Study on Microscopic Characteristics and Rock Mechanical Properties of Tight Sandstone after Acidification–Supercritical CO2 Composite Action: Case Study from Xujiahe Formation, China
Abstract
:1. Introduction
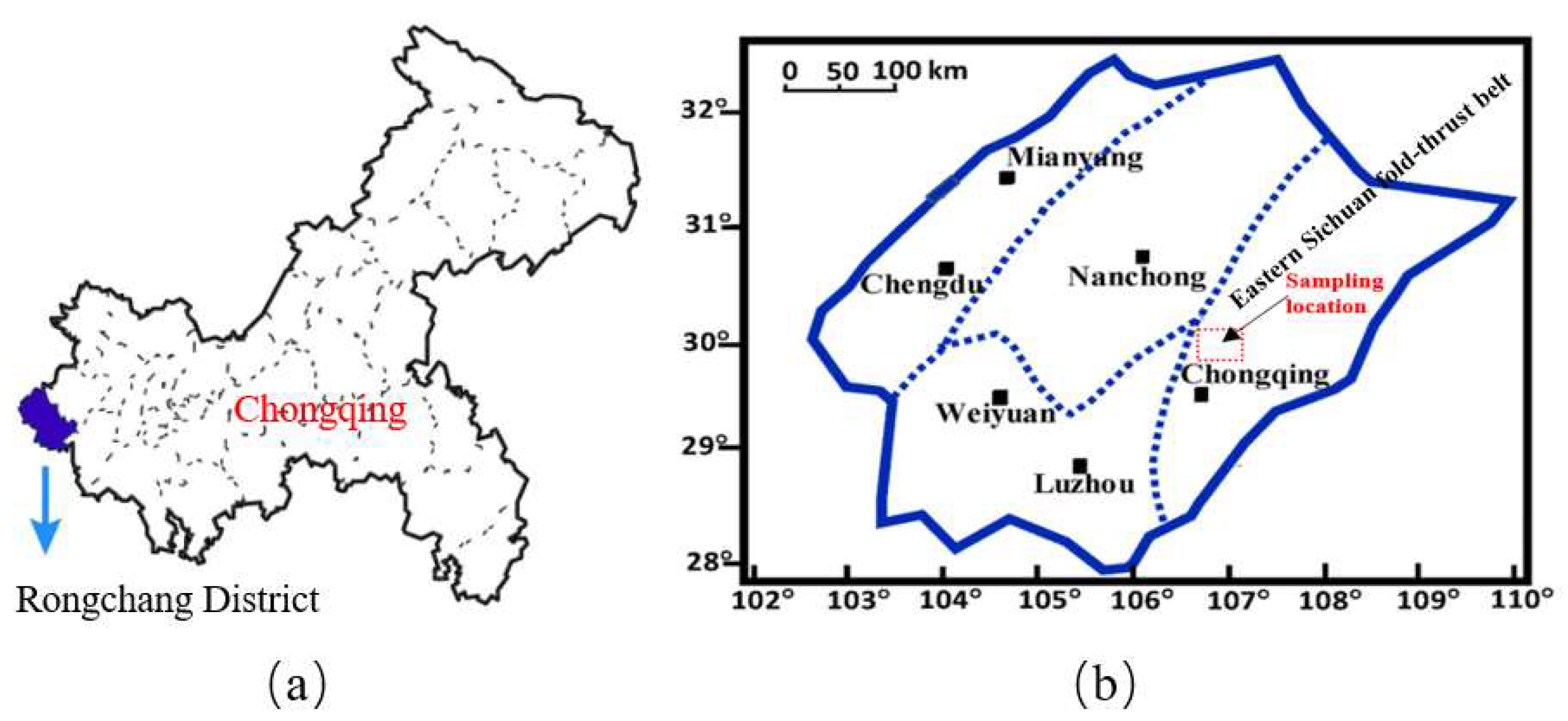
2. Materials and Equipment
2.1. Sample Preparation
2.2. Experimental Equipment and Procedure
3. Results and Discussion
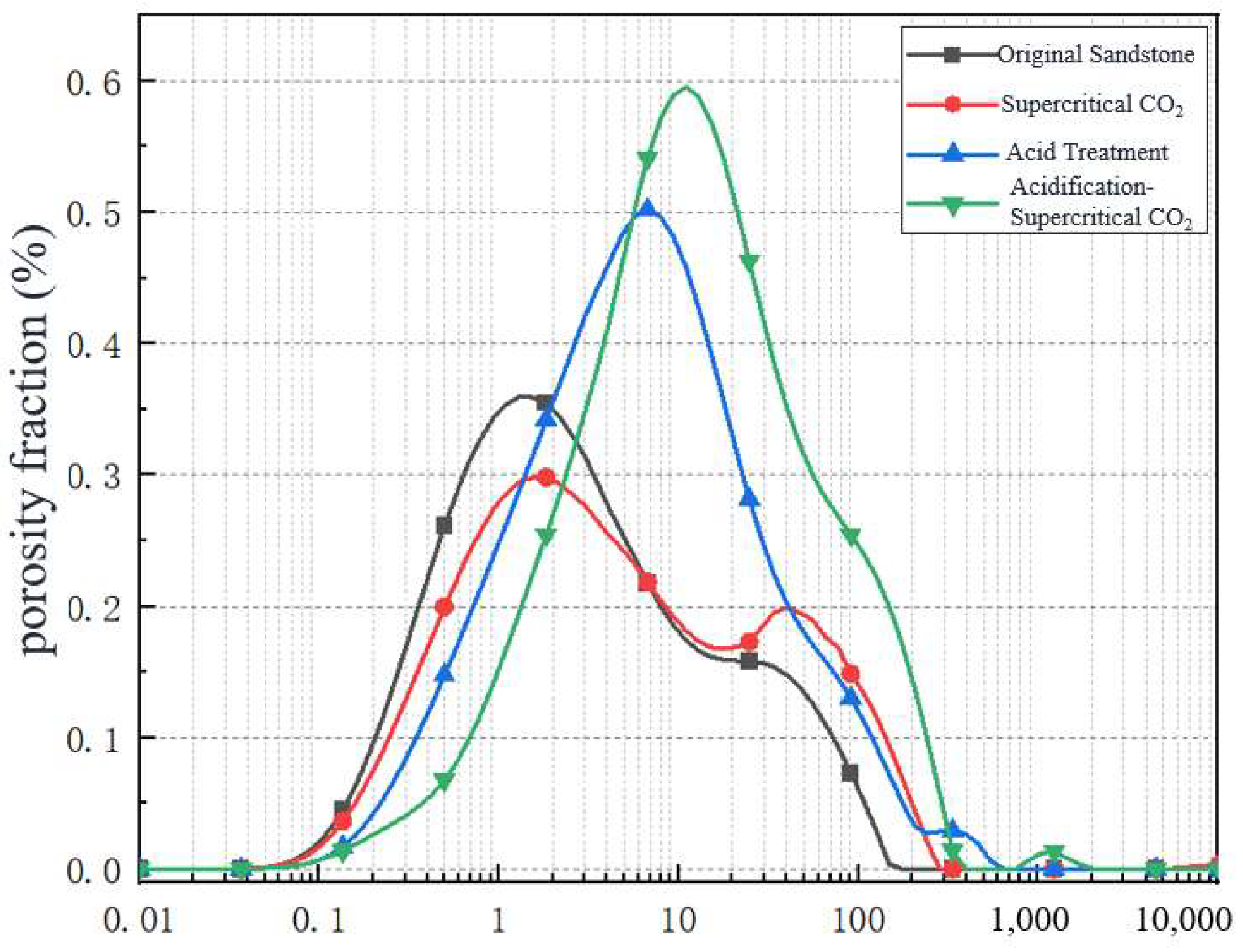
3.1. NMR Data Analysis
Porosity Change Analysis
3.2. Mechanical Property Analysis
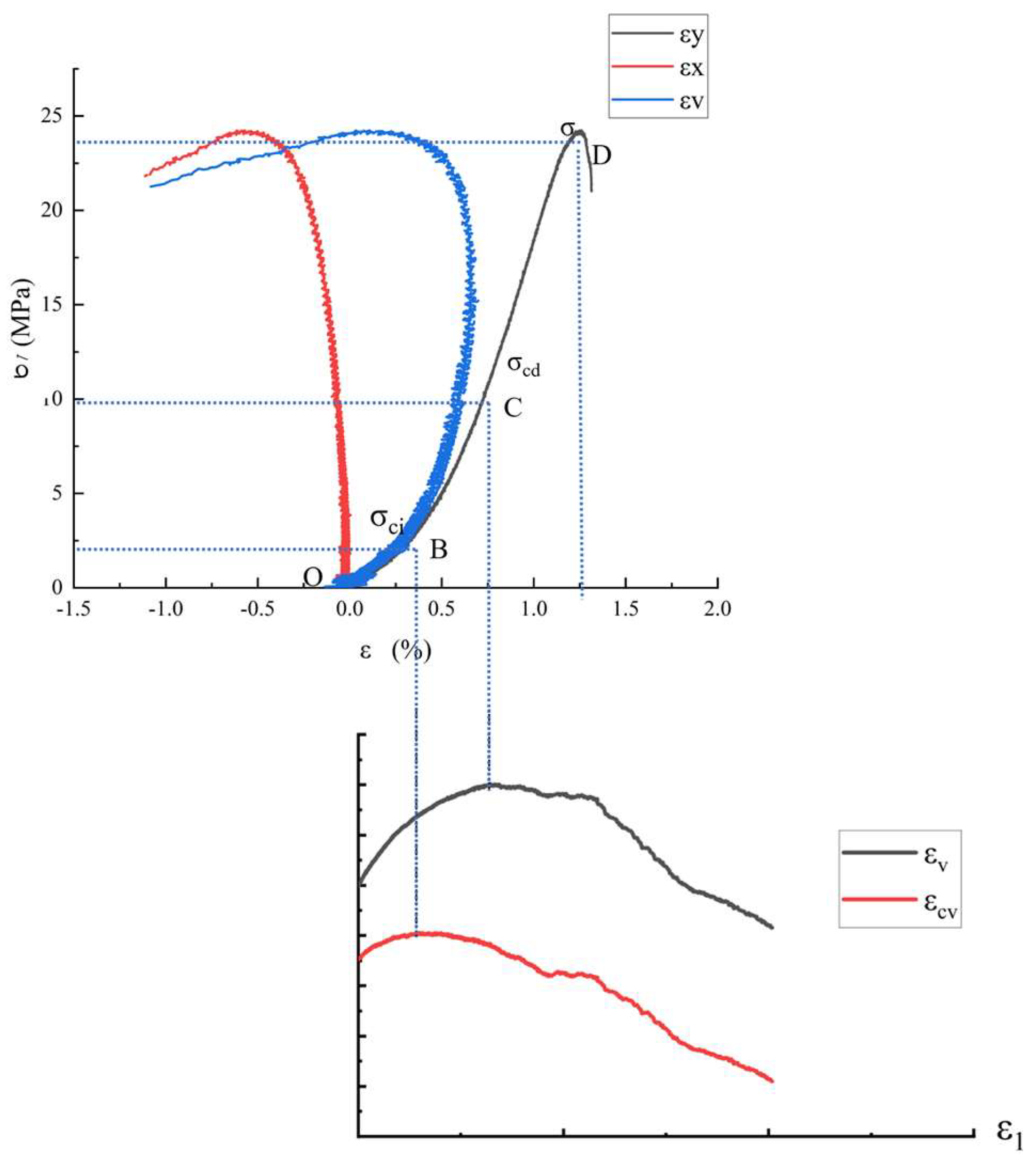
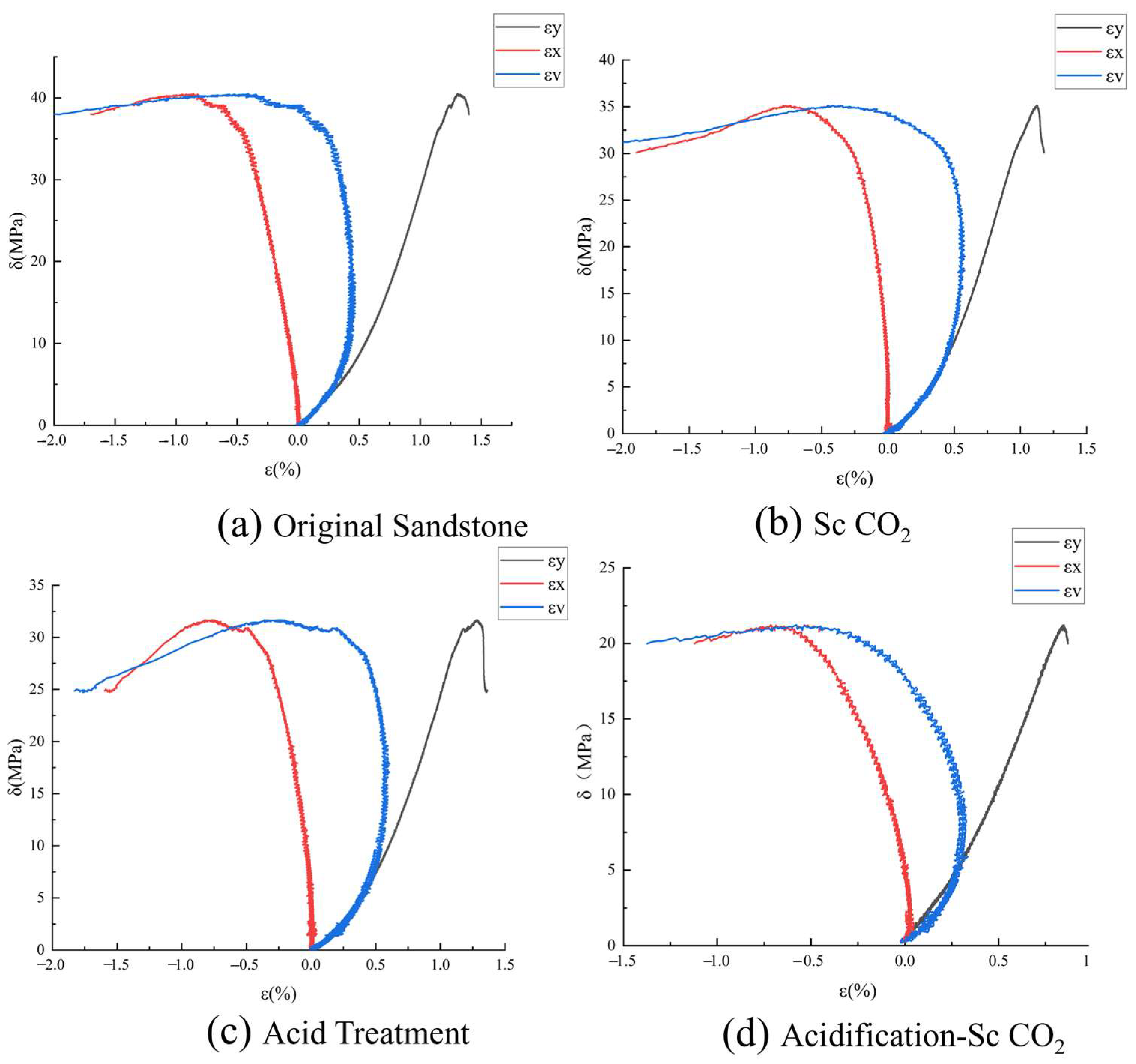
3.2.1. Stress–Strain Analysis
3.2.2. Determination and Analysis of Crack Initiation Stress and Damage Stress
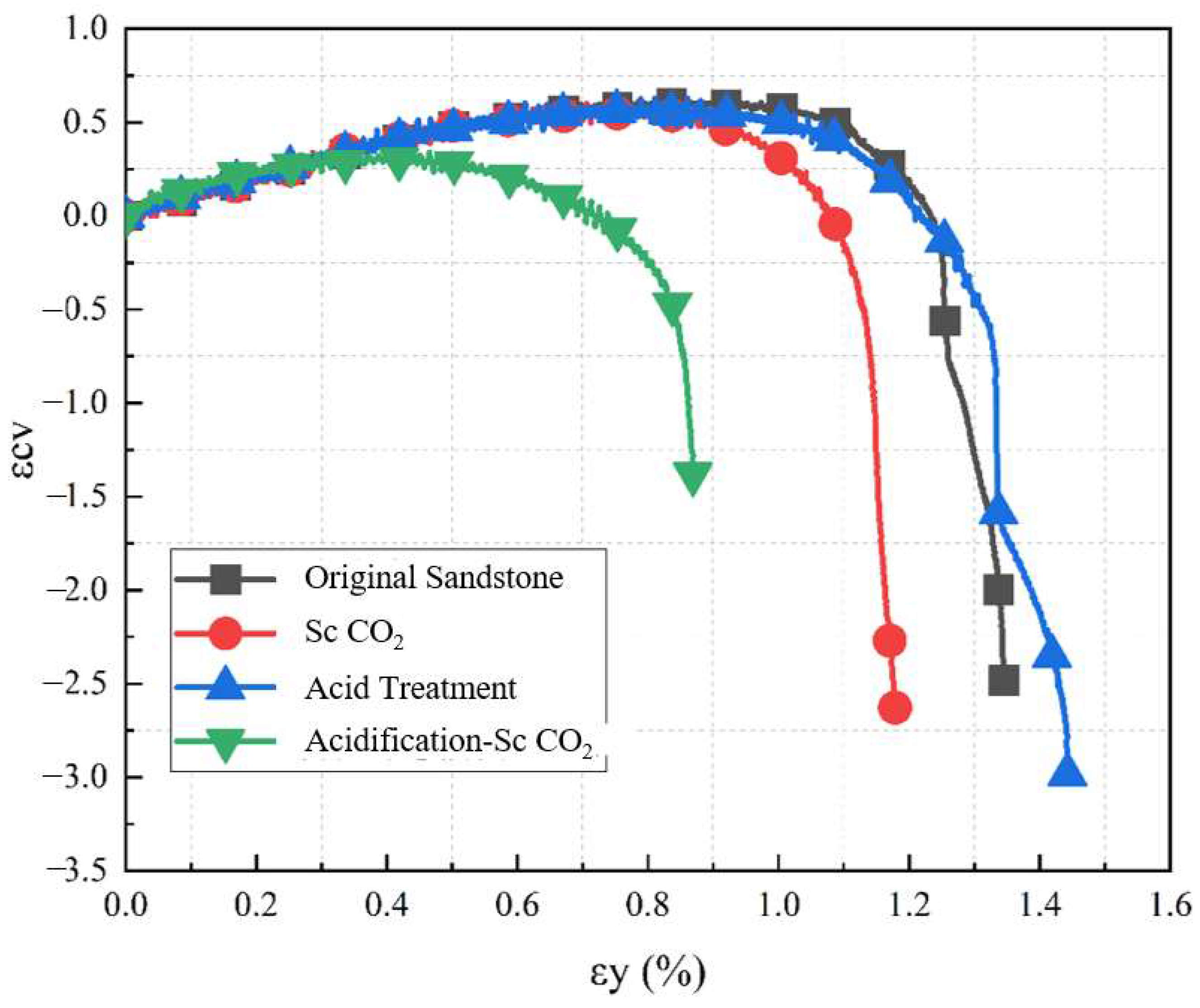
3.2.3. Analysis of Sandstone Strain Variation
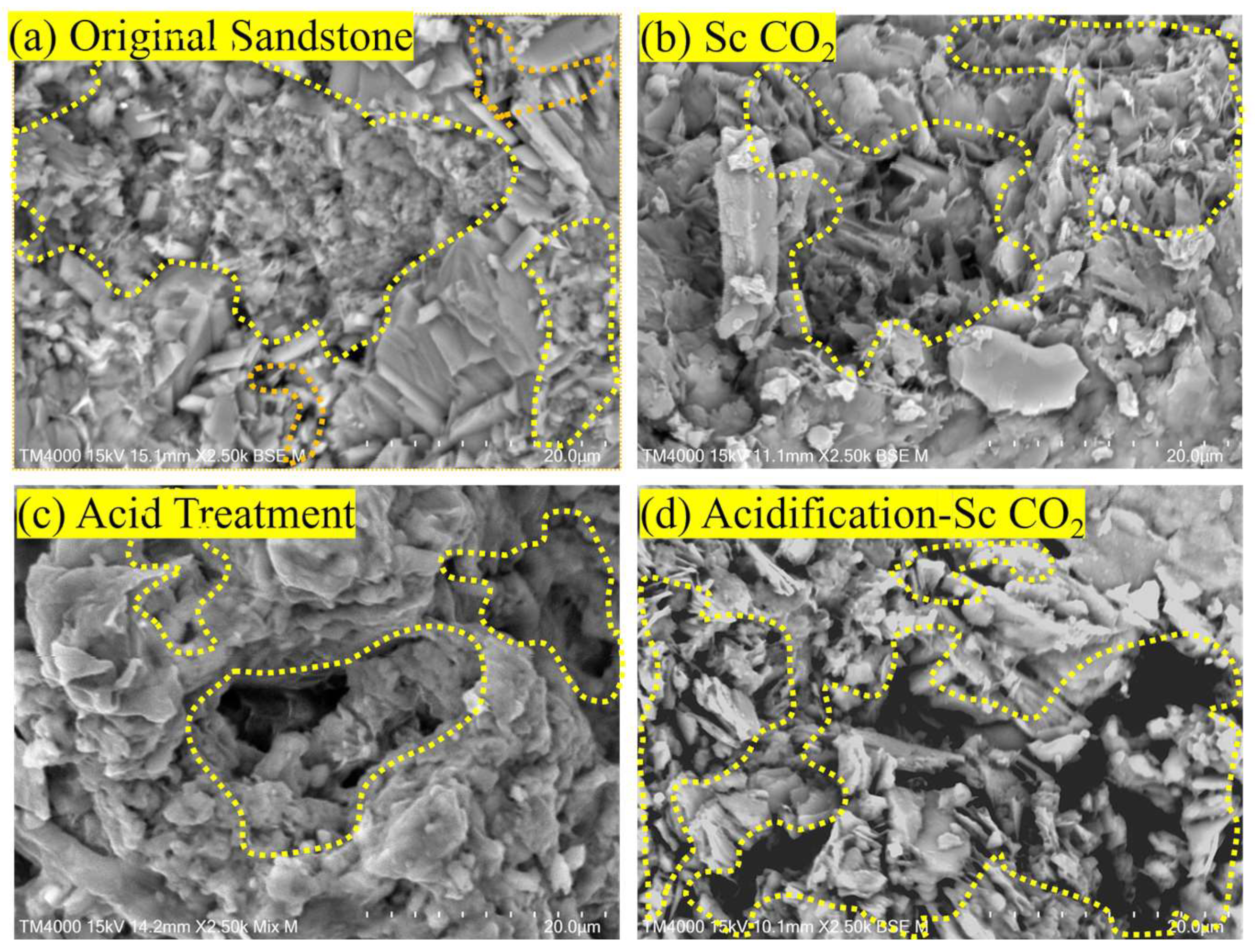
3.3. Mechanism of Sandstone Changes after Acidification–Sc CO2 Treatment
4. Conclusions
- (1)
- The continuity of the T2 spectral curve of the sandstone samples is not obvious. The acidification–supercritical CO2 composite treatment results in the dissolution of a large number of minerals after the composite treatment, which makes the pore size distribution more uniform, and the T2 spectral curve is trapezoidal, which is more favorable for the development of pores into macropores (T2 335 ms) and mesopores (T2 6.85 ms).
- (2)
- The acidification–supercritical CO2 composite treatment has a significant effect on the pore size distribution and permeability of the sandstone. Compared with the sandstone under untreated or single treatment conditions, the chlorite, feldspar, and carbonate contents of the sandstone after the composite treatment are significantly reduced; the morphology of the sandstone is dramatically changed, and the number of initial microcracks is also dramatically increased after the acidification–supercritical CO2 composite action.
- (3)
- After the acidization–supercritical CO2 composite treatment, the peak strength, initial stress, and damage stress of the sandstone decreases by 49.7%, 20%, and 49.5%, respectively. The composite treatment accelerates the growth rate of crack volume strain in the sandstone; enhances the ductility of the samples; causes overall deterioration of the samples, leading to frequent intercrossing of internal cracks; and exacerbates structural damage.
- (4)
- Acid pretreatment greatly increases the pore volume of the sandstone and then greatly increases the volume of supercritical CO2 and sandstone, while the residual acidic reactants and reaction products change the PH environment of supercritical CO2 and sandstone mineral solubility, so there is a physicochemical effect of acid treatment on the reaction between supercritical CO2 and sandstone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Qiao, D. Current situation of tight sand gas in China. In Proceedings of the International Conference on Energy, Environment and Sustainable Development (ICEESD 2011), Shanghai Univ Elect Power, Shanghai, China, 21–23 October 2012; Shanghai Univ Elect Power: Shanghai, China, 2011; p. 85. [Google Scholar]
- Zou, C.; Tao, S.; Han, W.; Zhao, Z.; Ma, W.; Li, C.; Bai, B.; Gao, X. Geological and Geochemical Characteristics and Exploration Prospect of Coal-Derived Tight Sandstone Gas in China: Case Study of the Ordos, Sichuan, and Tarim Basins. Acta Geol. Sin.-Engl. Ed. 2018, 92, 1609–1626. [Google Scholar] [CrossRef]
- Dai, J.; Ni, Y.; Wu, X. Tight gas in China and its significance in exploration and exploitation. Pet. Explor. Dev. 2012, 39, 277–284. [Google Scholar] [CrossRef]
- Ma, X.; Jia, A.; Tan, J.; He, D. Tight sand gas development technology and practices in China. Pet. Explor. Dev. 2012, 39, 611–618. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Talha, M.; Xia, Y.; Tang, M.; Xie, R. Sequence Stratigraphy of the Jurassic Strata and Occurrences of Potential Sandstone Reservoirs in the ST Gas Field, Northern West Siberia Basin. Appl. Sci. 2023, 13, 12096. [Google Scholar] [CrossRef]
- Li, N.; Chen, F.; Yu, J.; Han, P.; Kang, J. Pre-acid system for improving the hydraulic fracturing effect in low-permeability tight gas reservoir. J. Pet. Explor. Prod. Technol. 2021, 11, 1761–1780. [Google Scholar] [CrossRef]
- Tan, P.; Jin, Y.; Hou, B.; Zheng, X.; Guo, X.; Gao, J. Experiments and analysis on hydraulic sand fracturing by an improved true tri-axial cell. J. Pet. Sci. Eng. 2017, 158, 766–774. [Google Scholar] [CrossRef]
- Guo, C.; Xu, J.; Wei, M.; Jiang, R. Experimental study and numerical simulation of hydraulic fracturing tight sandstone reservoirs. Fuel 2015, 159, 334–344. [Google Scholar] [CrossRef]
- Zou, C.; Zhu, R.; Liu, K.; Su, L.; Bai, B.; Zhang, X.; Yuan, X.; Wang, J. Tight gas sandstone reservoirs in China: Characteristics and recognition criteria. J. Pet. Sci. Eng. 2012, 88, 82–91. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Cai, C.; Kang, Y.; Wang, X.; Huang, M.; Chen, F. Fracture Initiation of an Inhomogeneous Shale Rock under a Pressurized Supercritical CO2 Jet. Appl. Sci. 2017, 7, 1093. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, R.; Chen, M.; Kao, J.; Liu, X. Investigation on acid fracturing treatment in limestone formation based on true tri-axial experiment. Fuel 2019, 235, 473–484. [Google Scholar] [CrossRef]
- Zhao, H.; Xiong, Y.; Zhen, H.; Liu, C.; Li, X. Experimental investigation on the fracture propagation of three-stage acid fracturing of tight sandstone gas reservoir. J. Pet. Sci. Eng. 2022, 211, 110143. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y. Triaxial experimental investigation into the characteristics of acid-etched fractures and acid fracturing. J. Pet. Sci. Eng. 2021, 202, 108431. [Google Scholar] [CrossRef]
- Wang, W.; Liu, T.G.; Shao, J.F. Effects of Acid Solution on the Mechanical Behavior of Sandstone. J. Mater. Civ. Eng. 2016, 28, 04015089. [Google Scholar] [CrossRef]
- Gao, J.; Chen, G.; Mitani, Y.; Gong, S.; Feng, C. Study on the effect of hydro-chemical dissolution on shear properties of rock fractures using the improved discontinuous deformation analysis. Int. J. Numer. Anal. Methods Geomech. 2023, 47, 1457–1480. [Google Scholar] [CrossRef]
- Luo, T.; Fan, G.; Guo, B.; Zhang, S. Experimental study on the influence of hydro-chemical erosion on morphology parameters and shear properties of limestone fractures. Acta Geotech. 2021, 16, 3867–3880. [Google Scholar] [CrossRef]
- Zhang, H.; Zhong, Y.; Zhang, J.; Zhang, Y.; Kuang, J.; Yang, B. Experimental research on deterioration of mechanical properties of carbonate rocks under acidified conditions. J. Pet. Sci. Eng. 2020, 185, 106612. [Google Scholar] [CrossRef]
- Grgic, D.; Giraud, A. The influence of different fluids on the static fatigue of a porous rock: Poro-mechanical coupling versus chemical effects. Mech. Mater. 2014, 71, 34–51. [Google Scholar] [CrossRef]
- Martin, R.J. Time-Dependent Crack Growth in Quartz and Its Application to Creep of Rocks. J. Geophys. Res. 1972, 77, 1406–1419. [Google Scholar] [CrossRef]
- Linsong, Z.; Jianfen, D.; Ping, G.; Zhouhua, W. Influence of Supercritical CO2 on the Physical Property of Unconsolidated Sandstone Reservoir. Oilfield Chem. 2015, 32, 217–222. [Google Scholar]
- Weiguo, L.; Wei, H.; Jiwei, Y. Weakening and fracturing mechanism of mechanical properties of coal and rock caused by supercritical CO2. J. China Coal Soc. 2022, 47, 2557–2568. [Google Scholar]
- Quanzhong, L.; Haiyang, H.; Xiaofeng, J. Research on differences in pore structures of coal, shale and sandstone and their effects on methane adsorption. Coal Sci. Technol. 2022, 50, 157–163. [Google Scholar]
- Mahmoud, H. Automatic characterization and quantitative analysis of seismic facies in naturally fractured reservoir: Case study of Amguid Messaoud field, Algeria. Min. Miner. Depos. 2023, 17, 42–48. [Google Scholar] [CrossRef]
- Ping, Q.; Li, X. Analysis of the Fracture Characteristics and Crack Propagation Mechanism of Fractured Sandstone under Dynamic Loading. Appl. Sci. 2024, 14, 2042. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Wang, Z.; Tan, J.; Zhao, Y. Biocomposition characteristics and potential evaluation of hydrocarbon generation in the Xujiahe Formation source rocks in the Sichuan Basin. Nat. Gas Geosci. 2024, 1–17. Available online: https://www.cnki.com.cn/Article/CJFDTotal-TDKX20240305001.htm (accessed on 10 April 2024).
- Geng, B.; He, X.; Yan, J.; Zhang, F.; Cui, Y.; Gu, J.; Li, Z.; Wen, D. NMR Based Low Permeability Sandstone Storage Layer Pore Structure Quantification Inversion Method, Involves Forming Wellbore Profile with Sand Rock Storage Layer along Reverse Inversion Direction. CN104819923-B, 15 May 2015. [Google Scholar]
- Du, Y.; Li, T.; Wang, B.; Zhang, S.; Li, H.; Zhang, H.; Zhu, Q. Experimental study on mechanical characteristics and permeability evolution during the coupled hydromechanical failure of sandstone containing a filled fissure. Acta Geotech. 2023, 18, 4055–4075. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Shu, Z. Distribution and genesis of calcite-replaced and calcite-cemented tight reservoirs in Xujiahe Formation, Yuanba area, Northeast Sichuan. Acta Pet. Sin. 2019, 40, 692–705. [Google Scholar]
- Zhou, Z. Reservoir Characteristics and Factors Controlling the Physical Properties of the Sandstone in the Forth Member of Xujiahe Formation in the Bazhong Area, Northeastern Sichuan Basin. Master Thesis, China University of Petroleum, Beijing, China, 2020. [Google Scholar]


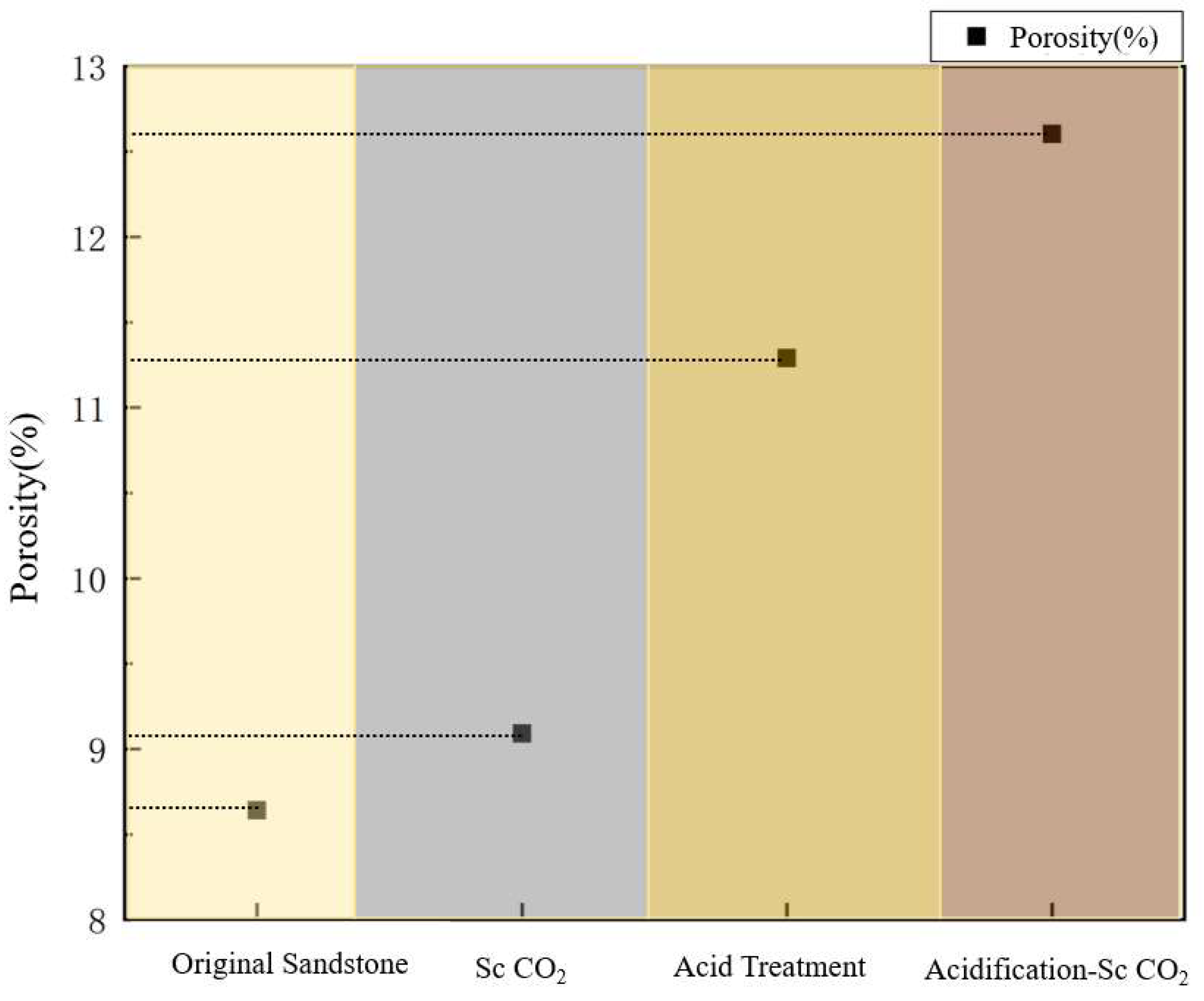






| Pretreatment Conditions | Porosity/% |
|---|---|
| Original sandstone | 8.64 |
| Sc CO2 | 9.08 |
| Acidification | 11.28 |
| Acidification-Sc CO2 | 12.60 |
| Pretreatment Conditions | Mineral Composition | |||
|---|---|---|---|---|
| Quartz (%) | Feldspar (%) | Clay Minerals (%) | Magnesite (%) | |
| Original sandstone | 42.1 | 2.4 | 51.6 | 3.9 |
| Sc CO2 | 45.7 | 2.3 | 48.1 | 3.9 |
| Acid treatment | 54.8 | 1.3 | 41.2 | 2.7 |
| Acidification–Sc CO2 | 62.5 | 0.7 | 34.2 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Huang, G.; Liang, Q.; Chen, Q. Study on Microscopic Characteristics and Rock Mechanical Properties of Tight Sandstone after Acidification–Supercritical CO2 Composite Action: Case Study from Xujiahe Formation, China. Appl. Sci. 2024, 14, 4108. https://doi.org/10.3390/app14104108
Zhao Y, Huang G, Liang Q, Chen Q. Study on Microscopic Characteristics and Rock Mechanical Properties of Tight Sandstone after Acidification–Supercritical CO2 Composite Action: Case Study from Xujiahe Formation, China. Applied Sciences. 2024; 14(10):4108. https://doi.org/10.3390/app14104108
Chicago/Turabian StyleZhao, Yunfei, Gun Huang, Qinming Liang, and Qiang Chen. 2024. "Study on Microscopic Characteristics and Rock Mechanical Properties of Tight Sandstone after Acidification–Supercritical CO2 Composite Action: Case Study from Xujiahe Formation, China" Applied Sciences 14, no. 10: 4108. https://doi.org/10.3390/app14104108




