Ethanolic Extracts of Six Cultivated Mushrooms as a Source of Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Solvent Extraction
2.3. Total Phenolics and Flavonoids Content
2.4. Antioxidant Capacity
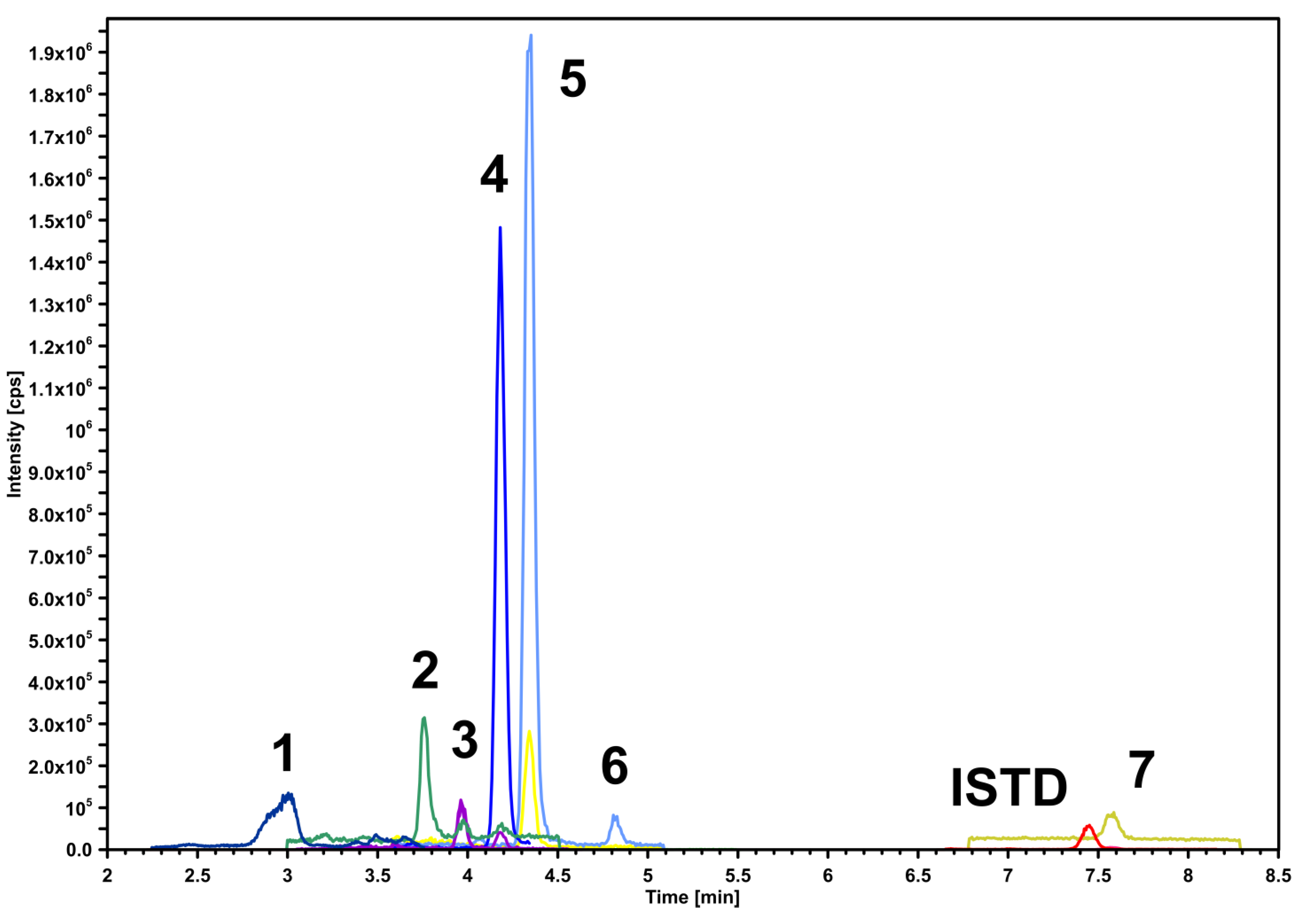
2.5. Determination of Phenolic Acids Profile
2.6. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield
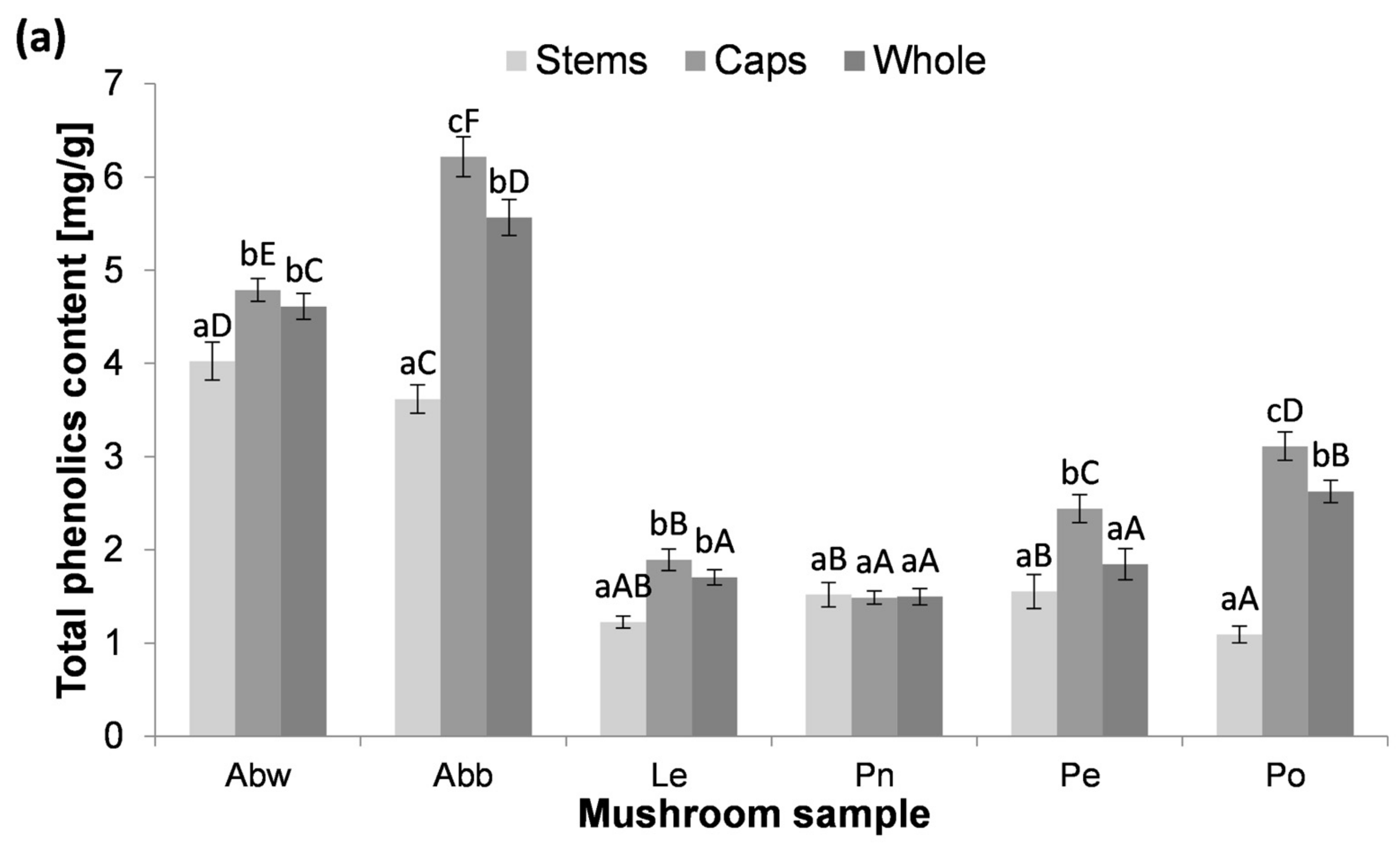
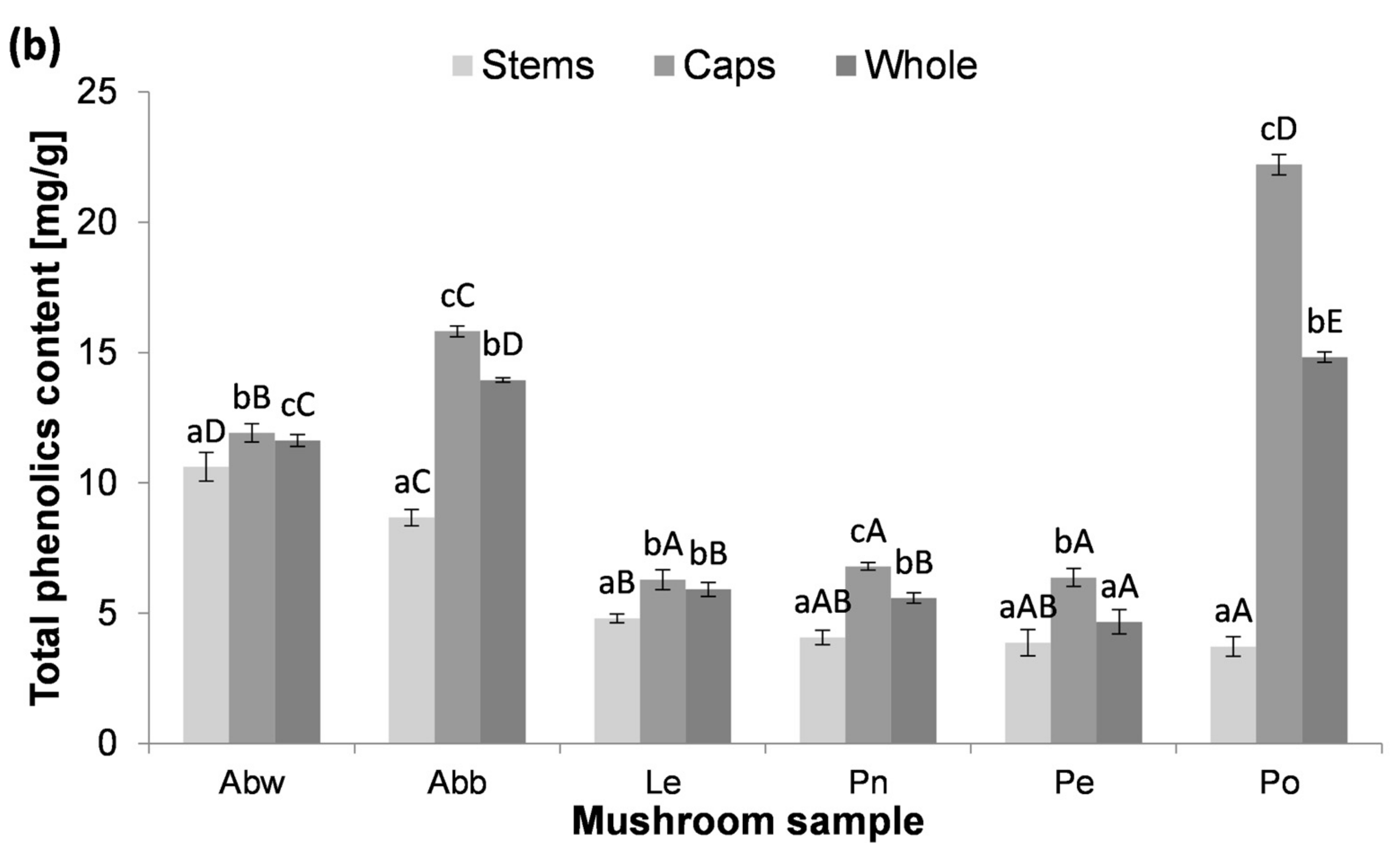
3.2. The Content of Total Phenolics
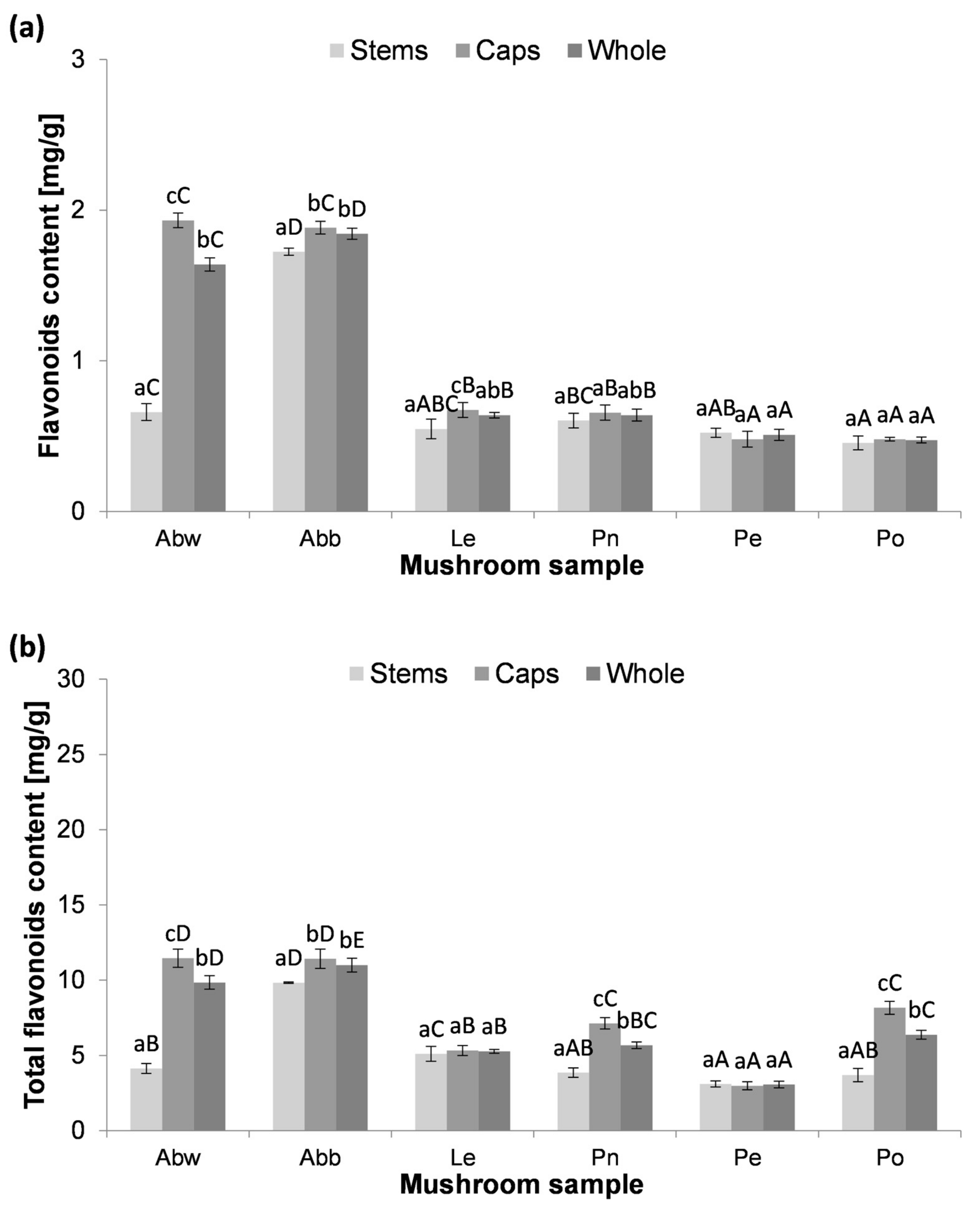
3.3. The Content of Total Flavonoids
3.4. Phenolic Acids Profile
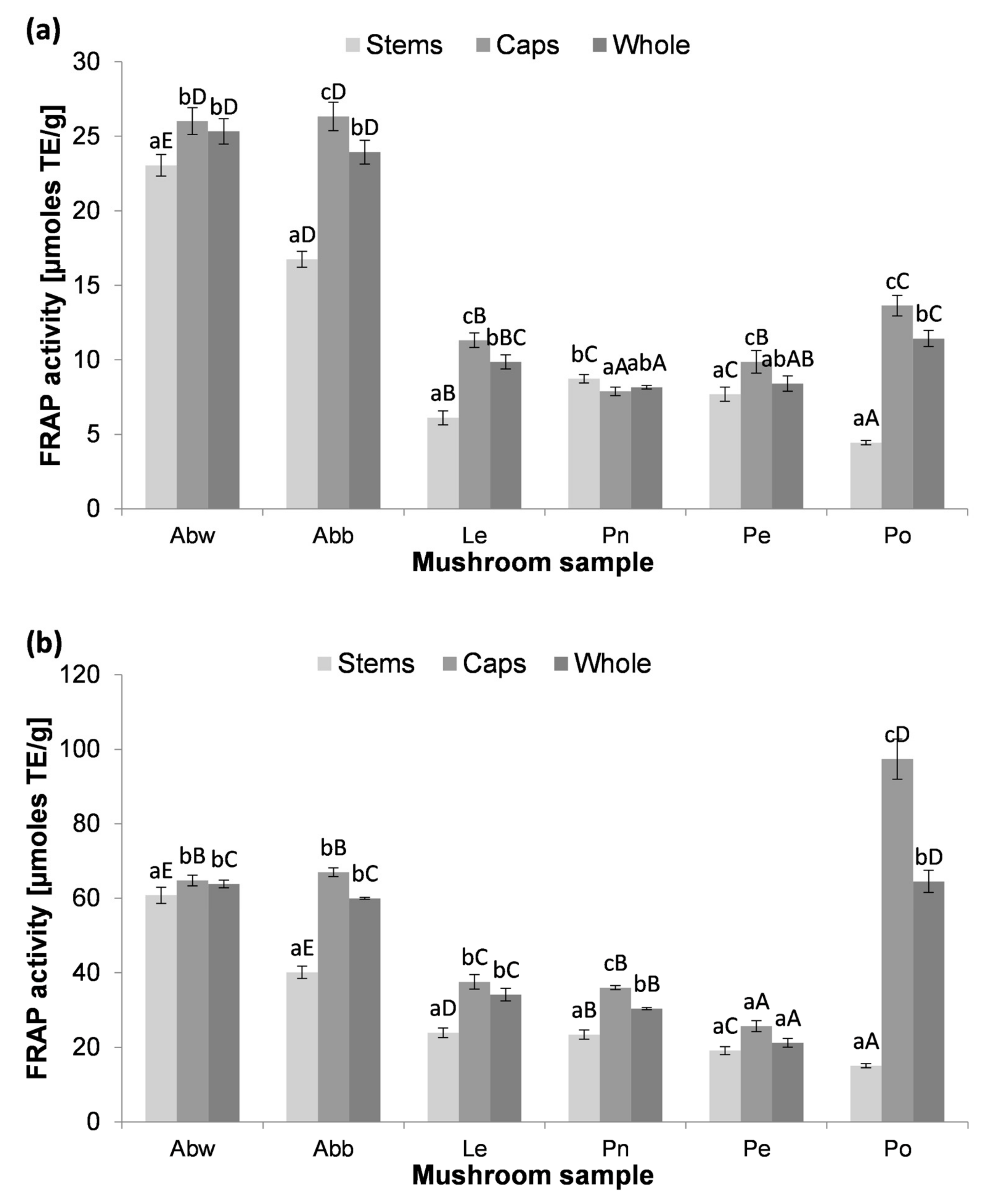
3.5. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sánchez, C. Modern Aspects of Mushroom Culture Technology. Appl. Microbiol. Biotechnol. 2004, 64, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Siwulski, M.; Sobieralski, K.; Sas-Golak, I. Wartość Odżywcza i Prozdrowotna Grzybów. Żywność Nauk. Technol. Jakość. 2014, 21, 16–28. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, S.; Sharma, V. Status and Trends in World Mushroom Production-III-World Production of Different Mushroom Species in 21st Century. Mushroom Res. 2021, 29, 75. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional Foods Based on Extracts or Compounds Derived from Mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Singh, M.P.; Rai, S.N.; Dubey, S.K.; Pandey, A.T.; Tabassum, N.; Chaturvedi, V.K.; Singh, N.B. Biomolecules of Mushroom: A Recipe of Human Wellness. Crit. Rev. Biotechnol. 2022, 42, 913–930. [Google Scholar] [CrossRef]
- Guillamón, E.; García-Lafuente, A.; Lozano, M.; D’arrigo, M.; Rostagno, M.A.; Villares, A.; Martínez, J.A. Edible Mushrooms: Role in the Prevention of Cardiovascular Diseases. Fitoterapia 2010, 81, 715–723. [Google Scholar] [CrossRef]
- Shaffique, S.; Kang, S.M.; Kim, A.Y.; Imran, M.; Khan, M.A.; Lee, I.J. Current Knowledge of Medicinal Mushrooms Related to Anti-Oxidant Properties. Sustain. 2021, 13, 7948. [Google Scholar] [CrossRef]
- Bhambri, A.; Srivastava, M.; Mahale, V.G.; Mahale, S.; Karn, S.K. Mushrooms as Potential Sources of Active Metabolites and Medicines. Front. Microbiol. 2022, 13, 837266. [Google Scholar] [CrossRef]
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Walton, J.; Viñas, I.; Tiwari, B.K. Ultrasound Assisted Extraction of Polysaccharides from Mushroom By-Products. Lwt 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Ribeiro, B.; Lopes, R.; Andrade, P.B.; Seabra, R.M.; Gonçalves, R.F.; Baptista, P.; Quelhas, I.; Valentão, P. Comparative Study of Phytochemicals and Antioxidant Potential of Wild Edible Mushroom Caps and Stipes. Food Chem. 2008, 110, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-Radical Scavenging Capacity and Reducing Power of Wild Edible Mushrooms from Northeast Portugal: Individual Cap and Stipe Activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Barroso, L.S.; Copetti, C.; Komeroski, M.R.; Saudades, J.d.O.; da Silva, V.L.; Doneda, D.; Camargo, L.d.R.; Flôres, S.H.; Rios, A.d.O.; de Oliveira, V.R. Physicochemical and Sensory Evaluation in Sautéed Caps and Stems of Edible Mushrooms. J. Culin. Sci. Technol. 2020, 18, 306–316. [Google Scholar] [CrossRef]
- Radzki, W.; Kalbarczyk, J. Water Soluble Polysaccharides Content in Three Species of Edible and Medicinal Mushrooms: Lentinula Edodes, Pleurotus ostreatus, Agaricus Blazei. Herba Pol. 2010, 56, 31–38. [Google Scholar]
- Takeuchi, T.; Pereira, C.; Braga, M.; Maróstica, M.; Leal, P.; Meireles, A. Low-Pressure Solvent Extraction (Solid–Liquid Extraction, Microwave Assisted, and Ultrasound Assisted) from Condimentary Plants. In Extracting Bioactive Compounds for Food Products; Meireles, A., Ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic Acid–Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The Determination of Flavonoid Contents Inmulberry and Their Scavenging Effects on Superoxides Radicals. Food Chem. 1998, 64, 555–559. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Szydłowska-Tutaj, M.; Szymanowska, U.; Tutaj, K.; Domagała, D.; Złotek, U. The Addition of Reishi and Lion’s Mane Mushroom Powder to Pasta Influences the Content of Bioactive Compounds and the Antioxidant, Potential Anti-Inflammatory, and Anticancer Properties of Pasta. Antioxidants 2023, 12, 738. [Google Scholar] [CrossRef]
- Radzki, W.; Sławinska, A.; Jabłonska-Ryś, E.; Michalak-Majewska, M. Effect of Blanching and Cooking on Antioxidant Capacity of Cultivated Edible Mushrooms: A Comparative Study. Int. Food Res. J. 2016, 23, 599–605. [Google Scholar]
- Huang, D.; Boxin, O.U.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant Properties of Phenolic Compounds Occurring in Edible Mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of Polyphenols and Ergothioneine in Cultivated Mushrooms and Correlation to Total Antioxidant Capacity. Food Chem. 2007, 105, 727–735. [Google Scholar] [CrossRef]
- Shreya, S.; Mohapatra, D.; Naik, G.G.; Bobde, Y.; Ghosh, B.; Sahu, A.N. In Vitro Antioxidant and Cytotoxic Potential of Pleurotus Mushroom and Activity-Based Correlation: A Comparative Study. J. Anal. Chem. 2023, 78, 456–463. [Google Scholar] [CrossRef]
- Babu, D.R.; Rao, G.N. Antioxidant Properties and Electrochemical Behavior of Cultivated Commercial Indian Edible Mushrooms. J. Food Sci. Technol. 2013, 50, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-H.; Jin, Y.-J.; Pyo, Y.-H. Antioxidant Properties and Ubiquinone Contents in Different Parts of Several Commercial Mushrooms. J. Korean Scociety Food Sci. Nutr. 2012, 41, 1235–1241. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, H.; Zhang, Y.; Ma, L.; Xu, X. Comparative Studies on Chemical Parameters and Antioxidant Properties of Stipes and Caps of Shiitake Mushroom as Affected by Different Drying Methods. J. Sci. Food Agric. 2013, 93, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, A.; Janczewska, A.; Kobus-Cisowska, J.; Dziedziński, M.; Siwulski, M.; Czarniecka-Skubina, E.; Stuper-Szablewska, K. The Effect of Light Conditions on the Content of Selected Active Ingredients in Anatomical Parts of the Oyster Mushroom (Pleurotus ostreatus L.). PLoS ONE 2022, 17, e0262279. [Google Scholar] [CrossRef]
- Barros, L.; Falcão, S.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Antioxidant Activity of Agaricus Sp. Mushrooms by Chemical, Biochemical and Electrochemical Assays. Food Chem. 2008, 111, 61–66. [Google Scholar] [CrossRef]
- Chu, M.; Khan, R.D.; Zhou, Y.; Agar, O.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Phenolic Compounds in Common Commercial Mushrooms and Their Potential Antioxidant Activities. Processes 2023, 11, 1711. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic Compound Concentration and Antioxidant Activities of Edible and Medicinal Mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Ucar, T.M.; Karadag, A. The Effects of Vacuum and Freeze-Drying on the Physicochemical Properties and in Vitro Digestibility of Phenolics in Oyster Mushroom (Pleurotus ostreatus). J. Food Meas. Charact. 2019, 13, 2298–2309. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Šamec, D.; Piljac, A.; Mešić, A.; Tkalčec, Z. Antioxidant Properties of Extracts of Wild Medicinal Mushroom Species from Croatia. Int. J. Med. Mushrooms 2011, 13, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Parrilla, E.; De La Rosa, L.A.; Martínez, N.R.; González Aguilar, G.A. Total Phenols and Antioxidant Activity of Commercial and Wild Mushrooms from Chihuahua, Mexico. Cienc. Tecnol. Aliment. 2007, 5, 329–334. [Google Scholar] [CrossRef]
- Mishra, K.K.; Pal, R.S.; Bhatt, J.C. Comparison of Antioxidant Properties in Cap and Stipe of Lentinula Edodes -A Medicinal Mushroom. Emir. J. Food Agric. 2015, 27, 562–569. [Google Scholar] [CrossRef]
- Prior, R.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Oboh, G.; Shodehinde, S.A. Distribution of Nutrients, Polyphenols and Antioxidant Activities in the Pilei and Stipes of Some Commonly Consumed Edible Mushrooms in Nigeria. Bull. Chem. Soc. Ethiop. 2009, 23, 391–398. [Google Scholar] [CrossRef]
- Lin, J.T.; Liu, C.W.; Chen, Y.C.; Hu, C.C.; Juang, L.D.; Shiesh, C.C.; Yang, D.J. Chemical Composition, Antioxidant and Anti-Inflammatory Properties for Ethanolic Extracts from Pleurotus Eryngii Fruiting Bodies Harvested at Different Time. Lwt 2014, 55, 374–382. [Google Scholar] [CrossRef]


| Species | Stems [%] | Caps [%] | S:C Ratio | Description of the Fruiting Bodies |
|---|---|---|---|---|
| A. bisporus (white) | 23 | 77 | 1:3.35 | closed caps, caps diameter 4–6 cm |
| A. bisporus (brown) | 21 | 62 | 1:2.95 | closed caps, caps diameter 2.5–4.5 cm |
| L. edodes | 27.8 | 72.2 | 1:2.59 | open caps, caps diameter 4–6 cm |
| P. nameko | 32 | 68 | 1:2.12 | closed caps, caps diameter 0.8–1.7 cm |
| P. eryngii | 67.4 | 32.6 | 1:0.48 | open caps, caps diameter 4.5–7 cm |
| P. ostreatus | 24.5 | 75.5 | 1:3.09 | open caps, caps length 3.5–9 cm |
| Compound | Retention Time (min) | Precursor Ion (m/z) | Product Ion 1 (m/z)/CE | Product Ion 2 (m/z)/CE | DP | CXP |
|---|---|---|---|---|---|---|
| 3,4-Dihydroxybenzoic acid | 3.00 | 153 | 108.9/−20 | 65.0/−30 | −30 | −11 |
| Caffeic acid | 3.75 | 179 | 135.0/−22 | 107.0/−32 | −30 | −9 |
| Syringic acid | 3.80 | 197 | 181.9/−18 | 122.9/−30 | −30 | −19 |
| 4-Hydroxybenzaldehyde | 4.18 | 121 | 91.9/−30 | 65.1/−32 | −30 | −11 |
| p-Coumaric acid | 4.33 | 163 | 119.0/−20 | 92.9/−42 | −20 | −9 |
| Ferulic acid | 4.74 | 193 | 177.9/−18 | 134/−22 | −30 | −11 |
| trans-Cinnamic-d7 acid | 7.40 | 153,9 | 110.0/−16 | - | −35 | −13 |
| trans-Cinnamic acid | 7.53 | 146.9 | 77.0/−28 | 103/−14 | −30 | −9 |
| Species | Stems | Caps | Whole |
|---|---|---|---|
| A. bisporus (white) | 37.9 ± 1.1 aC | 40.2 ± 1.4 bD | 39.7 ± 1.0 bC |
| A. bisporus (brown) | 41.7 ± 0.6 aD | 39.3 ± 0.3 aD | 39.9 ± 1.3 aC |
| L. edodes | 25.5 ± 0.6 aA | 30.1 ± 0.3 cC | 28.8 ± 0.1 bB |
| P. nameko | 37.3 ± 0.8 cC | 21.9 ± 0.2 aB | 26.8 ± 0.6 bB |
| P. eryngii | 40.2 ± 0.6 bD | 38.3 ± 0.3 aD | 39.6 ± 0.4 abC |
| P. ostreatus | 29.4 ± 0.5 cB | 14.0 ± 0.1 aA | 17.7 ± 0.7 bA |
| Species | Part | 3,4-DHBA | Caffeic Acid | Syringic Acid | p-Coumaric Acid | Ferulic Acid | t-Cinnamic Acid | 4-hydroxybenzaldehyde |
|---|---|---|---|---|---|---|---|---|
| A. bisporus | Stems | 0.05 ± 0.02/0.14 ± 0.04 | nd | 0.26 ± 0.01/0.69 ± 0.03 | 0.03 ± 0.01/0.07 ± 0.02 | 0.06 ± 0.00/0.16 ± 0.01 | 0.29 ± 0.02/0.76 ± 0.04 | 0.81 ± 0.03/2.14 ± 0.14 |
| (white) | Caps | 0.10 ± 0.04/0.26 ± 0.11 | 0.02 ± 0.00/0.04 ± 0.00 | 0.22 ± 0.02/0.55 ± 0.05 | 0.08 ± 0.01/0.19 ± 0.04 | 0.06 ± 0.00/0.14 ± 0.01 | 1.20 ± 0.12/2.99 ± 0.34 | 0.30 ± 0.08/0.74 ± 0.18 |
| Whole | 0.09 ± 0.03/0.24 ± 0.08 | 0.01 ± 0.00/0.03 ± 0.00 | 0.23 ± 0.01/0.58 ± 0.04 | 0.07 ± 0.01/0.17 ± 0.03 | 0.06 ± 0.00/0.15 ± 0.01 | 0.99 ± 0.10/2.50 ± 0.25 | 0.41 ± 0.05/1.04 ± 0.12 | |
| A. bisporus | Stems | 0.15 ± 0.02/0.36 ± 0.05 | 0.04 ± 0.02/0.11 ± 0.04 | 0.41 ± 0.03/0.98 ± 0.08 | 0.31 ± 0.02/0.73 ± 0.06 | 0.06 ± 0.01/0.15 ± 0.01 | 0.63 ± 0.08/1.50 ± 0.19 | 0.68 ± 0.03/1.63 ± 0.07 |
| (brown) | Caps | 0.09 ± 0.01/0.23 ± 0.02 | 0.35 ± 0.01/0.90 ± 0.05 | 0.35 ± 0.01/0.88 ± 0.04 | 0.62 ± 0.07/1.58 ± 0.24 | 0.06 ± 0.00/0.15 ± 0.00 | 0.62 ± 0.09/1.57 ± 0.24 | 0.51 ± 0.01/1.29 ± 0.08 |
| Whole | 0.10 ± 0.01/0.26 ± 0.03 | 0.28 ± 0.00/0.71 ± 0.03 | 0.36 ± 0.02/0.90 ± 0.03 | 0.55 ± 0.06/1.37 ± 0.19 | 0.06 ± 0.00/0.15 ± 0.00 | 0.62 ± 0.07/1.55 ± 0.18 | 0.55 ± 0.01/1.37 ± 0.07 | |
| L. edodes | Stems | nd | 0.23 ± 0.05/0.89 ± 0.22 | 2.18 ± 0.07/8.55 ± 0.49 | 0.02 ± 0.01/0.07 ± 0.02 | nd | 0.86 ± 0.06/3.35 ± 0.14 | 0.30 ± 0.01/1.17 ± 0.06 |
| Caps | nd | 0.47 ± 0.12/1.55 ± 0.39 | 0.68 ± 0.04/2.26 ± 0.11 | nd | nd | 0.83 ± 0.04/2.76 ± 0.12 | 0.29 ± 0.02/0.95 ± 0.07 | |
| Whole | nd | 0.41 ± 0.11/1.43 ± 0.36 | 1.02 ± 0.05/3.55 ± 0.16 | <0.01/0.01 ± 0.00 | nd | 0.84 ± 0.02/2.91 ± 0.09 | 0.29 ± 0.02/1.01 ± 0.07 | |
| P. nameko | Stems | 0.59 ± 0.02/1.57 ± 0.07 | 0.18 ± 0.01/0.48 ± 0.01 | 1.21 ± 0.03/3.24 ± 0.03 | 0.39 ± 0.04/1.04 ± 0.13 | 0.07 ± 0.00/0.20 ± 0.01 | 0.93 ± 0.04/2.49 ± 0.07 | 0.52 ± 0.02/1.39 ± 0.08 |
| Caps | 0.93 ± 0.03/4.26 ± 0.24 | 0.37 ± 0.02/1.71 ± 0.09 | 1.63 ± 0.02/7.45 ± 0.22 | 3.79 ± 0.34/17.33 ± 2.04 | nd | 3.16 ± 0.07/14.42 ± 0.09 | 1.12 ± 0.05/5.10 ± 0.34 | |
| Whole | 0.85 ± 0.03/3.18 ± 0.17 | 0.33 ± 0.01/1.23 ± 0.05 | 1.53 ± 0.02/5.72 ± 0.13 | 3.00 ± 0.27/11.22 ± 1.30 | 0.02 ± 0.00/0.06 ± 0.00 | 2.64 ± 0.06/9.86 ± 0.07 | 0.98 ± 0.04/3.65 ± 0.23 | |
| P. eryngii | Stems | 1.71 ± 0.05/4.27 ± 0.15 | nd | 0.17 ± 0.02/0.43 ± 0.04 | 0.05 ± 0.01/0.13 ± 0.03 | nd | 1.07 ± 0.55/2.66 ± 1.41 | 0.60 ± 0.04/1.50 ± 0.09 |
| Caps | 3.97 ± 0.09/10.37 ± 0.42 | nd | 0.07 ± 0.01/0.18 ± 0.02 | 0.23 ± 0.01/0.60 ± 0.04 | nd | 1.17 ± 0.71/3.06 ± 1.87 | 0.71 ± 0.01/1.84 ± 0.01 | |
| Whole | 3.45 ± 0.06/8.73 ± 0.15 | nd | 0.09 ± 0.01/0.23 ± 0.01 | 0.19 ± 0.01/0.48 ± 0.03 | nd | 1.15 ± 0.61/2.91 ± 1.56 | 0.68 ± 0.01/1.73 ± 0.03 | |
| P. ostreatus | Stems | 0.95 ± 0.03/3.24 ± 0.11 | 0.24 ± 0.01/0.81 ± 0.01 | 0.28 ± 0.00/0.95 ± 0.01 | 0.06 ± 0.01/0.22 ± 0.01 | nd | 0.47 ± 0.28/1.62 ± 0.98 | 0.60 ± 0.01/2.05 ± 0.02 |
| Caps | 2.31 ± 0.11/16.52 ± 1.83 | 0.09 ± 0.01/0.62 ± 0.09 | 0.05 ± 0.01/0.38 ± 0.06 | 0.07 ± 0.01/0.50 ± 0.09 | nd | 0.80 ± 0.05/5.71 ± 0.34 | 0.84 ± 0.03/5.99 ± 0.58 | |
| Whole | 1.99 ± 0.08/11.28 ± 0.84 | 0.12 ± 0.01/0.69 ± 0.06 | 0.10 ± 0.00/0.59 ± 0.04 | 0.07 ± 0.01/0.39 ± 0.05 | nd | 0.73 ± 0.09/4.10 ± 0.58 | 0.78 ± 0.02/4.43 ± 0.29 |
| Assay | FRAP | DPPH | TFC |
|---|---|---|---|
| TPC | 0.97 * | 0.83 * | 0.67 * |
| TFC | 0.69 * | 0.48 * | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzki, W.; Tutaj, K.; Skrzypczak, K.; Michalak-Majewska, M.; Gustaw, W. Ethanolic Extracts of Six Cultivated Mushrooms as a Source of Bioactive Compounds. Appl. Sci. 2024, 14, 66. https://doi.org/10.3390/app14010066
Radzki W, Tutaj K, Skrzypczak K, Michalak-Majewska M, Gustaw W. Ethanolic Extracts of Six Cultivated Mushrooms as a Source of Bioactive Compounds. Applied Sciences. 2024; 14(1):66. https://doi.org/10.3390/app14010066
Chicago/Turabian StyleRadzki, Wojciech, Krzysztof Tutaj, Katarzyna Skrzypczak, Monika Michalak-Majewska, and Waldemar Gustaw. 2024. "Ethanolic Extracts of Six Cultivated Mushrooms as a Source of Bioactive Compounds" Applied Sciences 14, no. 1: 66. https://doi.org/10.3390/app14010066







