Xu, Y. Enhanced Homing of CXCR-4 Modified Bone Marrow-Derived Mesenchymal Stem Cells to Acute Kidney Injury Tissues by Micro-Bubble-Mediated Ultrasound Exposure. Ultrasound Med. Biol. 2016, 42, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, L.; Wang, G.; Shen, W.; Xu, Y. Ultrasound-targeted stromal cell-derived factor-1-loaded microbubble destruction promotes mesenchymal stem cell homing to kidneys in diabetic nephropathy rats. Int. J. Nanomed. 2014, 9, 5639–5651. [Google Scholar]
- Zhang, Y.; Ye, C.; Wang, G.; Gao, Y.; Tan, K. Kidney-targeted transplantation of mesenchymal stem cells by ultrasound-targeted microbubble destruction promotes kidney repair in diabetic nephropathy rats. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, J.; Prausnitz, M.R. Can ultrasound enable efficient intracellular uptake of molecules? A retrospective literature review and analysis. Ultrasound Med. Biol. 2012, 38, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, D.; Xie, X.; Shen, P.; Zeng, Y. Utility of contrast-enhanced ultrasound with SonoVue in biopsy of small subpleural nodules. Int. J. Clin. Exp. Med. 2015, 8, 15991–15998. [Google Scholar] [PubMed]
- Dijkmans, P.A.; Juffermans, L.J.; van Dijk, J.; Musters, R.J.P.; Spreeuwenberg, M.D.; Kamp, O. Safety and feasibility of real time adenosine myocardial contrast echocardiography with emphasis on induction of arrhythmias: A study in healthy volunteers and patients with stable coronary artery disease. Echocardiography 2009, 26, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Beaton, C.; Cochlin, D.; Kumar, N. Contrast enhanced ultrasound should be the initial radiological investigation to characterize focal liver lesions. Eur. J. Surg. Oncol. 2010, 36, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Kaps, M.; Seidel, G.; Bokor, D.; Modrau, B.; Algermissern, C. Safety and ultrasound-enhancing potentials of a new sulfur hexafluoride-containing agent in the cerebral circulation. J. Neuroimaging 1999, 9, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, X.; Chen, X.; Liao, L.; Pan, R.; Zhou, N.; Di, N. Value of three-dimensional hystero—Salpingo-contrast sonography with SonoVue in the assessment of tubal patency. Ultrasound Obstet. Gynecol. 2012, 40, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.S.; Park, J.H. Ultrasound-mediated gene delivery. Expert Opin. Drug. Deliv. 2010, 7, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liang, K.; Xie, M.; Wang, X.; Lü, Q.; Zhang, J. Induced apoptosis with ultrasound-mediated microbubble destruction and shRNA targeting survivin in transplanted tumors. Adv. Ther. 2009, 26, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Z.; Li, H.L.; Du, L.F.; Wang, H.P.; Gu, Q. Comparative analysis of gene transfer to human and rat retinal pigment epithelium cell line by a combinatorial use of recombinant adeno-associated virus and ultrasound or/and microbubbles. Bosn. J. Basic Med. Sci. 2009, 9, 174–181. [Google Scholar] [PubMed]
| UCAs | Albunex | Optison | Levovist | SonoVue | Definity | Sonazoid |
|---|---|---|---|---|---|---|
| Manufacturer | Molecular Biosystems | Amersham | Schering AG. | Bracco Diagnostics | Bristol-Myers Squibb Medical Imaging | Nycomed/Amersham |
| Composition | Albumin | Albumin | Lipids/Galactose | Lipids | Lipid/Surfactant | Lipid |
| Gas core | Air | C3F8 | Air | SF6 | C3F8 | C4F10 |
| Size | 4.3 μm | 2–4.5 μm | 2–4 μm | 2–3 μm | 1.1–3.3 μm | 2–2.8 μm |
| Application | Cardiovascular imaging | Cardiovascular imaging, myocardial perfusion imaging, thermoablation of tumors, gene therapy | Cardiovascular imaging, general vascular imaging, abdominal visceral imaging, sonothrombolysis | Cardiovascular imaging, general vascular imaging, tumor imaging, carotid plaque imaging, ultrasonic positioning, gene therapy, drug delivery, sonothrombolysis | Cardiovascular imaging, myocardial perfusion imaging, general vascular imaging, tumor imaging, gene delivery, thrombolysis | Tumor imaging Guiding treatment and assessing treatment response |
| Reference | [9,10] | [8,11,12,13] | [14,15,16,17] | [18,19,20,21,22,23,24,25] | [11,26,27,28,29,30] | [31,32] |
| Target | Ligand | Tumor Model | Reference |
|---|---|---|---|
| VEGFR2/KDR | Heterodimeric peptide (BR55) | Colon carcinoma | [45] |
| VEGFR2 | Heterodimeric peptide (BR55) | Mammary carcinoma, Breast cancer, Prostate adenocarcinoma, Renal cell carcinoma | [46,47,48] |
| VEGFR2 | Antibody | Breast cancer, Pancreatic cancer, Squamous cell carcinoma, Angiosarcoma (SVR), Glioma (C6) | [49,50,51] |
| αvβ3 | Cyclic RGD peptide | Breast cancer | [33] |
| αvβ3 | RGD | Squamous cell carcinoma | [52] |
| αvβ3 | Echistatin | Glioma | [53] |
| αvβ3 | Knottin | Ovarian cancer | [54] |
| Endoglin (CD105) | Antibody | Pancreatic cancer | [50] |
| SFRP2 | Antibody | Angiosarcoma (SVR) | [39] |
| VEGFR2, αVβ3, Endoglin (CD105) | Antibody | Subcutaneous cancers (breast, ovarian, pancreatic cancer) | [55] |
| VEGFR2, αVβ3, P-selectin | Antibody | Breast cancer | [37] |
| αVβ3, ICAM-1 | Antibody | Prostate cancer | [56] |
| VEGFR2 + αvβ3 | Antibody | Ovarian cancer | [57] |
| VEGFR2 + αvβ3 + ICAM1 | Antibody | Human MDA-MB-231 breast cancer | [58] |
| CRPPR, ATWLPPR | Antibody | Breast cancer | [59] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, X.; Ye, J.; Wang, L.; Xu, S.; Zou, C.; Yang, Y.; Liu, Z. Advanced Microbubbles as a Multifunctional Platform Combining Imaging and Therapy. Appl. Sci. 2016, 6, 365. https://doi.org/10.3390/app6110365
Ni X, Ye J, Wang L, Xu S, Zou C, Yang Y, Liu Z. Advanced Microbubbles as a Multifunctional Platform Combining Imaging and Therapy. Applied Sciences. 2016; 6(11):365. https://doi.org/10.3390/app6110365
Chicago/Turabian StyleNi, Xianwei, Jinmin Ye, Liping Wang, Shunlong Xu, Chunpeng Zou, Yan Yang, and Zhe Liu. 2016. "Advanced Microbubbles as a Multifunctional Platform Combining Imaging and Therapy" Applied Sciences 6, no. 11: 365. https://doi.org/10.3390/app6110365






