Glycerol: A promising Green Solvent and Reducing Agent for Metal-Catalyzed Transfer Hydrogenation Reactions and Nanoparticles Formation
Abstract
:1. Introduction
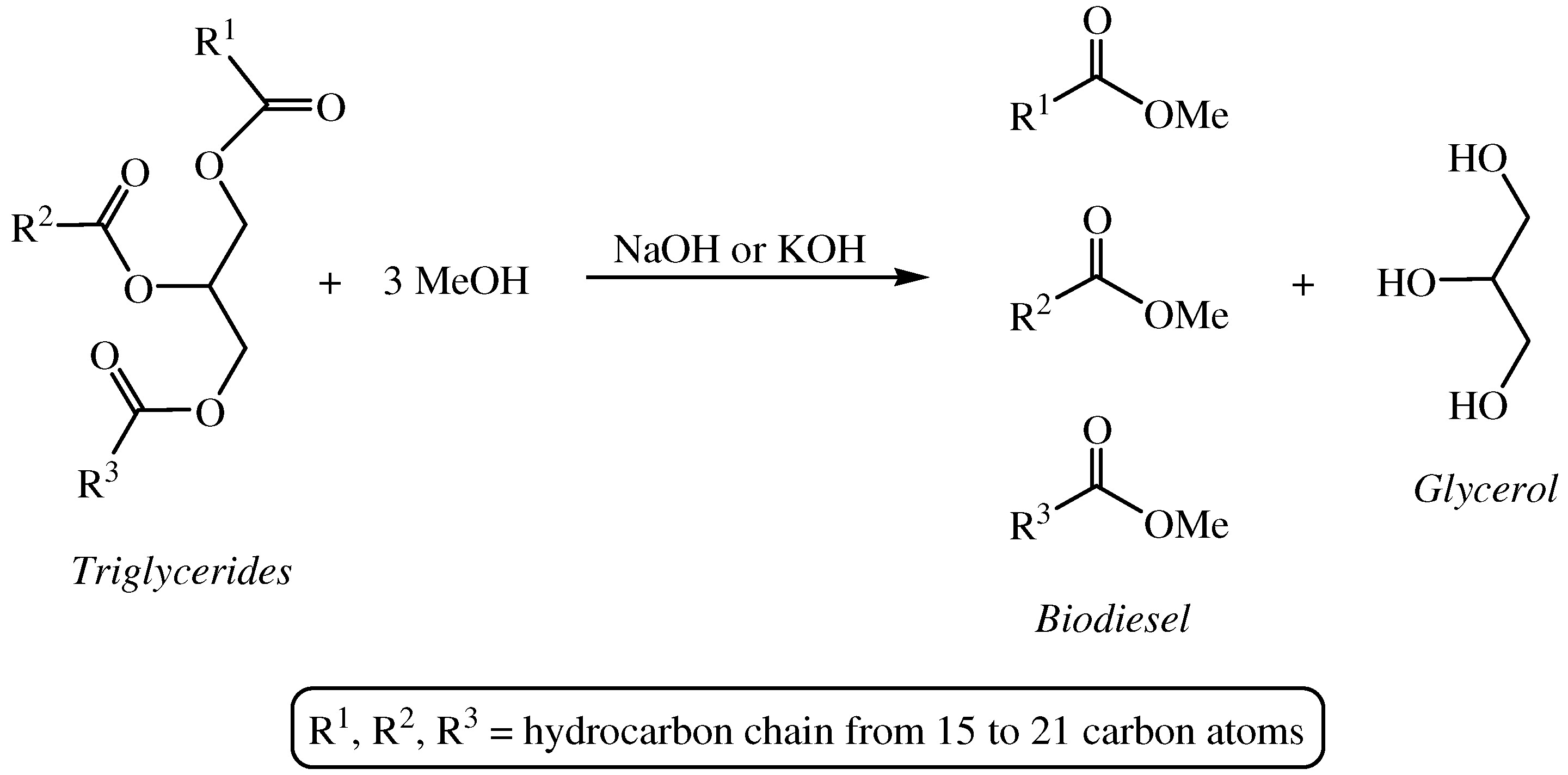

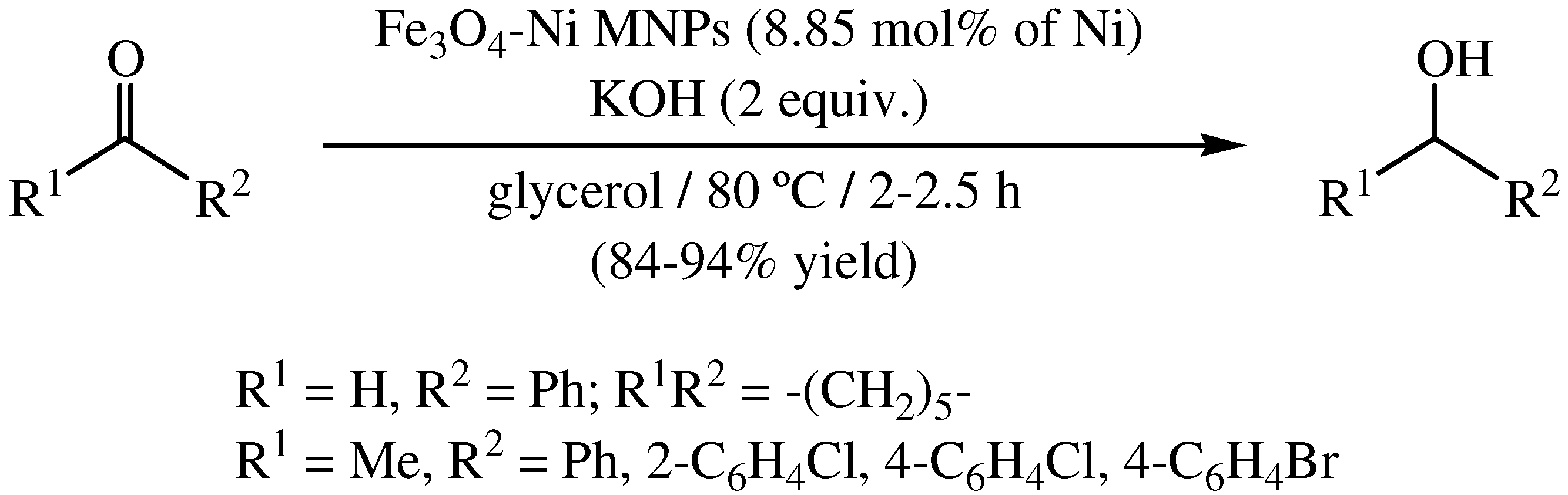
2. Transfer Hydrogenation of Carbonyl Compounds in Glycerol
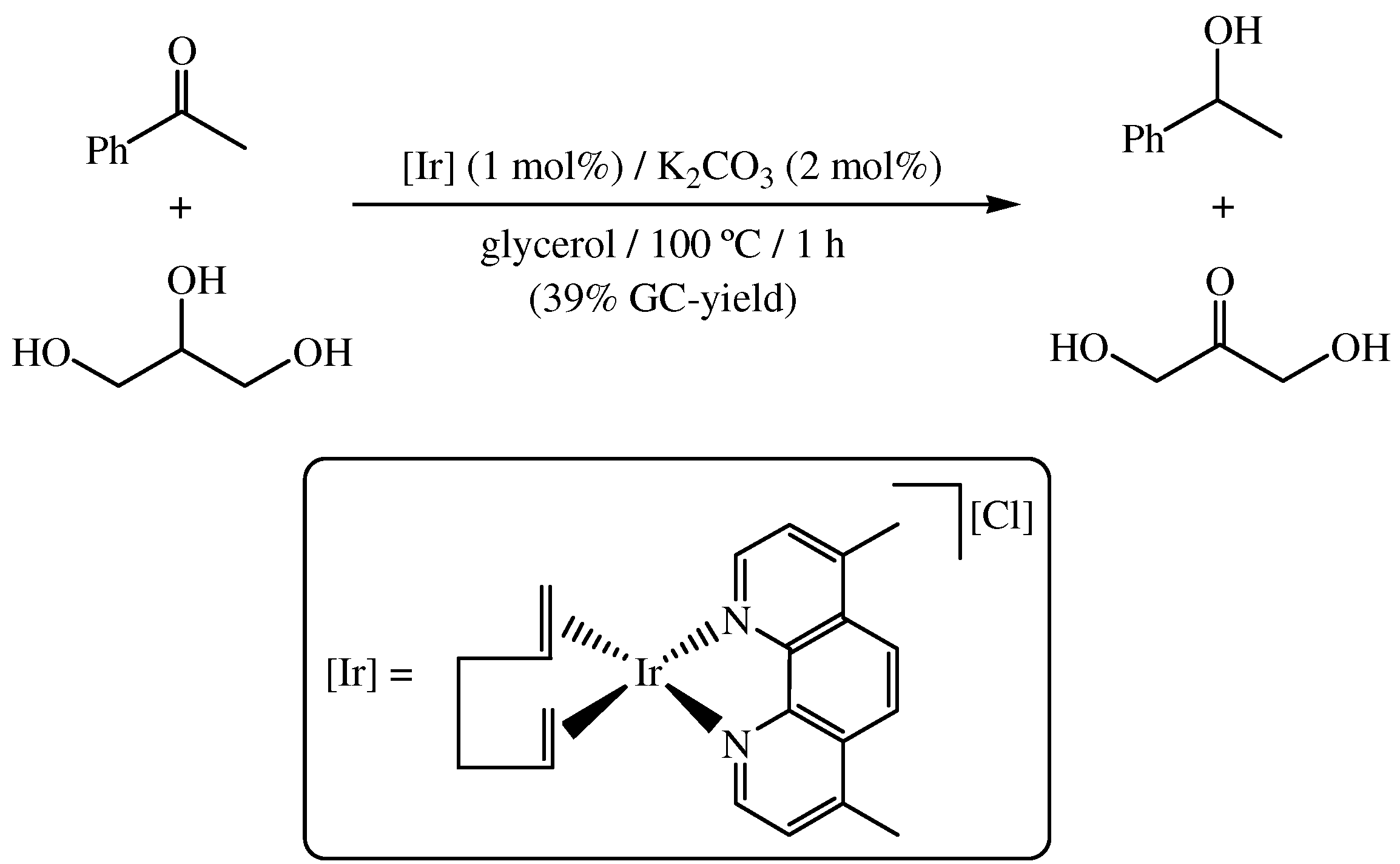


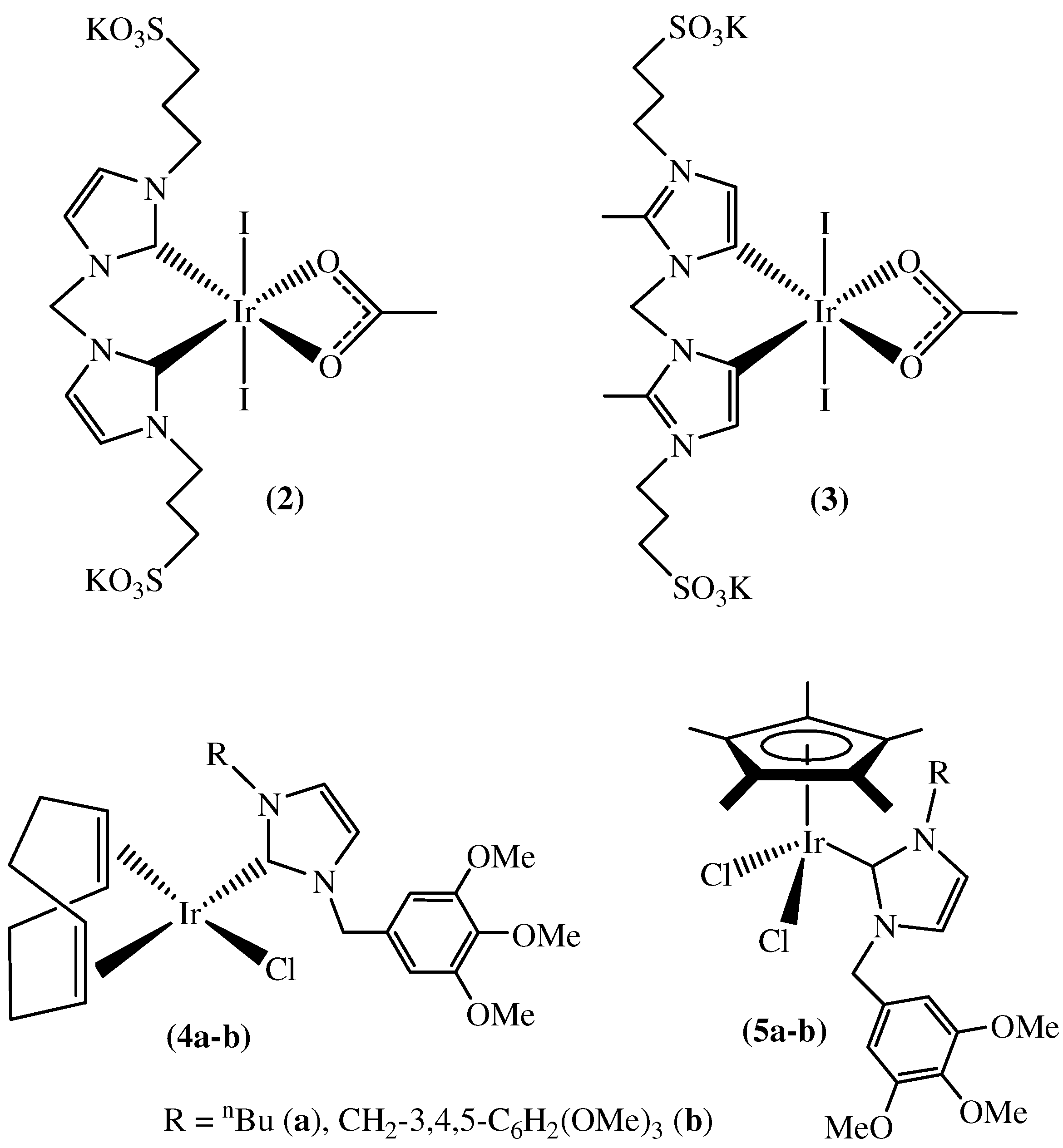
3. Transfer Hydrogenation of Olefins in Glycerol
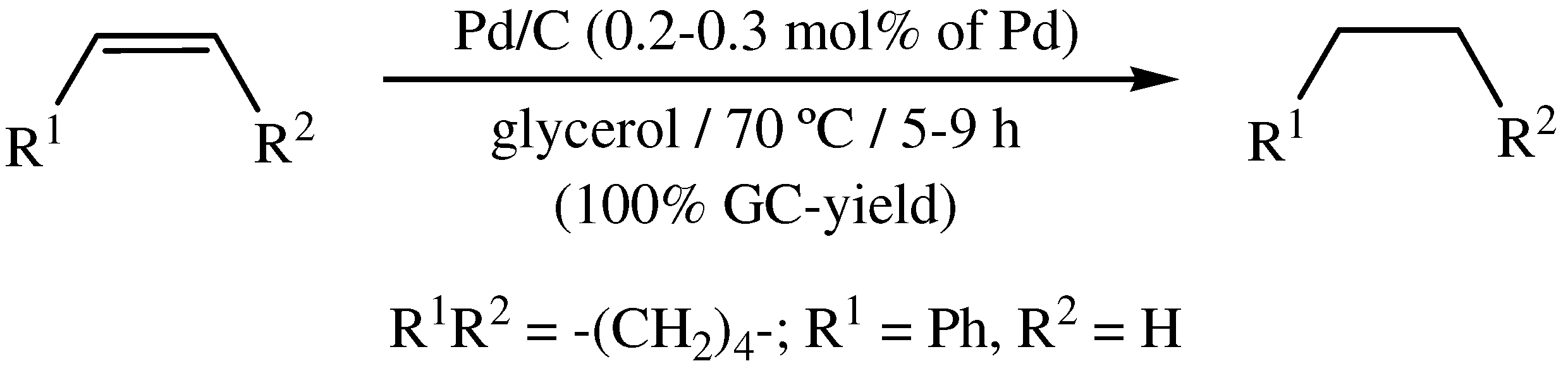
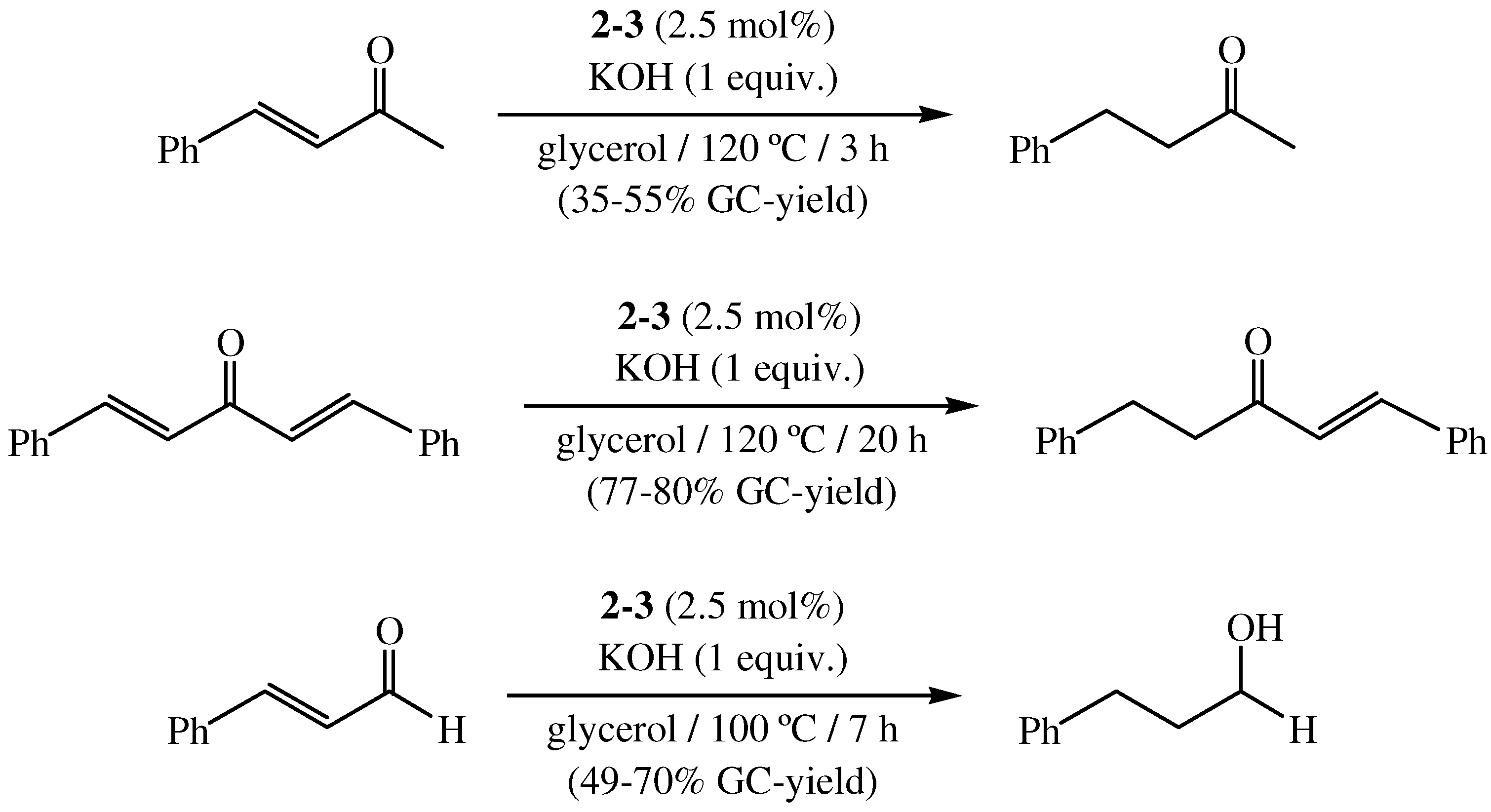
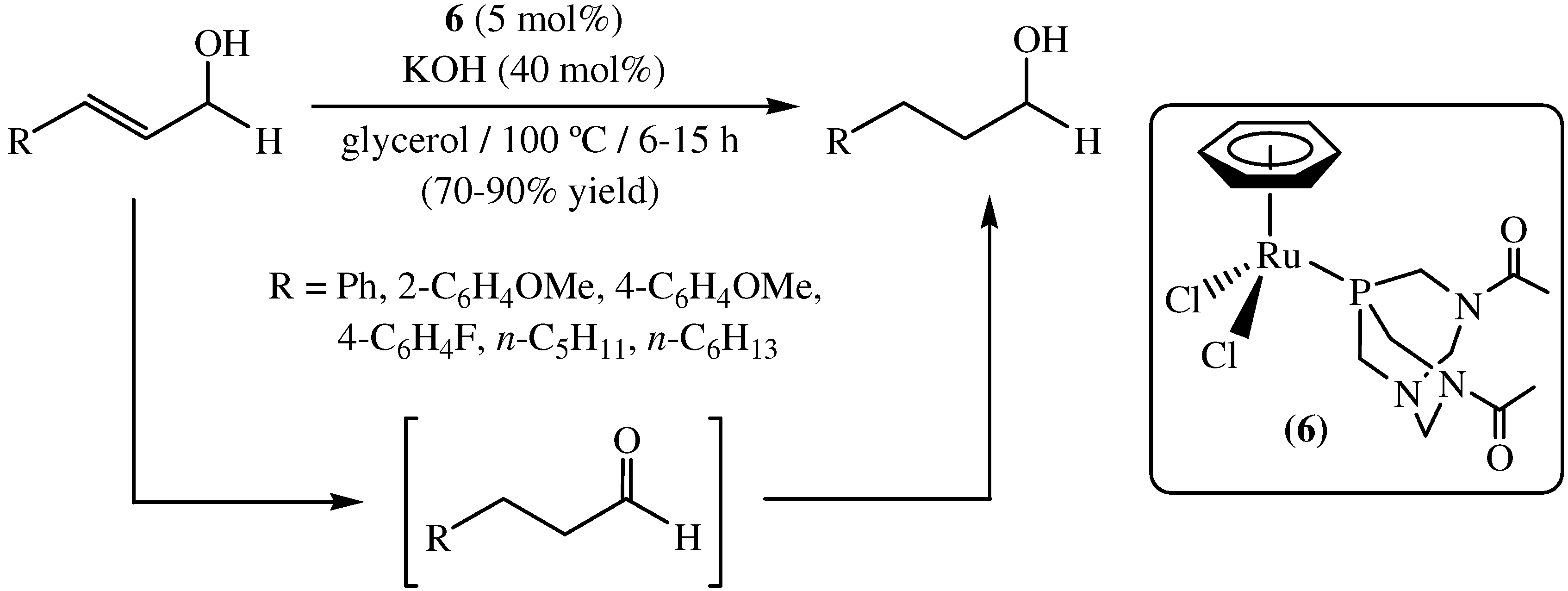

4. Transfer Hydrogenation of Nitroarenes in Glycerol

5. Transfer Hydrogenation of Other Organic Molecules in Glycerol

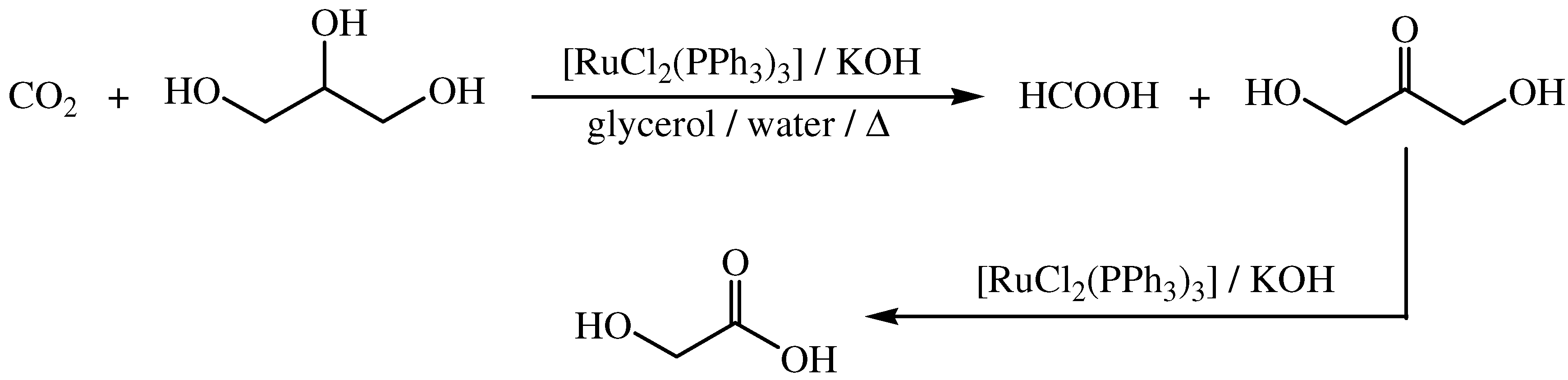
6. Glycerol as Solvent and Reductant for the Formation of Metal Nanoparticles
7. Conclusions and Perspectives
Acknowledgments
Conflict of Interest
References
- Zhou, C.-H.; Beltramini, J.N.; Fan, Y.-X.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar]
- Pagliaro, M.; Rossi, M. The Future of Glycerol: New Usages for a Versatile Raw Material, 2nd ed; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar]
- Johnson, D.T.; Taconi, K.A. The glycerin glut: Options for the value-added conversion of crude glycerol resulting from biodiesel production. Environ. Prog. 2007, 26, 338–348. [Google Scholar] [CrossRef]
- Jérôme, F.; Pouilloux, Y.; Barrault, J. Rational design of solid catalysts for the selective use of glycerol as a natural organic building block. ChemSusChem 2008, 1, 586–613. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Pina, C.D. Recent advances in the conversion of bioglycerol into value-added products. Eur. J. Lipid Sci. Technol. 2009, 111, 788–799. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Conteiro, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar]
- Mota, C.J.A.; da Silva, C.X.A.; Gonçalves, V.L.C. Glycerochemistry: New products and processes from glycerin of biodiesel production. Quim. Nova 2009, 32, 639–648. [Google Scholar] [CrossRef]
- Jérôme, F.; Barrault, J. Use of hybrid organic-siliceous materials for the selective conversion of glycerol. Eur. J. Lipid Sci. Technol. 2011, 113, 118–134. [Google Scholar] [CrossRef]
- Billamboz, M.; Lageay, J.C.; Hapiot, F.; Monflier, E.; Len, C. Novel strategy for the bis-butenolide synthesis via ring-closing metathesis. Synthesis 2012, 14, 137–143. [Google Scholar]
- Vaidya, P.D.; Rodrigues, A.E. Glycerol reforming for hydrogen production: A review. Chem. Eng. Technol. 2009, 32, 1463–1469. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.D.; Haryanto, A. Hydrogen production from glycerol: An update. Energy Convers. Manage. 2009, 50, 2600–2604. [Google Scholar] [CrossRef]
- Nahar, G.; Dupont, V. Hydrogen via stream reforming of liquid biofeedstock. Biofuels 2012, 3, 167–191. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Lancaster, M. Green Chemistry: An Introductory Text, 2nd ed; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Nelson, W.M. Green Solvents for Chemistry: Perspectives and Practice; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Clark, J.H.; Taverner, S.J. Alternatives solvents: Shades of green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Kerton, F.M. Alternative Solvents for Green Chemistry; RSC Publishing: Cambridge, UK, 2009. [Google Scholar]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar]
- Constable, D.J.C.; Jimenez-Gonzalez, C.; Henderson, R.K. Perspective on solvent use in the pharmaceutical industry. Org. Process Res. Dev. 2007, 11, 133–137. [Google Scholar] [CrossRef]
- Li, C.-J.; Chen, L. Organic chemistry in water. Chem. Soc. Rev. 2006, 35, 68–82. [Google Scholar]
- Li, C.-J. The development of catalytic nucleophilic additions of terminal alkynes in water. Acc. Chem. Res. 2010, 43, 581–590. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S. An efficient and expeditious Fmoc protection of amines and amino acids in aqueous media. Green Chem. 2011, 13, 3355–3359. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Crochet, P.; Cadierno, V. Metal-catalyzed amide bond forming reactions in an environmentally friendly aqueous medium: Nitrile hydrations and beyond. Green Chem. 2013, 15, 46–66. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Díaz-Álvarez, A.E.; Francos, J.; Lastra-Barreira, B.; Crochet, P.; Cadierno, V. Glycerol and derived solvents: New sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227. [Google Scholar]
- Wolfson, A.; Dlugy, C.; Tavor, D. Glycerol-based solvents in organic synthesis. Trends Org. Chem. 2011, 15, 41–50. [Google Scholar]
- Calvino-Casilda, V. Glycerol as an Alternative Solvent for Organic Reactions. In Green Solvents I: Properties and Applications in Chemistry; Mohammad, Ali, Inamuddin, Eds.; Springer Science & Business Media: Dordrecht, The Netherlands, 2012; pp. 187–207. [Google Scholar]
- Wolfson, A.; Tavor, D.; Cravotto, G. Is Glycerol a Sustainable Reaction Medium? In Glycerol: Production, Structure and Applications; Silva, S., Ferreira, C., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 233–248. [Google Scholar]
- Safaei, H.R.; Shekouhy, M.; Rahmanpur, S.; Shirinfeshan, A. Glycerol as a biodegradable and reusable promoting medium for the catalyst-free one-pot three component synthesis of 4H-pyrans. Green Chem. 2012, 14, 1696–1704. [Google Scholar] [CrossRef]
- Gu, Y.; Barrault, J.; Jérôme, F. Glycerol as an efficient promoting medium for organic reactions. Adv. Synth. Catal. 2008, 350, 2007–2012. [Google Scholar] [CrossRef]
- Wolfson, A.; Snezhko, A.; Meyouhas, T.; Tavor, D. Glycerol derivatives as green reaction mediums. Green Chem. Lett. Rev. 2012, 5, 7–12. [Google Scholar] [CrossRef]
- Francos, J.; Cadierno, V. Palladium-catalyzed cycloisomerization of (Z)-enynols into furans using green solvents: Glycerol vs. water. Green Chem. 2010, 12, 1552–1555. [Google Scholar] [CrossRef]
- Zassinovich, G.; Mestroni, G.; Gladiali, S. Asymmetric hydrogen transfer reactions promoted by homogeneous transition metal catalysts. Chem. Rev. 1992, 92, 1051–1069. [Google Scholar]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar]
- Samec, J.S.M.; Bäckvall, J.-E.; Andersson, P.G.; Brandt, P. Mechanistic aspects of transition metal-catalyzed hydrogen transfer reactions. Chem. Soc. Rev. 2006, 35, 237–248. [Google Scholar]
- Gladiali, S.; Taras, R. Reduction of Carbonyl Compounds by Hydrogen Transfer. In Modern Reduction Methods; Andersson, P.G., Munslow, I.J., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 135–158. [Google Scholar]
- Wills, M. Imino Reductions by Transfer Hydrogenation. In Modern Reduction Methods; Andersson, P.G., Munslow, I.J., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 271–296. [Google Scholar]
- Adkins, H.; Elofson, R.M.; Rossow, A.G.; Robinson, C.C. The oxidation potentials of aldehydes and ketones. J. Am. Chem. Soc. 1949, 71, 3622–3629. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzyńska, E.; Girardon, J.-S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Mimura, N.; Paula, S.; Dumeignil, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Farnetti, E.; Kašpar, J.; Crotti, C. A novel glycerol valorization route: Chemoselective dehydrogenation catalyzed by iridium derivatives. Green Chem. 2009, 11, 704–709. [Google Scholar]
- Crotti, C.; Kašpar, J.; Farnetti, E. Dehydrogenation of glycerol to hydroxyacetone catalyzed by iridium complexes with P-N ligands. Green Chem. 2010, 12, 1295–1300. [Google Scholar] [CrossRef]
- Tavor, D.; Sheviev, O.; Dlugy, C.; Wolfson, A. Tranfer hydrogenations of benzaldehyde using glycerol as solvent and hydrogen source. Can. J. Chem. 2010, 88, 305–308. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y.; Tavor, D. Glycerol as solvent and hydrogen donor in transfer hydrogenation-dehydrogenation reactions. Tetrahedron Lett. 2009, 50, 5951–5953. [Google Scholar] [CrossRef]
- Cravotto, G.; Orio, L.; Gaudino, E.C.; Martina, K.; Tavor, D.; Wolfson, A. Efficient synthetic protocols in glycerol under heterogeneous conditions. ChemSusChem 2011, 4, 1130–1134. [Google Scholar] [CrossRef]
- Azua, A.; Mata, J.A.; Peris, E. Iridium NHC based catalysts for transfer hydrogenation processes using glycerol as solvent and hydrogen donor. Organometallics 2011, 30, 5532–5536. [Google Scholar]
- Azua, A.; Mata, J.A.; Peris, E.; Lamaty, F.; Martinez, J.; Colacino, E. Alternative energy input for transfer hydrogenation using iridium NHC based catalysts in glycerol as hydrogen donor and solvent. Organometallics 2012, 31, 3911–3919. [Google Scholar] [CrossRef]
- Gawande, M.B.; Rathi, A.K.; Branco, P.S.; Nogueira, I.D.; Velhinho, A.; Shrikhande, J.J.; Indulkar, U.U.; Jayaram, R.V.; Ghumman, C.A.A.; Bundaleski, N.; et al. Regio- and chemoselective reduction of nitroarenes and carbonyl compounds over recyclable magnetic ferrite-nickel nanoparticles (Fe3O4-Ni) by using glycerol as a hydrogen source. Chem. Eur. J. 2012, 18, 12628–12632. [Google Scholar]
- Tavor, D.; Popov, S.; Dlugy, C.; Wolfson, A. Catalytic transfer hydrogenation of olefins in glycerol. Org. Commun. 2010, 3, 70–75. [Google Scholar]
- Díaz-Álvarez, A.E.; Crochet, P.; Cadierno, V. Ruthenium-catalyzed reduction of allylic alcohols using glycerol as solvent and hydrogen donor. Catal. Commun. 2011, 13, 91–96. [Google Scholar]
- Cadierno, V.; Francos, J.; Gimeno, J.; Nebra, N. Ruthenium-catalyzed reduction of allylic alcohols: An efficient isomerization/transfer hydrogenation tandem process. Chem. Commun. 2007, 2536–2538. [Google Scholar]
- Cadierno, V.; Crochet, P.; Francos, J.; García-Garrido, S.E.; Gimeno, J.; Nebra, N. Ruthenium-catalyzed isomerization/transfer hydrogenation in organic and aqueous media: A one-pot tandem process for the reduction of allylic alcohols. Green Chem. 2009, 11, 1992–2000. [Google Scholar]
- Díaz, G.C.; Perez, R.S.; Tapanes, N.C.O.; Aranda, D.A.G.; Arceo, A.A. Hydrolysis-hydrogenation of soybean oil and tallow. Nat. Sci. 2011, 3, 530–534. [Google Scholar]
- Tavor, D.; Gefen, I.; Dlugy, C.; Wolfson, A. Transfer hydrogenations of nitrobenzene using glycerol as solvent and hydrogen donor. Synth. Commun. 2011, 41, 3409–3416. [Google Scholar] [CrossRef]
- Chung, W.J.; Baskar, C.; Chung, D.G.; Han, M.D.; Lee, C.H. Catalytic Transfer Hydrogenation of Carboxylic Acids to Their Corresponding Alcohols by Using Glycerol as Hydrogen Donor. Repub. Korean Kongkae Taeho Kongbo Patent KR 2012006276, 18 January 2012. [Google Scholar]
- Dibenedetto, A.; Stufano, P.; Nocito, F.; Aresta, M. RuII-mediated hydrogen transfer from aqueous glycerol to CO2: From waste to value-added products. ChemSusChem 2011, 4, 1311–1315. [Google Scholar] [CrossRef]
- Sanz, A.; Azua, A.; Peris, E. “(η6-arene)Ru(bis-NHC)” complexes for the reduction of CO2 to formate with hydrogen and by transfer hydrogenation with iPrOH. Organometallics 2010, 39, 6339–6343. [Google Scholar]
- Toledano, A.; Serrano, L.; Labidi, J.; Pineda, A.; Balu, A.M.; Luque, R. Heterogeneously catalysed mild hydrogenolytic depolymerisation of lignin under microwave irradiation with hydrogen-donating solvents. ChemCatChem 2012. [Google Scholar] [CrossRef]
- Carroll, K.J.; Reveles, J.U.; Shultz, M.D.; Khanna, S.N.; Carpenter, E.E. Preparation of elemental Cu and Ni nanoparticles by the polyol method: An experimental and theoretical approach. J. Phys. Chem. C 2011, 115, 2656–2664 and references cited therein. [Google Scholar]
- Rele, M.; Kapoor, S.; Sharma, G.; Mukherjee, T. Reduction and aggregation of silver and thallium ions in viscous media. Phys. Chem. Chem. Phys. 2004, 6, 590–595. [Google Scholar] [CrossRef]
- Ullah, M.H.; Kim, I.; Ha, C.-S. In-situ preparation of binary-phase silver nanoparticles at a high Ag+ concentration. J. Nanosci. Nanotechnol. 2006, 6, 777–782. [Google Scholar] [CrossRef]
- Ullah, M.H.; Kim, I.; Ha, C.-S. Preparation and optical properties of silver nanoparticles at a high Ag+ concentration. Mater. Lett. 2006, 60, 1496–1501. [Google Scholar]
- Grace, A.N.; Pandian, K. One pot synthesis of polymer protected Pt, Pd, Ag and Ru nanoparticles and nanoprisms under reflux and microwave mode of heating in glycerol—A comparative study. Mat. Chem. Phys. 2007, 104, 191–198. [Google Scholar]
- Sarkar, A.; Kapoor, S.; Mukherjee, T. Synthesis and characterization of silver nanoparticles in viscous solvents and its transfer into non-polar solvents. Res. Chem. Intermed. 2010, 36, 411–421. [Google Scholar] [CrossRef]
- Dzido, G.; Jarzębski, A.B. Fabrication of silver nanoparticles in a continuous flow, low temperature microwave-assisted polyol process. J. Nanopart. Res. 2011, 13, 2533–2541. [Google Scholar] [CrossRef]
- Preuksarattanawut, T.; Asavavisithchai, S.; Nisaratanoporn, E. Fabrication of silver hollow microspheres by sodium hydroxide in glycerol solution. Mat. Chem. Phys. 2011, 130, 481–486. [Google Scholar] [CrossRef]
- Garcia, A.C.; Gasparotto, L.H.S.; Gomes, J.F.; Tremiliosi-Filho, G. Straightforward synthesis of carbon-supported Ag nanoparticles and their application for the oxygen reduction reaction. Electrocatalysis 2012, 3, 147–152. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Han, S.-B.; Ko, A.R.; Kim, H.-S.; Park, K.-W. Glycerol-mediated synthesis of Pd nanostructures with dominant {111} facets for enhanced electrocatalytic activity. Catal. Commun. 2011, 15, 137–140. [Google Scholar]
- Koo, M.; Bae, J.-S.; Kim, H.-C.; Nam, D.-G.; Ko, C.H.; Yeum, J.H.; Oh, W. Electrochemical oxidation of some basic alcohols on multiwalled carbon nanotube-platinum composites. Bull. Mater. Sci. 2012, 35, 545–550. [Google Scholar] [CrossRef]
- Selvaraj, V.; Vinoba, M.; Alagar, M. Electrocatalytic oxidation of ethylene glycol on Pt and Pt-Ru nanoparticles modified multi-walled carbon nanotubes. J. Colloid Interf. Sci. 2008, 322, 537–544. [Google Scholar] [CrossRef]
- Gasparotto, L.H.S.; Garcia, A.C.; Gomes, J.F.; Tremiliosi-Filho, G. Electrocatalytic performances of environmentally friendly synthesized gold nanoparticles towards the borohydride electro-oxidation reaction. J. Power Sources 2012, 218, 73–78. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Oh, S.-E.; Park, K.-W. Highly active Pt-Pd alloy catalyst for oxygen reduction reaction in buffer solution. Electrochem. Commun. 2011, 13, 1300–1303. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Ko, A.-R.; Han, S.-B.; Kim, H.-S.; Park, K.-W. Synthesis of octahedral Pt-Pd alloy nanoparticles for improved catalytic activity and stability in methanol electrooxidation. Phys. Chem. Chem. Phys. 2011, 13, 5569–5572. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Ko, A.-R.; Kim, D.-Y.; Han, S.-B.; Park, K.-W. Octahedral Pt-Pd alloy catalysts with enhanced oxygen reduction activity and stability in proton exchange membrane fuel cells. RSC Adv. 2012, 2, 1119–1125. [Google Scholar]
- Nekooi, P.; Ahmadi, R.; Amini, M.K. Preparation of CoSe nanoparticles by microwave-assisted polyol method: Effect of Se/Co ratio, support type and synthesis conditions on oxygen reduction activity. J. Iran Chem. Soc. 2012, 9, 715–722. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y. Hydrothermal reduction route to Mn(OH)2 and MnCO3 nanocrystals. Mat. Chem. Phys. 2003, 82, 419–422. [Google Scholar] [CrossRef]
- Chen, X.; Wang, F.; Xu, J. Preparation of VO2(B) nanoflake with glycerol as reductant agent and its catalytic application in the aerobic oxidation of benzene to phenol. Top. Catal. 2011, 54, 1016–1023. [Google Scholar] [CrossRef]
- Kou, J.; Varma, R.S. Speedy fabrication of diameter-controlled Ag nanowires using glycerol under microwave irradiation conditions. Chem. Commun. 2013, 49, 692–694. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Díaz-Álvarez, A.E.; Cadierno, V. Glycerol: A promising Green Solvent and Reducing Agent for Metal-Catalyzed Transfer Hydrogenation Reactions and Nanoparticles Formation. Appl. Sci. 2013, 3, 55-69. https://doi.org/10.3390/app3010055
Díaz-Álvarez AE, Cadierno V. Glycerol: A promising Green Solvent and Reducing Agent for Metal-Catalyzed Transfer Hydrogenation Reactions and Nanoparticles Formation. Applied Sciences. 2013; 3(1):55-69. https://doi.org/10.3390/app3010055
Chicago/Turabian StyleDíaz-Álvarez, Alba E., and Victorio Cadierno. 2013. "Glycerol: A promising Green Solvent and Reducing Agent for Metal-Catalyzed Transfer Hydrogenation Reactions and Nanoparticles Formation" Applied Sciences 3, no. 1: 55-69. https://doi.org/10.3390/app3010055





