Synthesis and Anticancer Activity of Novel Thiazole-5-Carboxamide Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
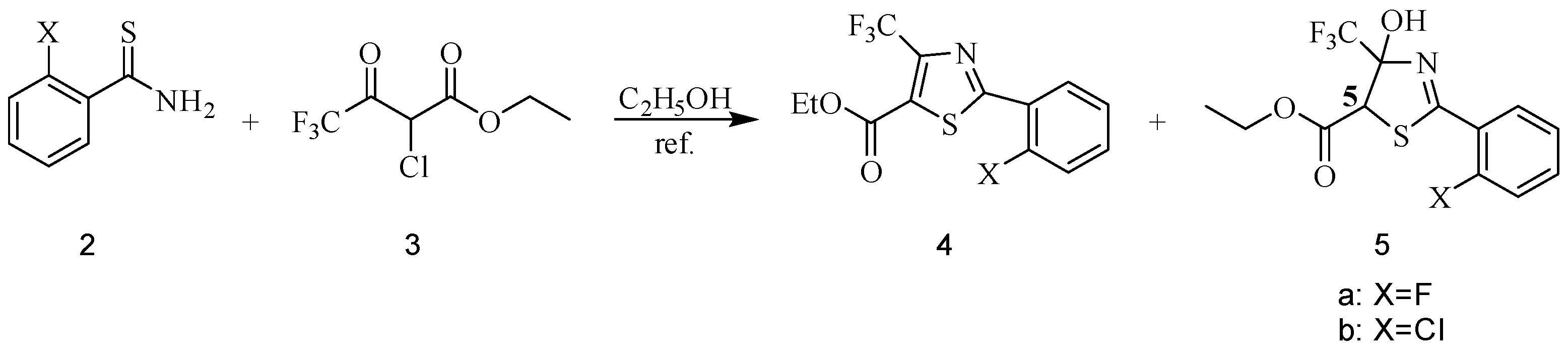
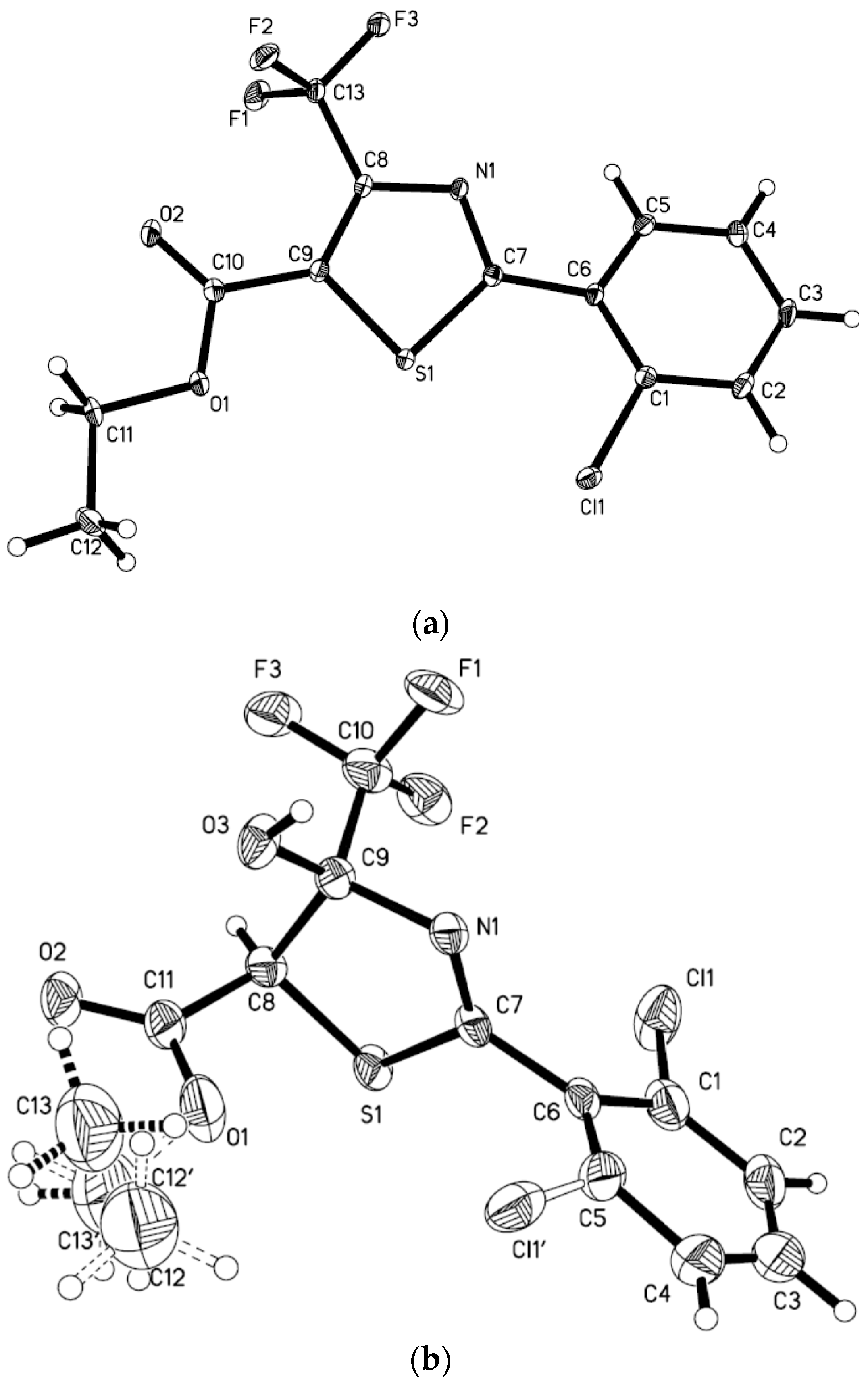
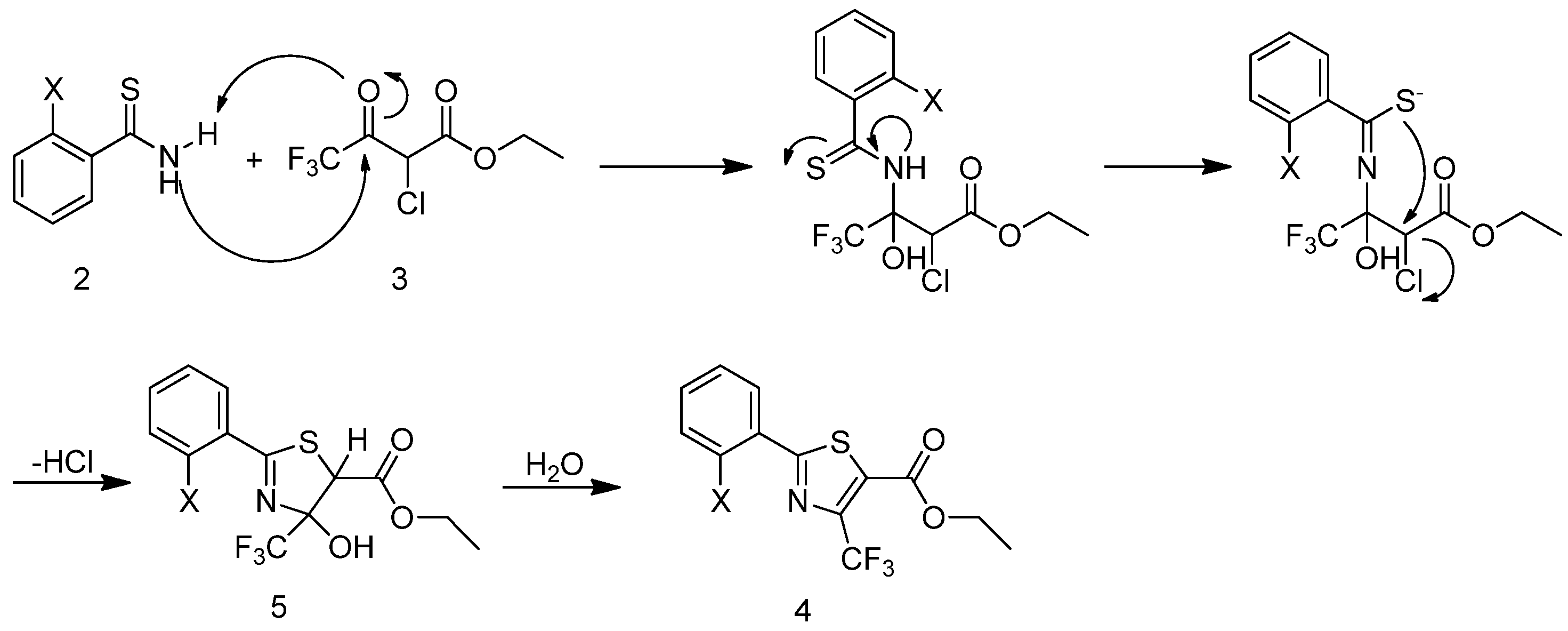
2.2. Anticancer Activities
| No. | A-549 | Bel7402 | HCT-8 |
|---|---|---|---|
| 7a | 36 | 10 | 2 |
| 7b | 24 | 0 | 19 |
| 7c | 16 | 0 | −7 |
| 7d | −2 | 6 | −5 |
| 7e | 4 | 9 | 17 |
| 7f | −19 | 12 | 40 |
| 8a | 27 | 6 | 1 |
| 8b | 27 | 22 | 2 |
| 8c | 48 | 5 | 28 |
| 8d | 37 | 16 | 8 |
| 8f | 40 | 16 | 2 |
| 5-Fluorouracil | 57 | 75 | 79 |
3. Experimental Section
3.1. Instruments
3.2. Synthesis
3.2.1. Preparation of 1
3.2.2. Preparation of 2 [40]
3.2.3. Preparation of 3 [42]
3.2.4. Preparation of 4 [44]
3.2.5. Preparation of 6 (Scheme 3)

3.2.6. Synthesis of Target Compounds (7a–f)
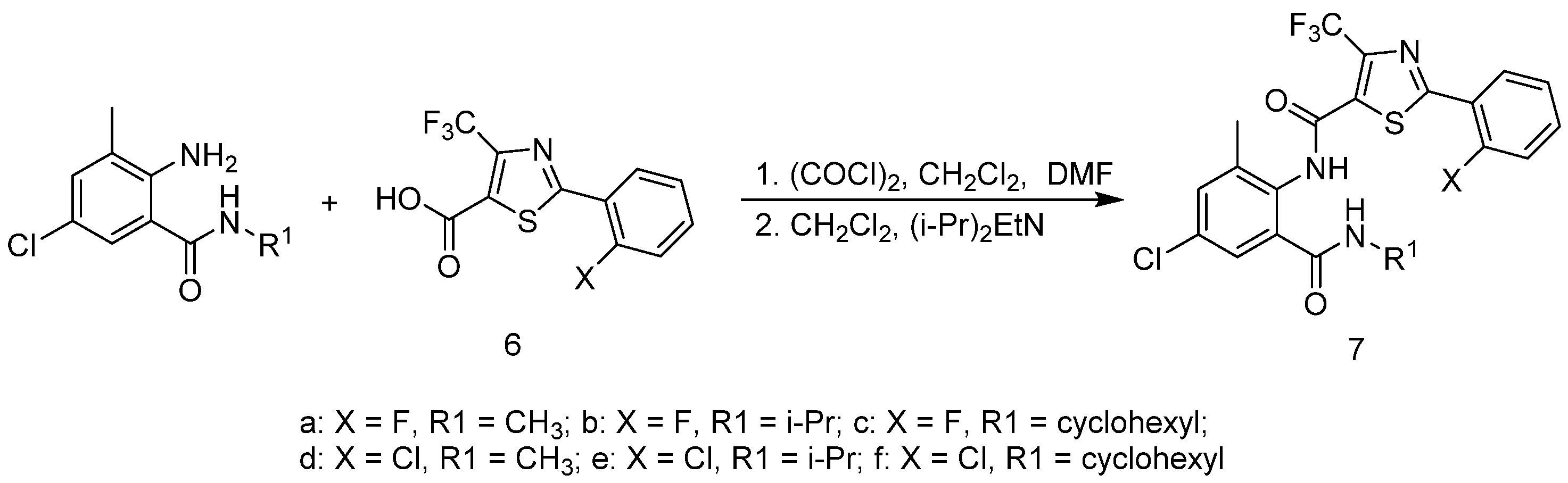
3.2.7. Synthesis of Target Compounds (8a–g)
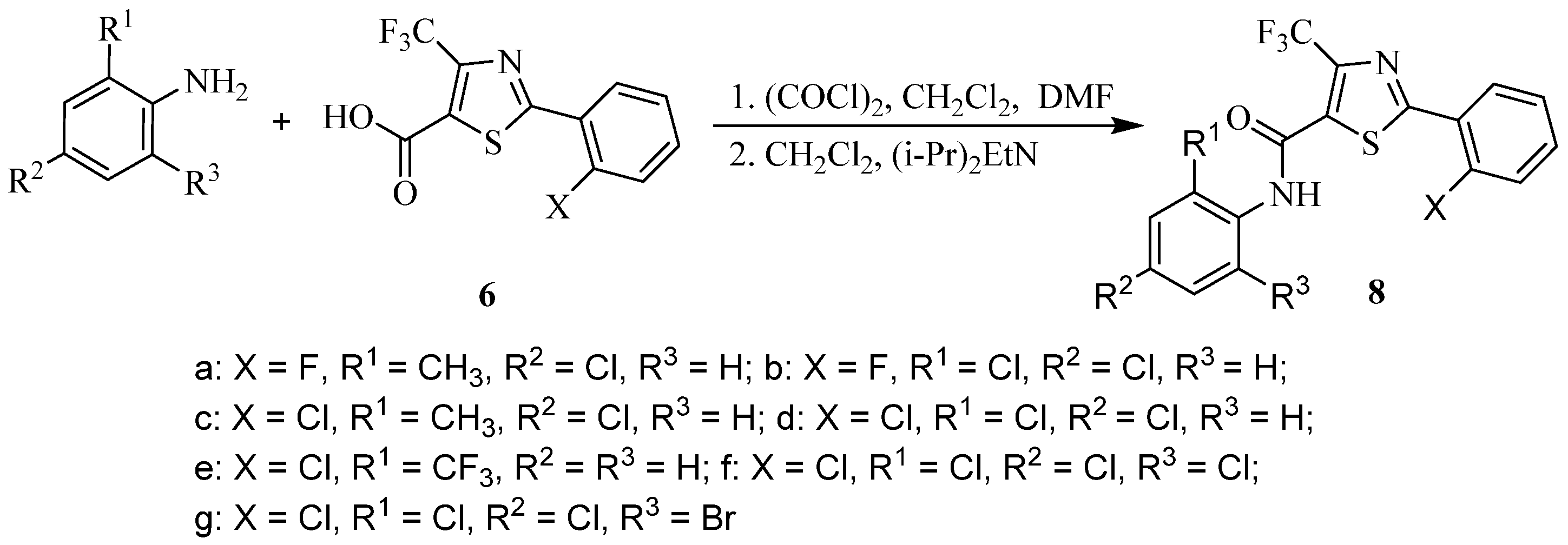
3.3. Anticancer Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M. Novel thiazole derivatives: A patent review (2008–2012. Part 2). Exp. Opin. Ther. Pat. 2014, 24, 759–777. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Oanh, D.T.K.; Dung, P.T.P.; Hue, V.T.M.; Park, S.H.; Han, B.W.; Kim, Y.; Hong, J.T.; Han, S.B.; Nam, N.H. New benzothiazole/thiazole-containing hydroxamic acids as potent histone deacetylase inhibitors and antitumor agents. Med. Chem. 2013, 9, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Al-Said, M.S. Antitumor activity of novel pyridine, thiophene and thiazole derivatives. Arc. Pharm. Res. 2012, 35, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Li, D.D.; Wang, R.R.; Sun, J.; Dong, J.J.; Du, Q.R.; Fang, F.; Zhang, W.M.; Zhu, H.L. Design and synthesis of thiazole derivatives as potent FabH inhibitors with antibacterial activity. Eur. J. Med. Chem. 2014, 75, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.D.; Yang, H.W.; Ma, S.; Li, W.W.; Zhang, C.H.; Wang, W.J.; Xiang, R.; Li, L.L.; Yang, S.Y. Discovery of 6-phenylimidazo [2,1-b]thiazole derivatives as a new type of FLT3 inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 4534–4538. [Google Scholar] [CrossRef] [PubMed]
- Geronikaki, A.; Hadjipavlou-Litina, D.; Zablotskaya, A.; Segal, I. Organosilicon-containing thiazole derivatives as potential lipoxygenase inhibitors and anti-inflammatory agents. Bioinorg. Chem. Appl. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Thoma, G.; Veenstra, S.; Strang, R.; Blanz, J.; Vangrevelinghe, E.; Berghausen, J.; Lee, C.C.; Zerwes, H.G. Orally bioavailable Syk inhibitors with activity in a rat PK/PD model. Bioorg. Med. Chem. Lett. 2015, 25, 4642–4647. [Google Scholar] [CrossRef] [PubMed]
- Barradas, J.S.; Errea, M.I.; D’Accorso, N.B.; Sepulveda, C.S.; Damonte, E.B. Imidazo [2,1-b]thiazole carbohydrate derivatives: Synthesis and antiviral activity against Junin virus, agent of Argentine hemorrhagic fever. Eur. J. Med. Chem. 2011, 46, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Grozav, A.; Găină, L.I.; Pileczki, V.; Crisan, O.; Silaghi-Dumitrescu, L.; Therrien, B.; Zaharia, V.; Berindan-Neagoe, I. The Synthesis and Antiproliferative Activities of New Arylidene-Hydrazinyl-Thiazole Derivatives. Int. J. Mol. Sci. 2014, 15, 22059–22072. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Özdemir, A.; Turan-Zitouni, G.; Ilgın, S.; Atlı, Ö.; Demirci, F.; Kaplancıklı, Z.A. Synthesis and in Vitro Evaluation of New Nitro-Substituted Thiazolyl Hydrazone Derivatives as Anticandidal and Anticancer Agents. Molecules 2014, 19, 14809–14820. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kumar, R.; Srivastava, C.; Dureja, P.; Khurana, J.M. Synthesis, biological activities and SAR studies of novel 1-ethyl-7-methyl-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid based diacyl and sulfonyl acyl hydrazines. Pest Manag. Sci. 2014, 70, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Tan, C.X.; Weng, J.Q. Phase transfer-catalyzed, one-pot synthesis of some novel N-pyrimidinyl-N′-picotinyl thiourea derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 552–557. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhao, W.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Liu, X.H. Synthesis and Biological Activity of Acylthiourea Derivatives Contain 1,2,3-Thiadiazole and 1,3,4-Thiadiazole. Lett. Drug Des. Discov. 2015, 12, 314–318. [Google Scholar] [CrossRef]
- Liu, X.H.; Weng, J.Q.; Wang, B.L.; Li, Y.H.; Tan, C.X.; Li, Z.M. Microwave-assisted synthesis of novel fluorinated 1,2,4-triazole derivatives, and study of their biological activity. Res. Chem. Intermed. 2014, 40, 2605–2612. [Google Scholar] [CrossRef]
- Chen, K.; Liu, Q.; Ni, J.P.; Zhu, H.J.; Li, Y.F.; Wang, Q. Synthesis, insecticidal activities and structure-activity relationship studies of novel anthranilic diamides containing pyridylpyrazole-4-carboxamide. Pest Manag. Sci. 2015, 71, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Pan, L.; Ma, Y.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Shi, Y.X.; Li, B.J.; Li, Z.M.; Zhang, Y.G. Design, synthesis, biological activities, and 3D-QSAR of new N,N′-diacylhydrazines containing 2-(2,4-dichlorophenoxy)propane moiety. Chem. Biol. Drug Des. 2011, 78, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Samala, G.; Nallangi, R.; Devi, P.B.; Saxena, S.; Yadav, R.; Sridevi, J.P.; Yogeeswari, P.; Sriram, D. Identification and development of 2-methylimidazo[1,2-a]pyridine-3-carboxamides as mycobacterium tuberculosis pantothenate synthetase inhibitors. Bioorg. Med. Chem. 2014, 22, 4223–4232. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-L.; Xu, J.-Y.; Xiong, L.-X.; Li, Z.-M. Synthesis, Structure and Insecticidal Activities of Some Novel Amides Containing N-Pyridylpyrazole Moeities. Molecules 2012, 17, 10414–10428. [Google Scholar] [CrossRef] [PubMed]
- Neagoie, C.; Krchnak, V. Piperazine amide linker for cyclative cleavage from solid support: Traceless synthesis of dihydroquinoxalin-2-ones. ACS Comb. Sci. 2012, 14, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.H.; Elhusseiny, A.F.; Elkony, Y.M.; Mansour, El-S.M. Synthesis and characterization of thermally stable aromatic polyamides and poly(1,3,4-oxadiazole-amide)s nanoparticles containing pendant substituted bezamides. Chem. Cent. J. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Nakao, H.; Harayama, H.; Yamaguchi, M.; Tohnishi, M.; Morimoto, M.; Fujioka, S. Preparation of Phthalamide Derivatives as Insecticides. WO 2,002,088,074, 7 November 2002. [Google Scholar]
- Lahm, G.P.; Selby, T.P.; Frendenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilicdiamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, dimeric crystal, and fungicidal activity of 1-(4-methylphenyl)-2-(5-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-4-phenyl-4H-1,2,4-triazol-3-ylthio)ethanone. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 558–564. [Google Scholar] [CrossRef]
- Tan, C.X.; Weng, J.Q.; Liu, Z.X.; Liu, X.H.; Zhao, W.G. Synthesis, crystal structure and fungicidal activity of a novel 1,2,3-thiadiazole compound. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 990–996. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhai, Z.W.; Xu, X.Y.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Chen, J. Facile and efficient synthesis and biological activity determination of novel 1,2,4-triazolo[4,3-a]pyridin-3(2H)-one derivatives via microwave irradiation. Bioorg. Med. Chem. Lett. 2015, 25, 5524–5528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Yang, M.Y.; Hu, B.Z.; Sun, Z.H.; Liu, X.H.; Weng, J.Q.; Tan, C.X. Microwave-assisted synthesis of novel 8-chloro-[1,2,4]triazolo [4,3-a]pyridine derivatives. Turk. J. Chem. 2015, 39, 867–873. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo[4,3-a]pyridine derivatives. Pest Manag. Sci. 2015, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Zhao, W.; Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, crystal structure and antifungal activity of 4-(5-((2,4-dichlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine. Chin. J. Struct. Chem. 2015, 34, 203–207. [Google Scholar]
- Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-a]pyridines. Chem. Biol. Drug Des. 2014, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.W.; Shi, Y.X.; Yang, M.Y.; Zhao, W.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhang, Y.G. Microwave assisted synthesis and antifungal activity of some novel thioethers containing 1,2,4-triazolo[4,3-a]pyridine moiety. Lett. Drug Des. Discov. 2016, 13. [Google Scholar] [CrossRef]
- Zhai, Z.W.; Yang, M.Y.; Sun, Z.H.; Liu, X.H.; Weng, J.Q.; Tan, C.X. Facile and efficient synthesis of novel 1,2,3-thiadiazole derivatives using microwave irradiation. J. Chem. Res. 2015, 39, 340–342. [Google Scholar] [CrossRef]
- Shen, Z.H.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhao, W.G. Synthesis, Crystal Structure, DFT Studies and Biological Activity of a Novel Schiff Base Containing Triazolo[4,3-a]Pyridine Moiety. Chin. J. Struct. Chem. 2016. [Google Scholar] [CrossRef]
- Zhai, Z.W.; Shi, Y.X.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Liu, X.H.; Li, B.J.; Zhang, Y.G. Synthesis, Crystal Structure, DFT Studies and Antifungal Activity of 5-(4-Cyclopropyl-5-((3-fluorobenzyl)sulfonyl)-4H-1,2,4-triazol-3-yl)-4-methyl-1,2,3-thiadiazole. Chin. J. Struct. Chem. 2016. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhai, Z.W.; Sun, Z.H.; Yu, S.J.; Liu, X.H.; Weng, J.Q.; Tan, C.X.; Zhao, W.G. A facile one-pot synthesis of novel 1,2,4-triazolo[4,3-a]pyridine derivativescontaining the trifluoromethyl moiety using microwave irradiation. J. Chem. Res. 2015, 39, 521–523. [Google Scholar] [CrossRef]
- Sierra, M.L.; Beneton, V.; Boullay, A.B.; Boyer, T.; Brewster, A.G.; Donche, F.; Forest, M.C.; Fouchet, M.H.; Gellibert, F.J.; Grillot, D.A.; et al. Substituted 2-[(4-aminomethyl)phenoxy]-2-methylpropionic acid PPARalpha agonists. 1. Discovery of a novel series of potent HDLc raising agents. J. Med. Chem. 2007, 50, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Tenn, W.J.; Murphy, J.L.; Bim-Merle, J.K.; Brown, J.A.; Junia, A.J.; Price, M.A.; Nagorski, R.W. Amidates as leaving groups: Structure/reactivity correlation of the hydroxide-dependent E1cB-like breakdown of carbinolamides in aqueous solution. J. Org. Chem. 2007, 72, 6075–6083. [Google Scholar] [CrossRef] [PubMed]
- Coats, S.J.; Link, J.S.; Hlasta, D.J. Alternative solvents for elevated-temperature solid-phase parallel synthesis. Application to thionation of amides. Org. Lett. 2003, 5, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Crane, L.J.; Anastassiadou, M.; Stigliani, J.-L.; Baziard-Mouysset, G.; Payard, M. Reactions of some ortho and para halogenated aromatic nitriles with ethylenediamine: Selective synthesis of imidazolines. Tetrahedron 2004, 60, 5325–5330. [Google Scholar] [CrossRef]
- Liu, C.-L.; Li, Z.-M.; Zhong, B. Synthesis and biological activity of novel 2-methyl-4-trifluoromethyl-thiazole-5-carboxamide derivatives. J. Fluor. Chem. 2004, 125, 1287–1290. [Google Scholar] [CrossRef]
- Guo, J.; Erickson, G.A.; Fitzgerald, R.N.; Matsuoka, R.T.; Rafferty, S.W.; Sharp, M.J.; Sickles, B.R.; Wisowaty, J.C. An efficient synthesis of a potent PPARpan agonist. J. Org. Chem. 2006, 71, 8302–8305. [Google Scholar] [CrossRef] [PubMed]
- Sznaidman, M.L.; Haffner, C.D.; Maloney, P.R.; Fivush, A.; Chao, E.; Goreham, D.; Sierra, M.L.; LeGrumelec, C.; Xu, H.E.; Montana, V.G.; et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)—Synthesis and biological activity. Bioorg. Med. Chem. Lett. 2003, 13, 1517–1521. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.-X.; Liu, A.-L.; Li, Z.-M.; Dong, W.-L.; Liu, X.-H.; Sun, N.-B. Synthesis and Anticancer Activity of Novel Thiazole-5-Carboxamide Derivatives. Appl. Sci. 2016, 6, 8. https://doi.org/10.3390/app6010008
Cai W-X, Liu A-L, Li Z-M, Dong W-L, Liu X-H, Sun N-B. Synthesis and Anticancer Activity of Novel Thiazole-5-Carboxamide Derivatives. Applied Sciences. 2016; 6(1):8. https://doi.org/10.3390/app6010008
Chicago/Turabian StyleCai, Wen-Xi, Ai-Lin Liu, Zheng-Ming Li, Wei-Li Dong, Xing-Hai Liu, and Na-Bo Sun. 2016. "Synthesis and Anticancer Activity of Novel Thiazole-5-Carboxamide Derivatives" Applied Sciences 6, no. 1: 8. https://doi.org/10.3390/app6010008
APA StyleCai, W.-X., Liu, A.-L., Li, Z.-M., Dong, W.-L., Liu, X.-H., & Sun, N.-B. (2016). Synthesis and Anticancer Activity of Novel Thiazole-5-Carboxamide Derivatives. Applied Sciences, 6(1), 8. https://doi.org/10.3390/app6010008





