Synthesis and Physical and Chemical Properties of Hypergolic Chemicals such as N,N,N-Trimethylhydrazinium and 1-Ethyl-4-Methyl-1,2,4-Triazolium Salts
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
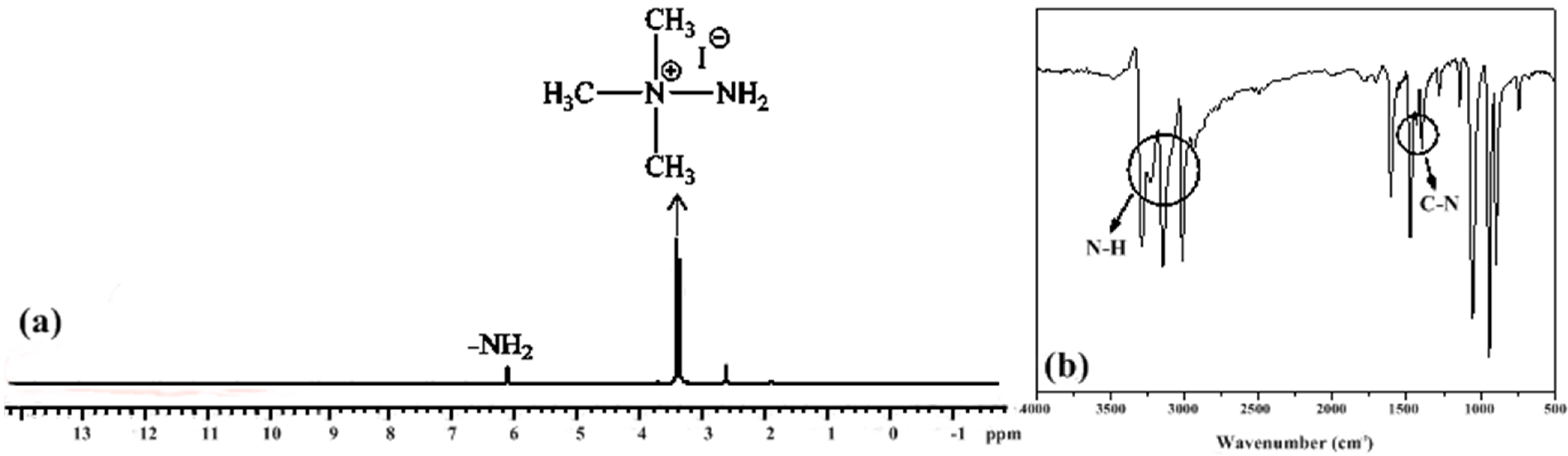
2.2. Synthesis of N,N,N-Trimethylhydrazinium Salts, [TMH]+[X]−
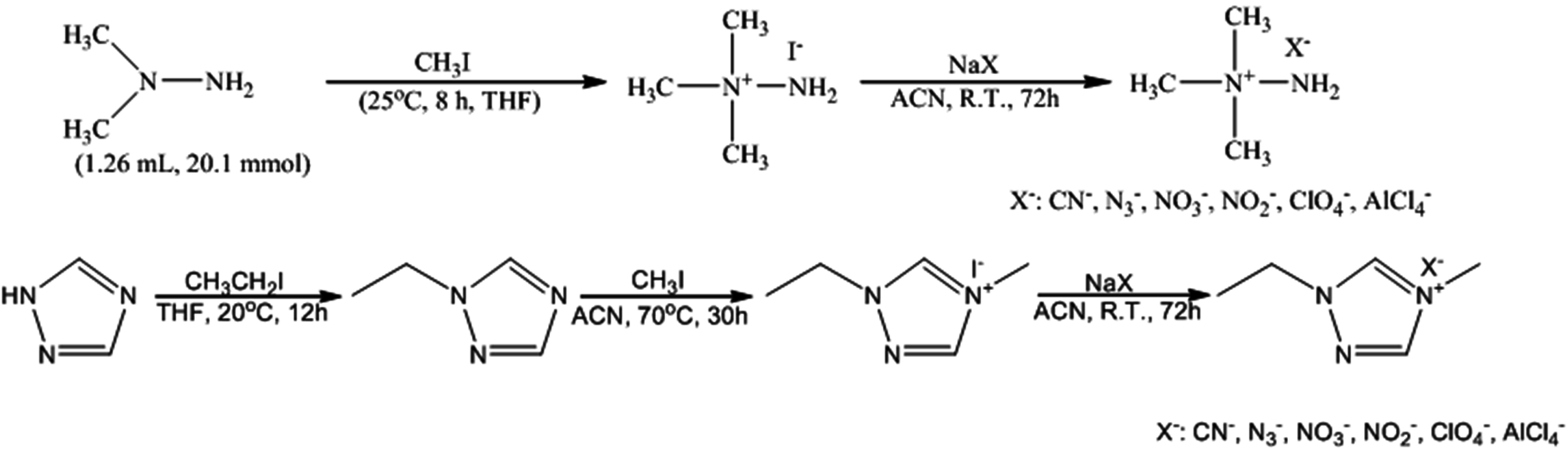
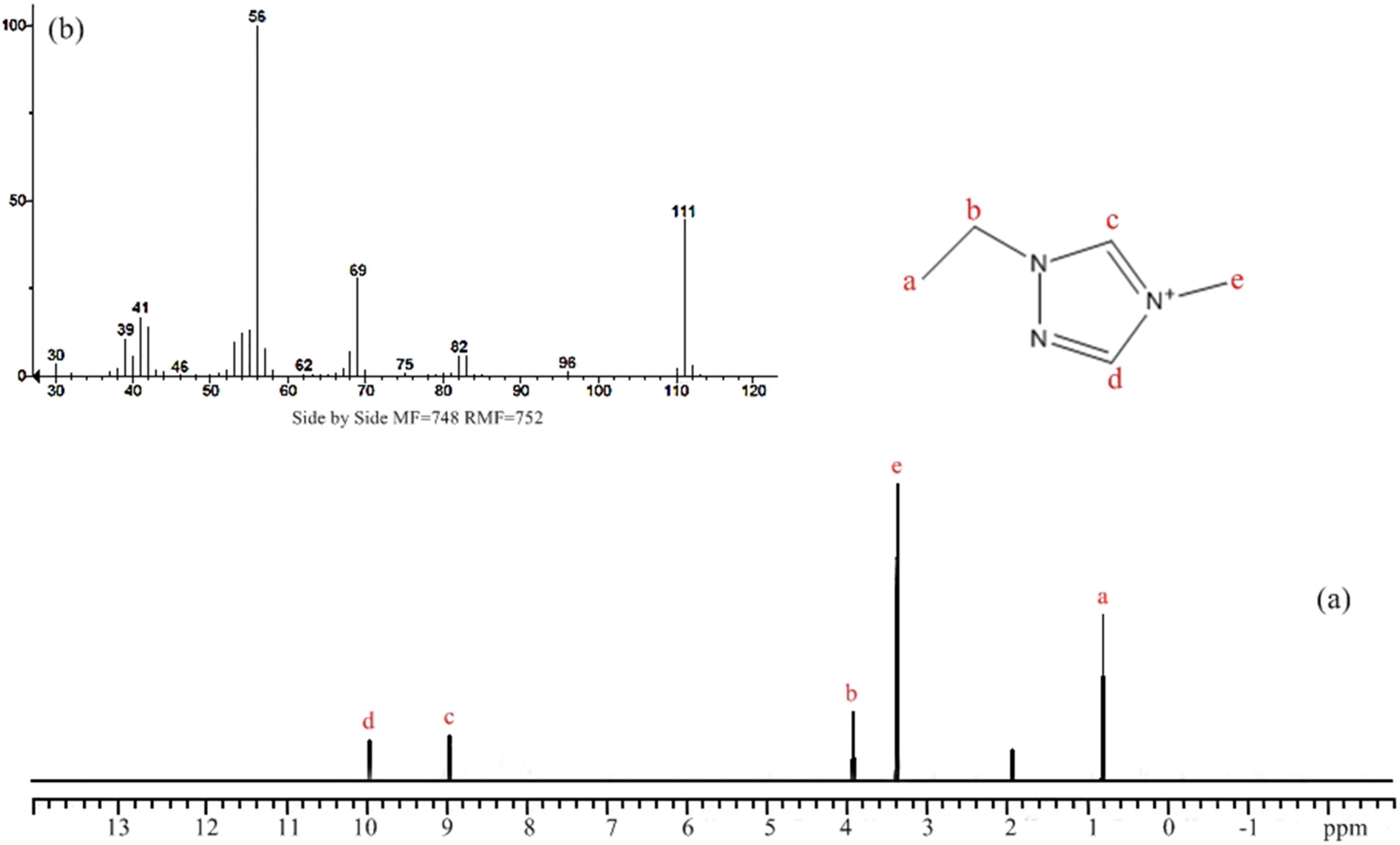
2.3. Synthesis of 1-Ethyl-4-Methyl-1,2,4-Triazolium Salts, [EMT]+[X]−
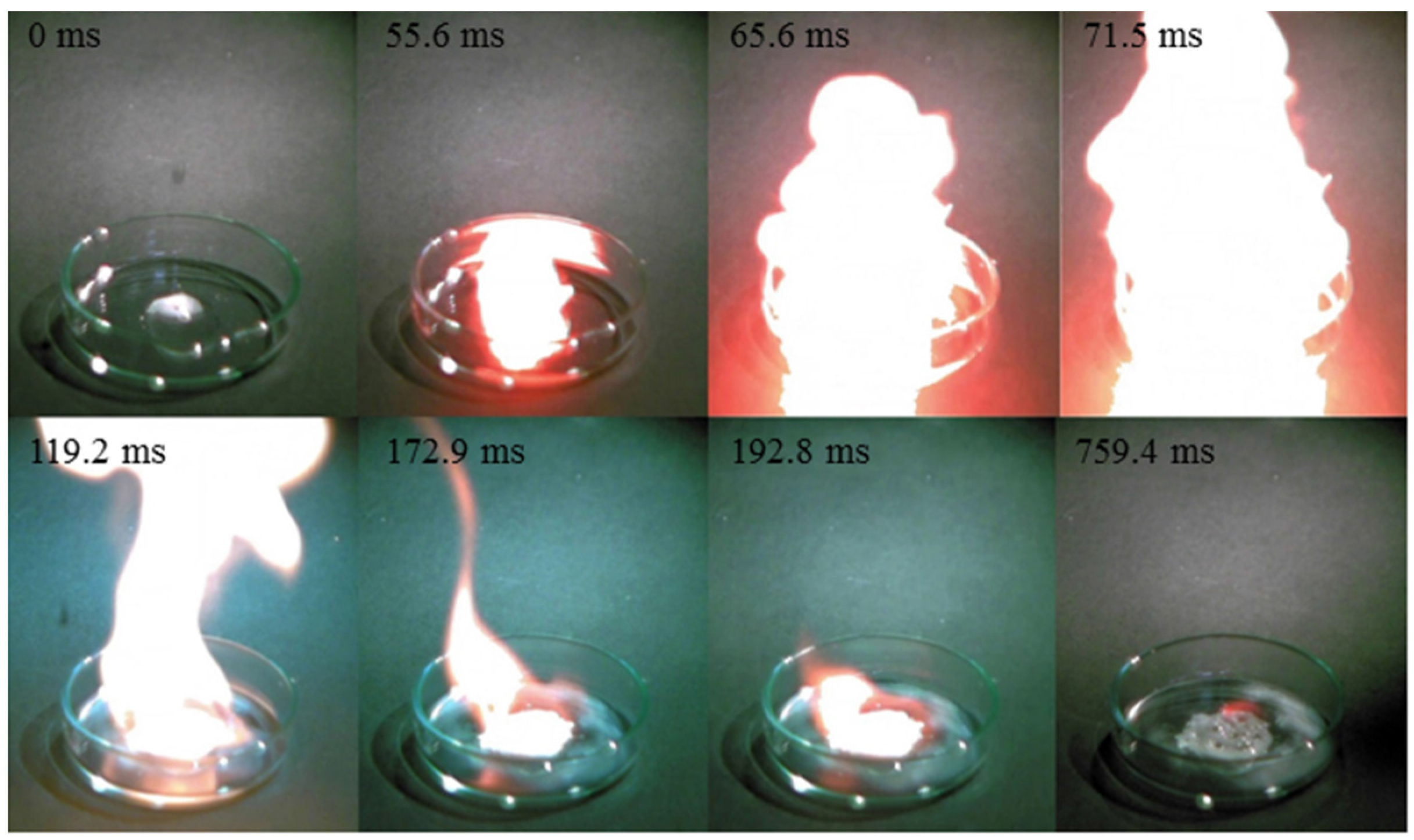
3. Results and Discussion
| Cation | Anion | Td b (°C) | d c (g/cm3) | ∆Hd d (kJ/mol) | η e (cP) | ID f (ms) |
|---|---|---|---|---|---|---|
| C3H11N2+ | N3− | 198.8 | 1.10 | −18.31 | 216 | 55.6 |
| CN− | 192.0 | 1.09 | −50.65 | 220 | 97.4 | |
| NO2− | 190.7 | 1.13 | −41.19 | 243 | 175.0 | |
| NO3− | 195.2 | 1.15 | −46.63 | 185 | 497.2 | |
| ClO4− | 191.0 | 1.13 | −37.63 | 176 | 970.2 | |
| AlCl4− | 184.3 | 1.14 | −80.06 | 162 | 341.0 | |
| I− | 194.2 | 1.09 | −64.52 | 203 | 190.3 |
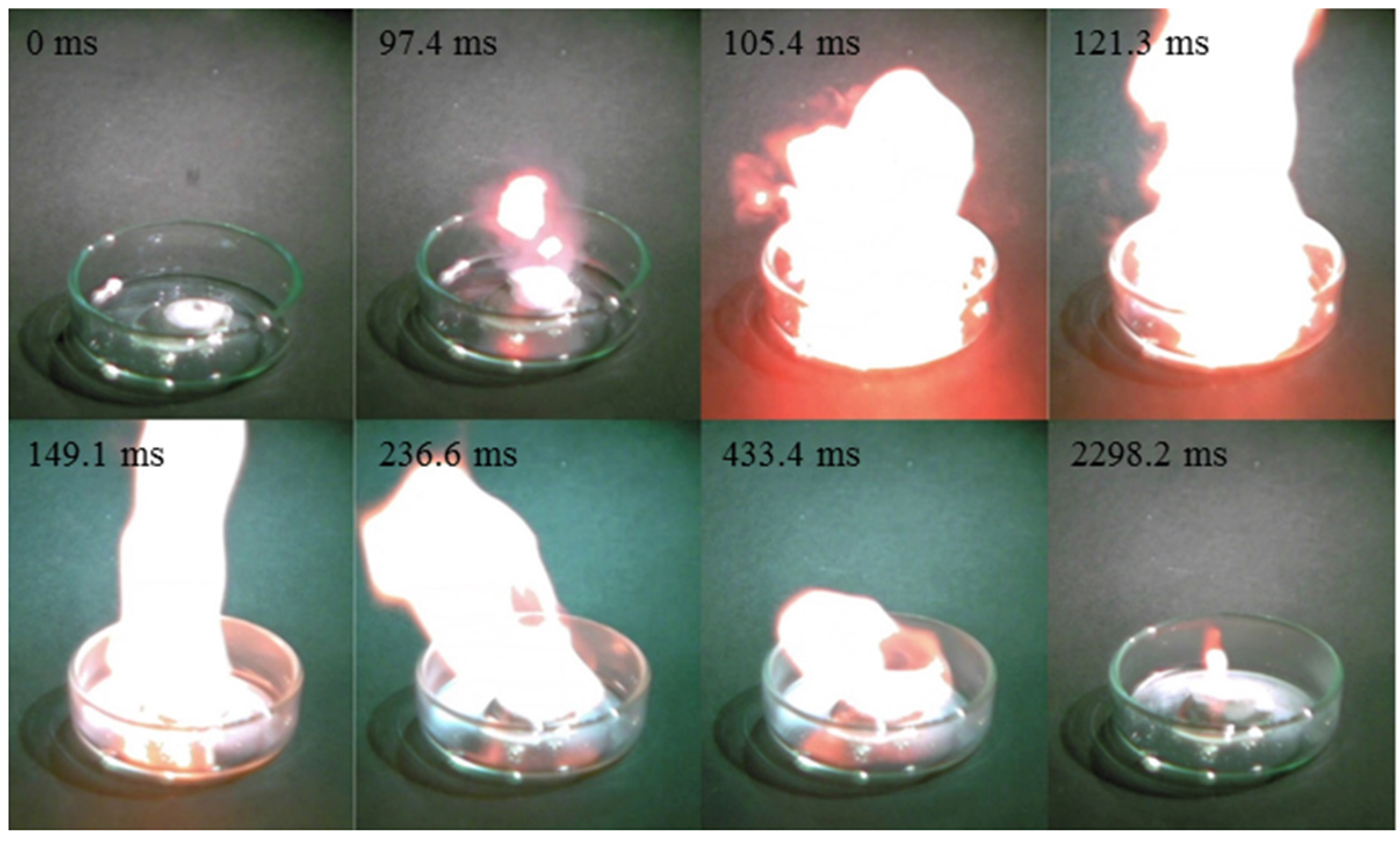
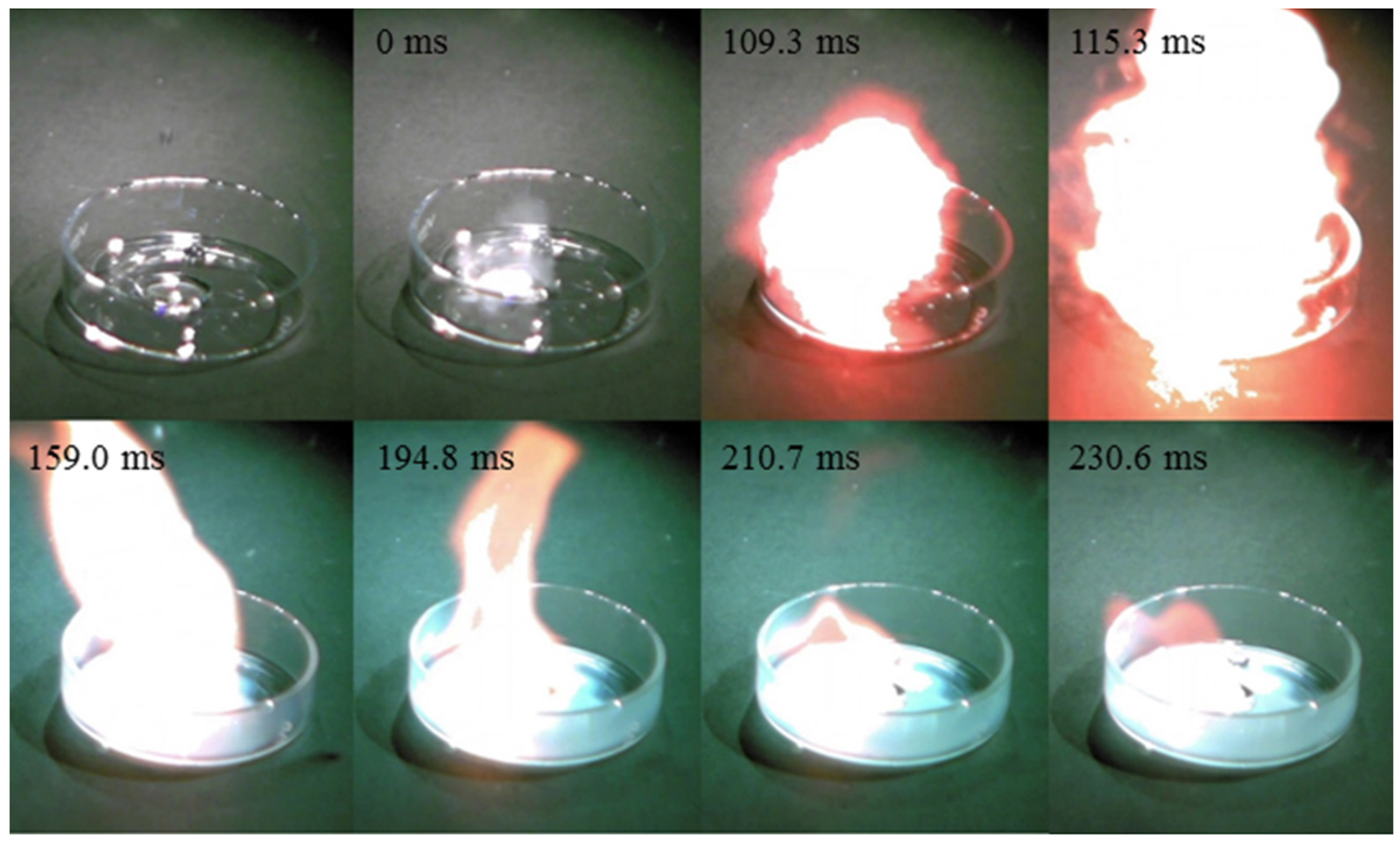
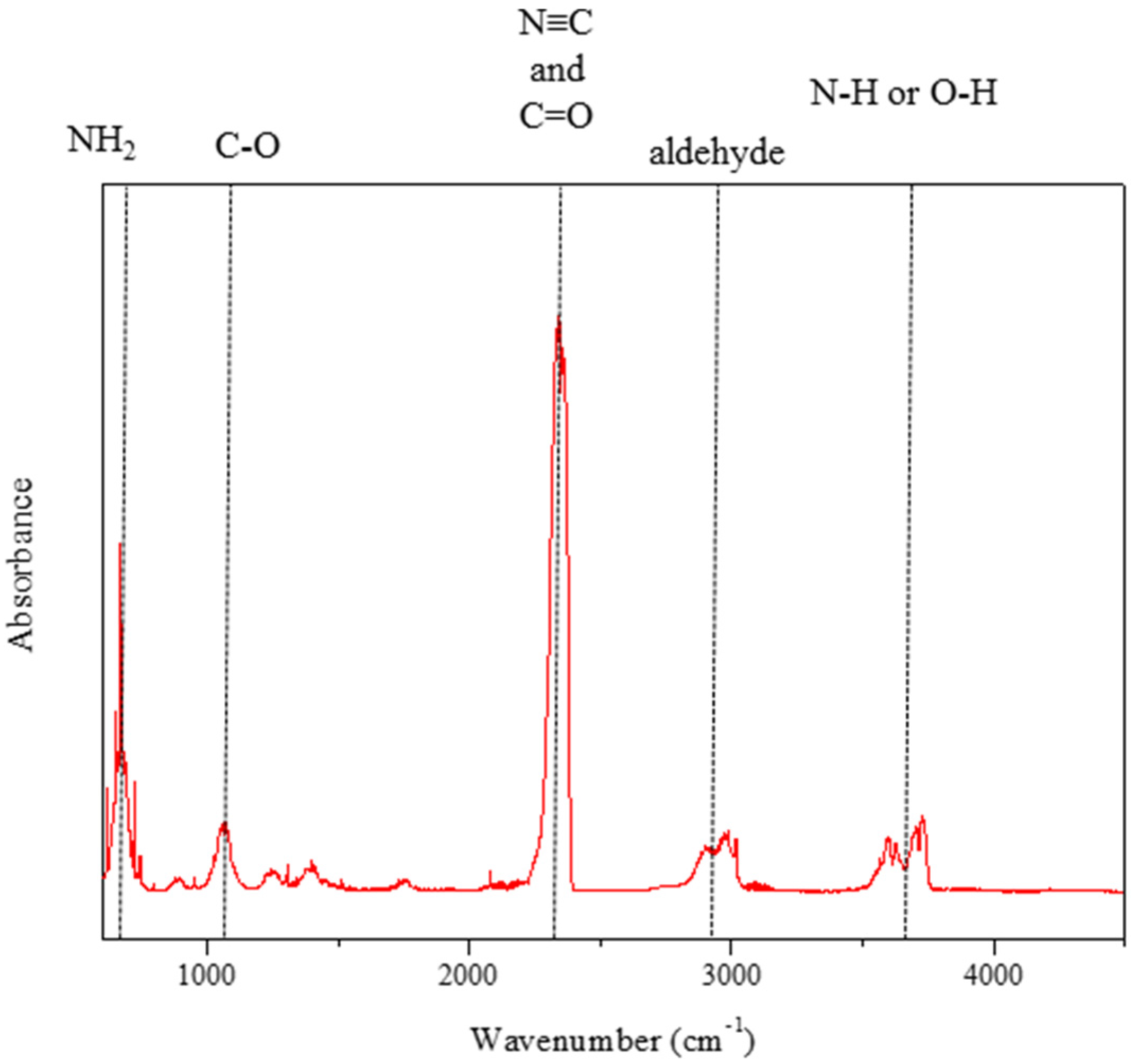
| Cation | Anion | Td b (°C) | d c (g/cm3) | ∆Hd d (kJ/mol) | η e (cP) | ID f (ms) |
|---|---|---|---|---|---|---|
| C5H10N3+ | N3− | 121 | 1.15 | −36.5 | 201 | 18.0 |
| CN− | 175 | 1.19 | −37.8 | 250 | 32.6 | |
| NO2− | 119 | 1.29 | −53.2 | 248 | 489.0 | |
| NO3− | 117 | 1.11 | −22.2 | 115 | 426.0 | |
| ClO4− | 207 | 1.21 | −105.3 | 165 | 524.0 | |
| AlCl4− | 232 | 1.21 | −32.9 | 191 | 27.6 | |
| I− | 136 | 1.22 | −82.4 | 938 | 7.97 |
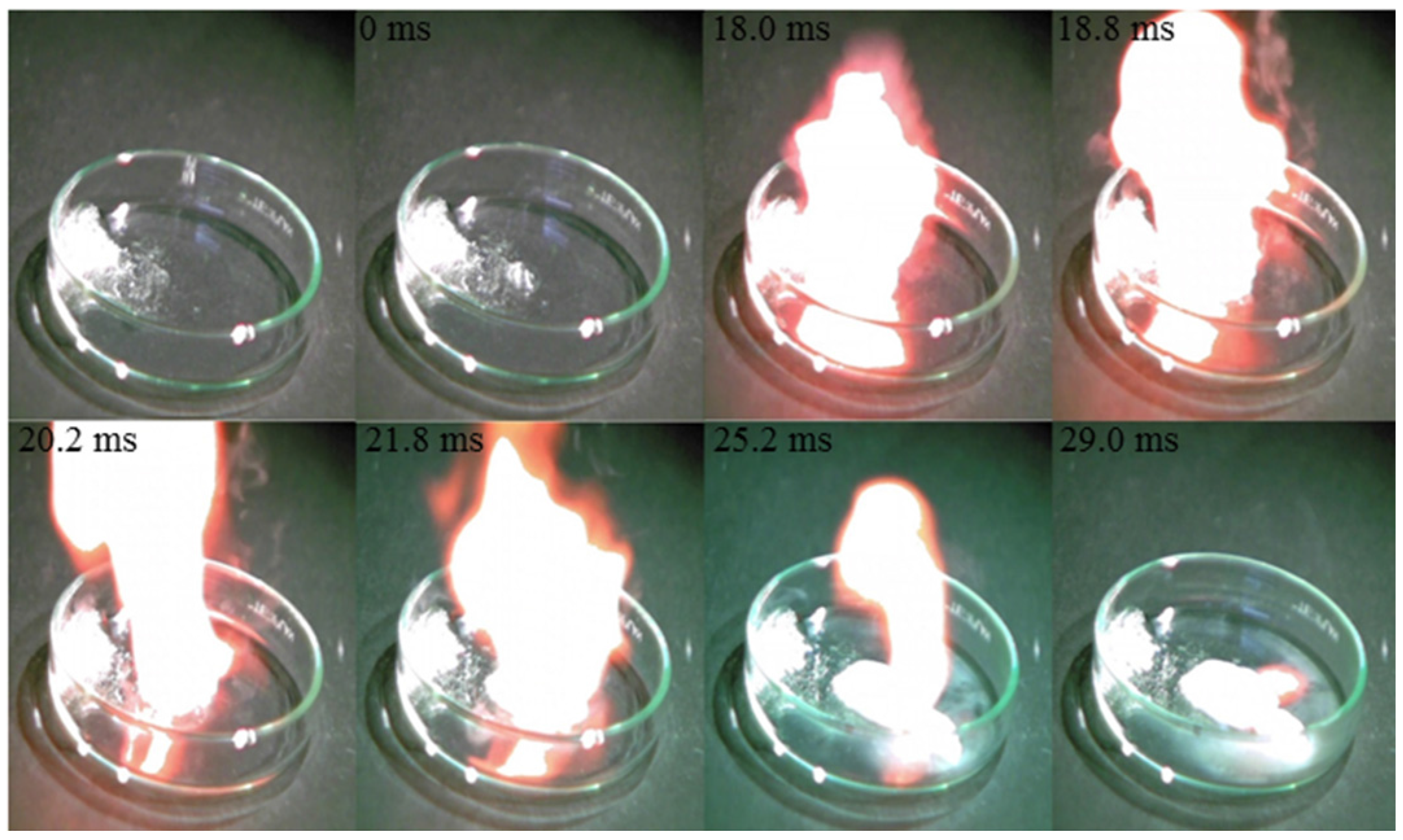
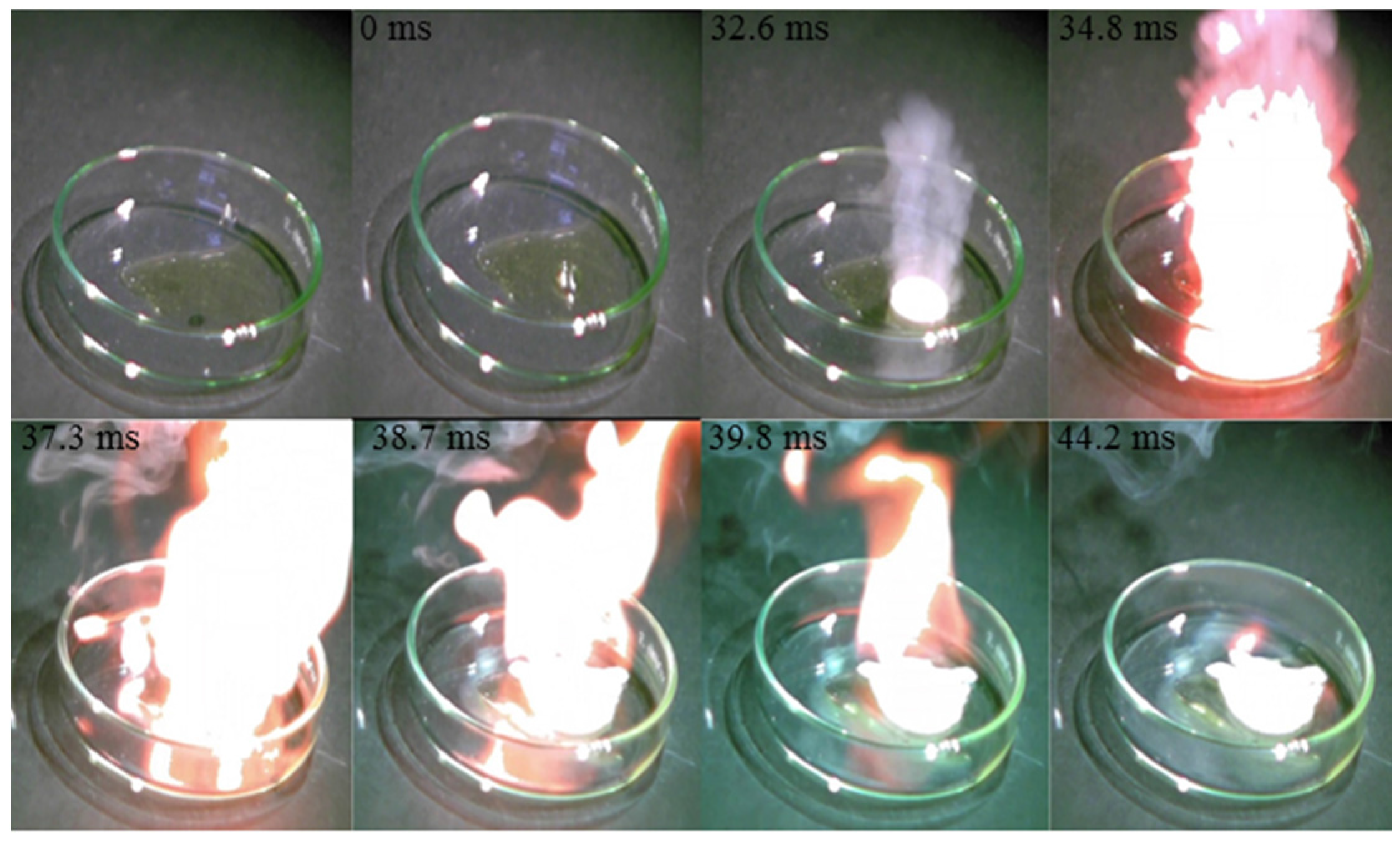
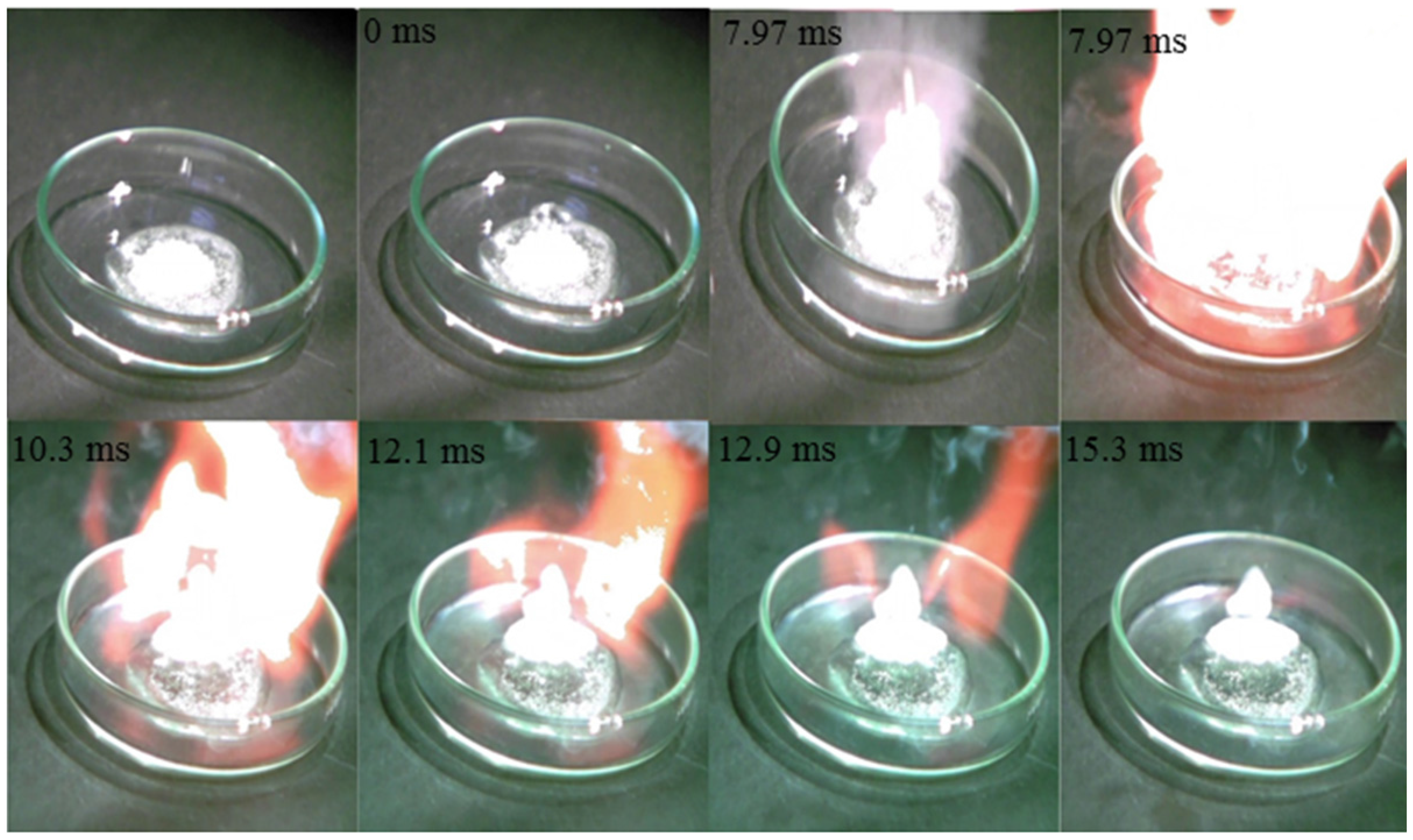
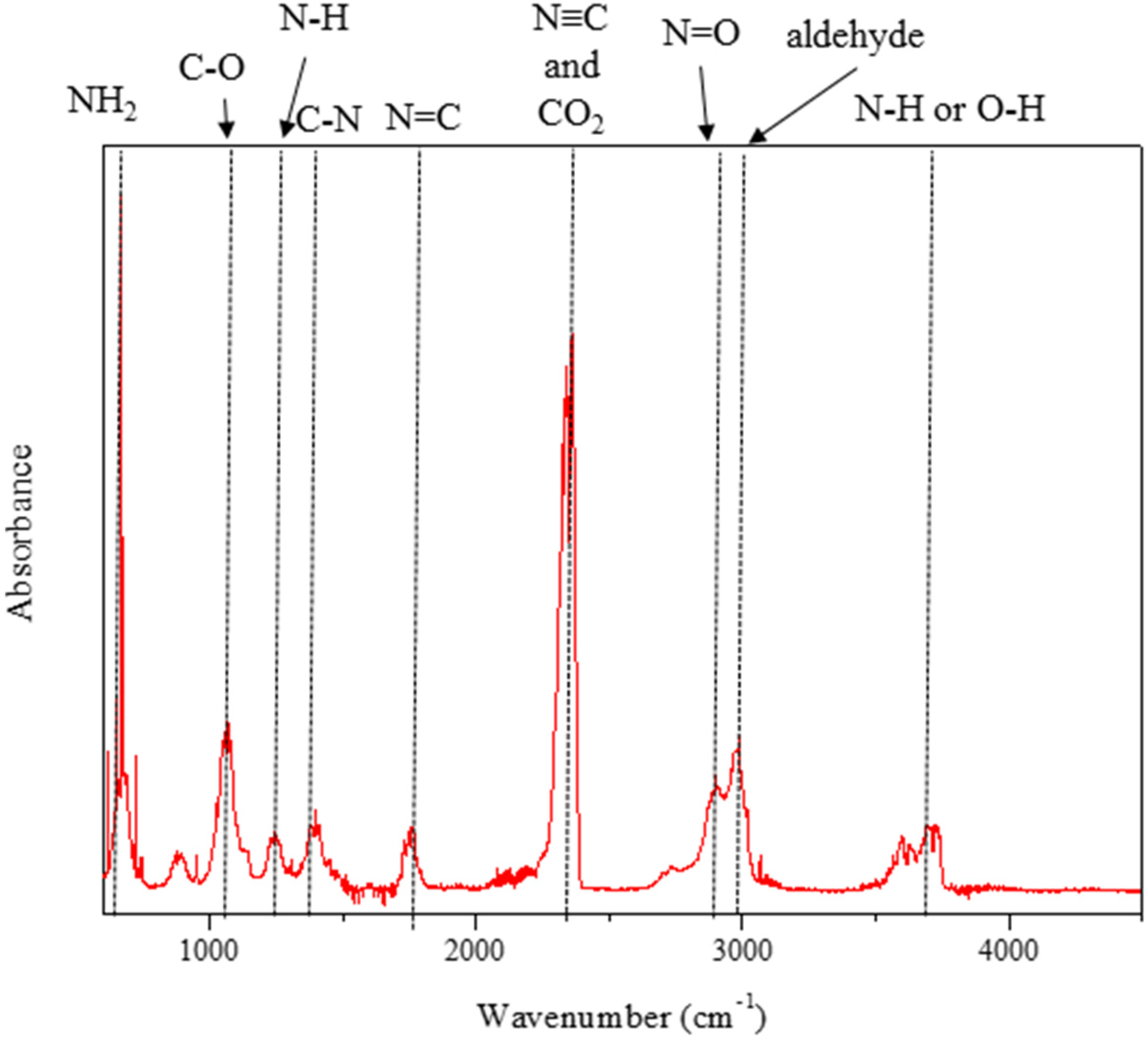
4. Conclusions
- (1)
- The synthesized hypergolic chemicals with [TMH]+[X]− and [EMT]+[X]− have good thermal stability.
- (2)
- The mixture of hypergolic chemicals with [TMH]+[N3]− have a low ID time of 55.6 ms.
- (3)
- The mixture of hypergolic chemicals with [EMT]+[N3]−, [EMT]+[CN]−, and [EMT]+[I]− have very short ID times (18.0, 32.6, and 7.97 ms, respectively).
- (4)
- Toxic nitro group-contained chemicals were not detected after detonation between hypergolic chemicals and H2O2 as oxidizer.
- (5)
- A synthesized mixture hypergolic chemicals such as [TMH]+[N3]−, [EMT]+[N3]−, [EMT]+[CN]−, and [EMT]+[I]− could be used as rocket propellant liquid fuel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Badgujar, D.M.; Talawar, M.B.; Asthana, S.N.; Mahulikar, P.P. Advances in science and technology of modern energetic materials: An overview. J. Hazard. Mater. 2008, 151, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Sebastiao, E.; Cook, C.; Hub, A.; Murugesu, M. Recent developments in the field of energetic ionic liquids. J. Mater. Chem. A 2014, 2, 8153–8173. [Google Scholar] [CrossRef]
- Liau, Y.-C.; Kim, E.S.; Yang, V. A comprehensive analysis of laser-induced ignition of RDX monopropellant. Combust. Flame 2001, 126, 1680–1698. [Google Scholar] [CrossRef]
- Talawar, M.B.; Sivabalan, R.; Mukundan, T.; Muthurajan, H.; Sikder, A.K.; Gandhe, B.R.; Rao, A.S. Environmentally compatible next generation green energetic materials (GEMs). J. Hazard. Mater. 2009, 161, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Ninga, H.; Yudea, L.; Hongpengb, Z.; Chunpenga, L. Research on the TNT equivalence of aluminized explosive. Procedia Eng. 2012, 43, 449–452. [Google Scholar] [CrossRef]
- Sarangapani, R.; Ramavat, V.; Reddy, S.; Subramanian, P.; Sikder, A.K. Rheology studies of NTO-TNT based melt-cast dispersions and influence of particle-dispersant interactions. Powder Technol. 2015, 273, 118–124. [Google Scholar] [CrossRef]
- Sabaté, C.M.; Delalu, H. Energetic salts of symmetrical dimethylhydrazine (SDMH). Eur. J. Inorg. Chem. 2012, 2012, 866–877. [Google Scholar] [CrossRef]
- Pagoria, P.F.; Mitchell, A.R.; Schmidt, R.D. 1,1,1-Trimethylhydrazinium iodide: A novel, highly reactive reagent for aromatic amination via vicarious nucleophilic substitution of hydrogen. J. Org. Chem. 1996, 61, 2934–2935. [Google Scholar] [CrossRef] [PubMed]
- Badgujara, D.M.; Talawar, M.B.; Harlapurc, S.F.; Asthanab, S.N.; Mahulikara, P.P. Synthesis, characterization and evaluation of 1,2-bis(2,4,6-trinitrophenyl) hydrazine: A key precursor for the synthesis of high performance energetic materials. J. Hazard. Mater. 2009, 172, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Klapötke, T.M.; Rienäckera, C.M.; Zewenb, H. Calculated and experimentally obtained heats of combustion of hydrazinium nitrate, monomethylhydrazinium nitrate, and N,N-dimethylhydrazinium nitrate. Z. Anorg. Allg. Chem. 2002, 628, 2372–2374. [Google Scholar] [CrossRef]
- Davis, S.M.; Yilmaz, N. Advances in hypergolic propellants: Ignition, hydrazine, and hydrogen peroxide research. Adv. Aerosp. Eng. 2014. [Google Scholar] [CrossRef]
- Daimon, W.; Tanaka, M.; Kimura, I. The mechanisms of explosions induced by contact of hypergolic liquid propellants, hydrazine and nitrogen tetroxide. Symp. Int. Combust. 1985, 20, 2065–2071. [Google Scholar] [CrossRef]
- Lai, K.-Y.; Zhu, R.; Lin, M.C. Why mixtures of hydrazine and dinitrogen tetroxide are hypergolic? Chem. Phys. Lett. 2012, 537, 33–37. [Google Scholar] [CrossRef]
- Li, S.; Gao, H.; Shreeve, J.M. Borohydride ionic liquids and borane/ionic-liquid solutions as hypergolic fuels with superior low ignition-delay times. Angew. Chem. Int. Ed. Engl. 2014, 53, 2969–2972. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Hawkins, T.; Rosander, M.; Vaghjiani, G.; Chambreau, S.; Drake, G. Ionic liquids as hypergolic fuels. Energy Fuels 2008, 22, 2871–2872. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, P.; Zhang, J.; Shreeve, J.M. Cyanoborohydride-based ionic liquids as green aerospace bipropellant fuels. Chem. Eur. J. 2014, 20, 6909–6914. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Hawkins, T.; Ahmed, Y.; Rosander, M.; Hudgens, L.; Mills, J. Green bipropellants: Hydrogen-rich ionic liquids that are hypergolic with hydrogen peroxide. Angew. Chem. Int. Ed. Engl. 2011, 50, 5886–5888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shreeve, J.M. Dicyanoborate-based ionic liquids as hypergolic fluids. Angew. Chem. Int. Ed. Engl. 2011, 123, 965–967. [Google Scholar] [CrossRef]
- Chaturvedi, D. Recent developments on task specific ionic liquids. Curr. Org. Chem. 2011, 15, 1236–1248. [Google Scholar] [CrossRef]
- Gao, H.; Joo, Y.H.; Twamley, B.; Zhuo, Z.; Shreeve, J.M. Hypergolic ionic liquids with the 2,2-dialkyltriazanium cation. Angew. Chem. Int. Ed. Engl. 2009, 121, 2830–2833. [Google Scholar] [CrossRef]
- Hawkins, T.W.; Schneider, S.; Drake, G.W.; Vaghjiani, G.; Chambreau, S. Hypergolic Fuels. U.S. Patent 8,034,202, 10 October 2011. [Google Scholar]
- He, L.; Tao, G.-H.; Parrish, D.A.; Shreeve, J.M. Nitrocyanamide-based ionic liquids and their potential applications as hypergolic fuels. Chem. Eur. J. 2010, 16, 5736–5743. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.-H.; Shreeve, J.M. 1,3-Diazido-2-(azidomethyl)-2-propylammonium Salts. Inorg. Chem. 2009, 48, 8431–8438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Qi, C.; Li, S.-H.; Zhang, H.-J.; Sun, C.-H.; Yu, Y.-Z.; Pang, S.-P. 1,1′-Azobis-1,2,3-triazole: A high-nitrogen compound with stable N8 structure and photochromism. J. Am. Chem. Soc. 2010, 132, 12172–12173. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, S.-H.; Li, Y.-C.; Yang, Y.-Z.; Yua, Y.; Pang, S.-P. Nitrogen-rich salts based on polyamino substituted N,N-azo-1,2,4-triazole: A new family of high performance energetic materials. J. Mater. Chem. 2014, A2, 15978–15986. [Google Scholar] [CrossRef]
- Chupakhin, O.N.; Charushin, V.N.; van der Plas, H.C. Nucleophilic Aromatic Substitution of Hydrogen; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Zhang, Y.; Gao, H.; Guo, Y.; Joo, Y.-H.; Shreeve, J.M. Hypergolic N,N-dimethylhydrazinium ionic liquids. Chem. Eur. J. 2010, 16, 3114–3120. [Google Scholar] [CrossRef] [PubMed]
- Colthup, N.B.; Daly, L.H.; Wiberley, S.E. Introduction to Infrared, Raman Spectroscopy; Academic Press: Boston, MA, USA, 1990. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Son, G.-H.; Na, T.-K.; Choi, S.-H. Synthesis and Physical and Chemical Properties of Hypergolic Chemicals such as N,N,N-Trimethylhydrazinium and 1-Ethyl-4-Methyl-1,2,4-Triazolium Salts. Appl. Sci. 2015, 5, 1547-1559. https://doi.org/10.3390/app5041547
Kim Y-S, Son G-H, Na T-K, Choi S-H. Synthesis and Physical and Chemical Properties of Hypergolic Chemicals such as N,N,N-Trimethylhydrazinium and 1-Ethyl-4-Methyl-1,2,4-Triazolium Salts. Applied Sciences. 2015; 5(4):1547-1559. https://doi.org/10.3390/app5041547
Chicago/Turabian StyleKim, Young-Seok, Gi-Hyuk Son, Tae-Kyung Na, and Seong-Ho Choi. 2015. "Synthesis and Physical and Chemical Properties of Hypergolic Chemicals such as N,N,N-Trimethylhydrazinium and 1-Ethyl-4-Methyl-1,2,4-Triazolium Salts" Applied Sciences 5, no. 4: 1547-1559. https://doi.org/10.3390/app5041547






