Preparation of Nanoemulgels Containing Lemon Essential Oil and Pectin: Physical Stability and Rheological Properties
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulgel Preparation
2.3. Particle Size Distribution
2.4. Apparent Viscosity of the Emulgel
2.5. Physical Stability
2.6. Statistical Analysis
3. Results and Discussion
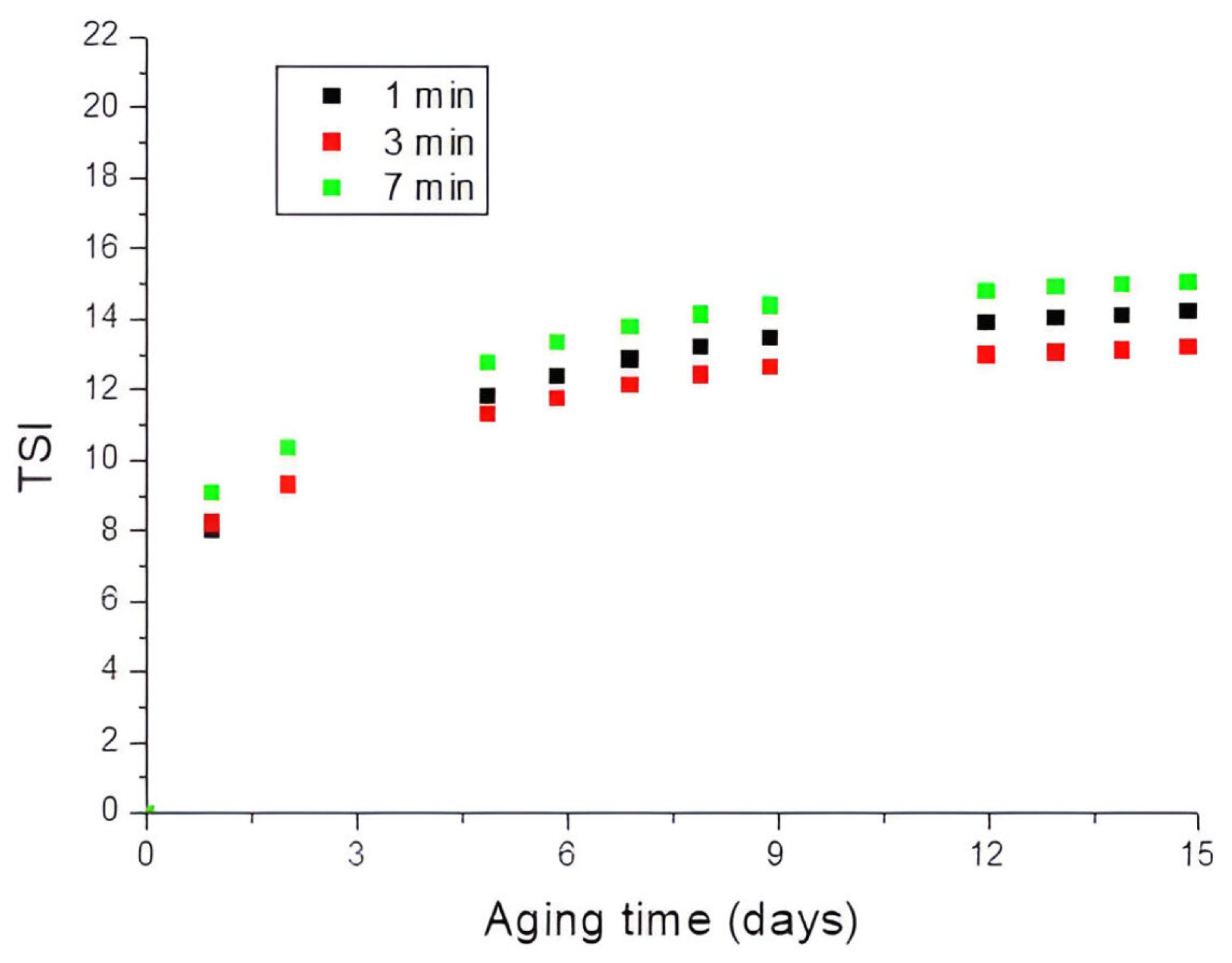
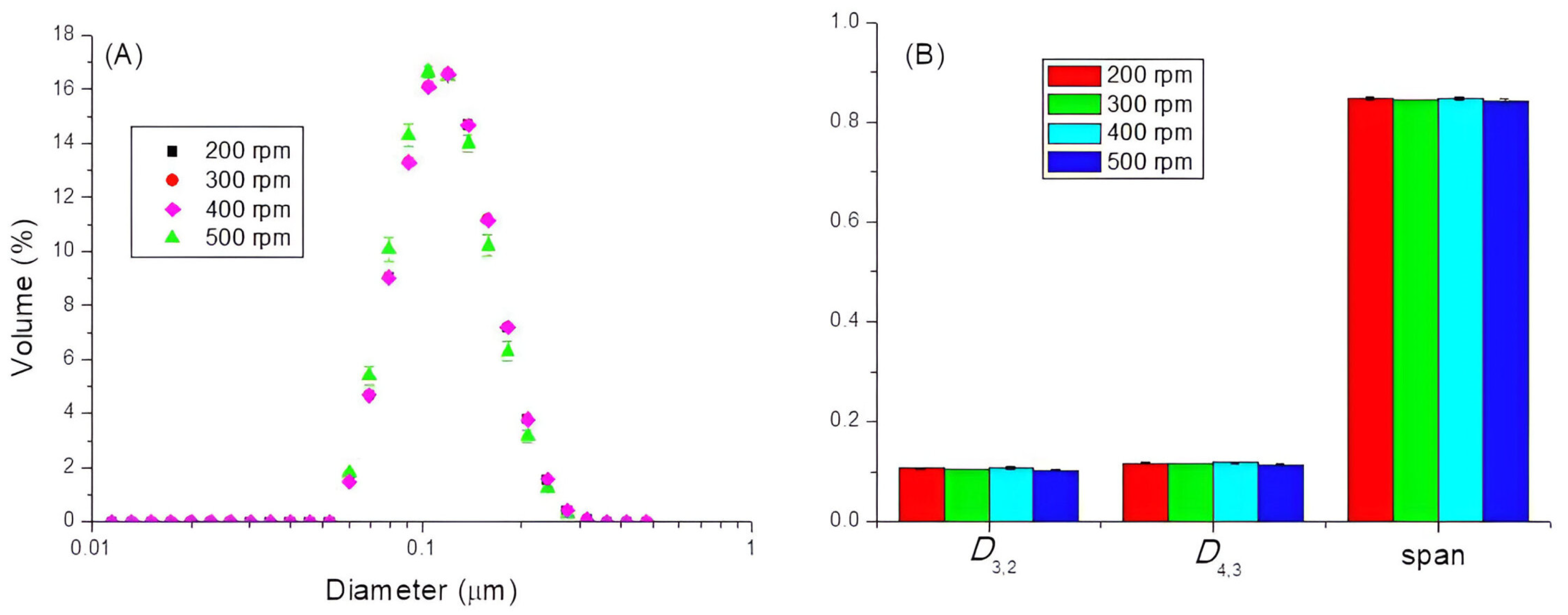
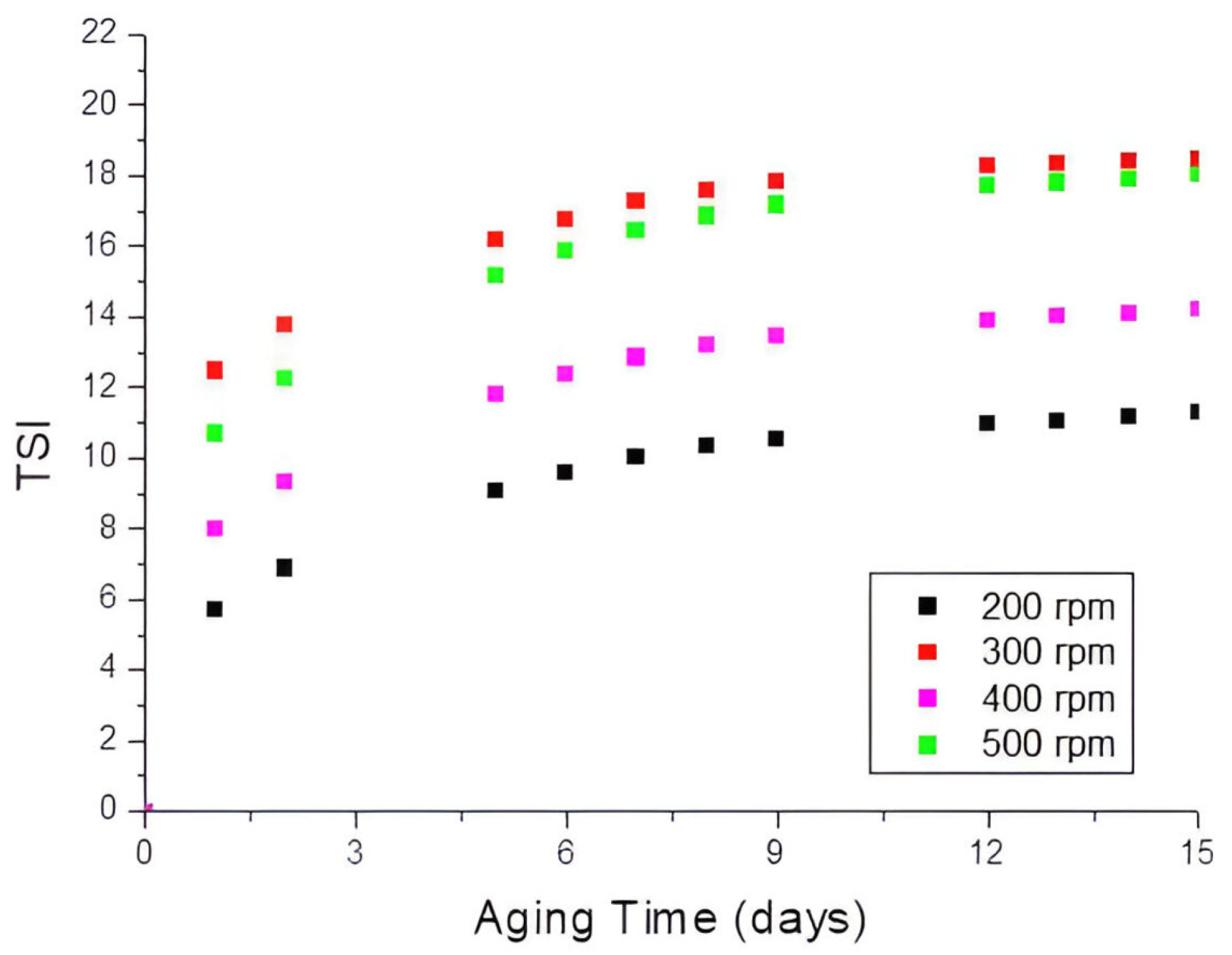
3.1. Influence of the Stirring Time and the Rotational Rate
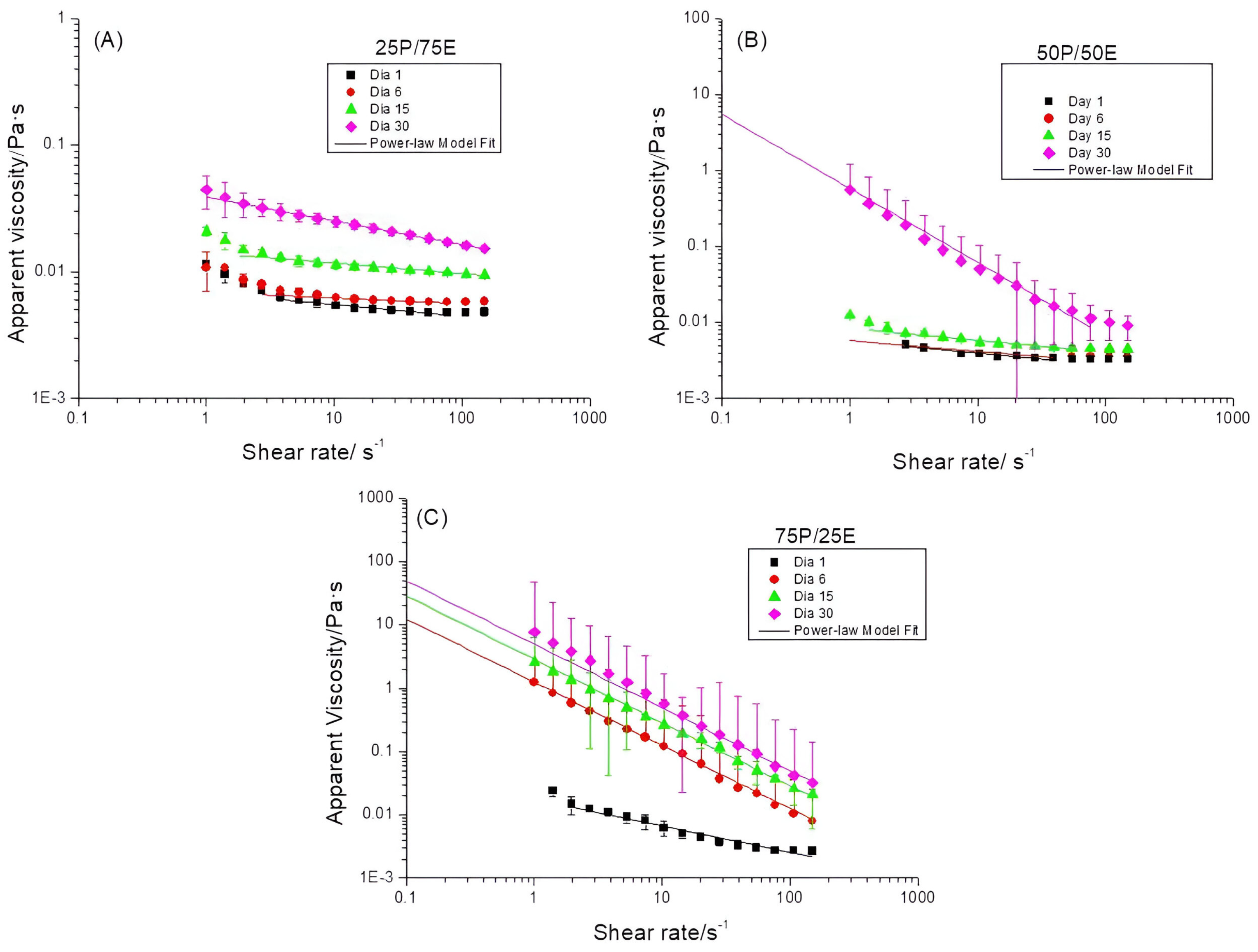
3.2. Influence of the Pectin/Emulsion Mass Ratio
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Dhal, S.; Pal, A.; Gramza-Michalowska, A.; Kim, D.; Mohanty, B.; Sagiri, S.S.; Pal, K. Formulation and Characterization of Emulgel-Based Jelly Candy: A Preliminary Study on Nutraceutical Delivery. Gels 2023, 9, 466. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.; Moraes, I.C.; Pinho, S.C. Rheology of Emulsion-Filled Gels Applied to the Development of Food Materials. Gels 2016, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Milutinov, J.; Krstonošić, V.; Ćirin, D.; Pavlović, N. Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers 2023, 15, 2302. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.I. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004, 6, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Khalil, E.; Schaefer, U.; Sallam, A. Release characteristics of diclofenac diethylamine from emulgels containing Pluronic F127. J. Drug Deliv. Sci. Technol. 2006, 16, 381–387. [Google Scholar] [CrossRef]
- Srivastava, M.; Neupane, Y.R.; Kumar, P.; Kohli, K. Nanoemulgel (NEG) of Ketoprofen with eugenol as oil phase for the treatment of ligature-induced experimental periodontitis in Wistar rats. Drug Deliv. 2016, 23, 2228–2234. [Google Scholar] [CrossRef]
- Ambhore, N.P.; Dandagi, P.M.; Gadad, A.P.; Mandora, P. Formulation and Characterization of Tapentadol Loaded Emulgel for Topical Application. Indian J. Pharm. Educ. Res. 2017, 51, 525–535. [Google Scholar] [CrossRef]
- Daudt, R.M.; Back, P.I.; Cardozo, N.S.M.; Marczak, L.D.F.; Külkamp-Guerreiro, I.C. Pinhão starch and coat extract as new natural cosmetic ingredients: Topical formulation stability and sensory analysis. Carbohydr. Polym. 2015, 134, 573–580. [Google Scholar] [CrossRef]
- Chang, W.-C.; Hu, Y.-T.; Huang, Q.; Hsieh, S.-C.; Ting, Y. Development of a topical applied functional food formulation: Adlay bran oil nanoemulgel. LWT 2020, 117, 108619. [Google Scholar] [CrossRef]
- Muñoz, J.; Prieto-Vargas, P.; García, M.C.; Alfaro-Rodríguez, M.C. Effect of a Change in the CaCl2/Pectin Mass Ratio on the Particle Size, Rheology and Physical Stability of Lemon Essential Oil/W Emulgels. Foods 2023, 12, 1137. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Deveda, P.; Vyas, N.; Chauhan, J. Development of antifungal emulsion based gel for topical fungal infection. Int. J. Pharm. Res. Dev. 2011, 3, 18–25. [Google Scholar]
- Abdullah; Liu, L.; Javed, H.U.; Xiao, J. Engineering emulsion gels as functional colloids emphasizing food applications: A review. Front. Nutr. 2022, 9, 890188. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of Emulsion Gels for the Delivery of Functional Food Ingredients: From Structure to Functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Yuan, F.; Gao, Y.; Mao, L. Emulsion gels with different proteins at the interface: Structures and delivery functionality. Food Hydrocoll. 2021, 116, 106637. [Google Scholar] [CrossRef]
- Yalçınöz, Ş.; Erçelebi, E. Potential applications of nano-emulsions in the food systems: An update. Mater. Res. Express 2018, 5, 062001. [Google Scholar] [CrossRef]
- Kirkeskov, B.; Christensen, R.; Bugel, R.; Bliddal, H.; Danneskiold-Samsøe, B.; Christensen, L.P.; Anders, J.R. The effects of rose hip (Rosa canina) on plasma antioxidative activity and C-reactive protein in patients with rheumatoid arthritis and normal controls: A prospective cohort study. Phytomedicine 2011, 18, 953–958. [Google Scholar] [CrossRef]
- Ammad, F.; Moumen, O.; Gasem, A.; Othmane, S.; Hisashi, K.-N.; Zebib, B.; Merah, O. The potency of lemon (Citrus limon L.) essential oil to control some fungal diseases of grapevine wood. Comptes Rendus Biol. 2018, 341, 97–101. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation Strategies to Improve the Bioavailability of Poorly Absorbed Drugs with Special Emphasis on Self-Emulsifying Systems. ISRN Pharm. 2013, 2013, 848043. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Halima, N.B.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Liang, Z.; Zhu, H.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou Y. B. chang against Listeria monocytogenes. Food Control 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Tmušić, N.; Stanojević, L.; Stanojević, J.; Cvetković, D. Essential oils content, composition and antioxidant activity of lemon balm, mint and sweet basil from Serbia. LWT 2022, 153, 112210. [Google Scholar] [CrossRef]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutierrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E.S. Chemical constituents, in vitro antioxidant activity and in silico study on NADPH oxidase of Allium sativum L. (garlic) essential oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef]
- Pucci, M.; Raimondo, S.; Zichittella, C.; Tinnirello, V.; Corleone, V.; Aiello, G.; Moschetti, M.; Conigliaro, A.; Fontana, S.; Alessandro, R. Biological Properties of a Citral-Enriched Fraction of Citrus limon Essential Oil. Foods 2020, 9, 1290. [Google Scholar] [CrossRef]
- Sharma, N.; Tripathi, A. Effects of Citrus sinensis (L.) Osbeck epicarp essential oil on growth and morphogenesis of Aspergillus niger (L.) Van Tieghem. Microbiol. Res. 2008, 163, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Kharat, P.; Chary, P.S.; Bhavana, V.; Rajana, N.; Devabattula, G.; Godugu, C.; Singh, S.B.; Mehra, N.K. Thymoquinone-Loaded Essential Oil–Based Emulgel as an Armament for Anti-psoriatic Activity. AAPS PharmSciTech 2023, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Patel, D.K.; Rai, V.K.; Pal, A.; Yadav, N.P. Development of emulgel formulation for vaginal candidiasis: Pharmaceutical characterization, in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2018, 48, 490–498. [Google Scholar] [CrossRef]
- da Silva Campelo, M.; Melo, E.O.; Arrais, S.P.; do Nascimento, F.B.S.A.; Gramosa, N.V.; de Aguiar Soares, S.; Ribeiro, M.E.N.P.; da Silva, C.R.; Júnior, H.V.N.; Ricardo, N.M.P.S. Clove essential oil encapsulated on nanocarrier based on polysaccharide: A strategy for the treatment of vaginal candidiasis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125732. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Elghamry, H.A.; Khalifa, N.E.; Khojali, W.M.A.; Khafagy, E.-S.; Shawky, S.; El-Horany, H.E.-S.; El-Housiny, S. Development and Optimization of Erythromycin Loaded Transethosomes Cinnamon Oil Based Emulgel for Antimicrobial Efficiency. Gels 2023, 9, 137. [Google Scholar] [CrossRef]
- Azam, F.; Alqarni, M.H.; Alnasser, S.M.; Alam, P.; Jawaid, T.; Kamal, M.; Khan, S.; Alam, A. Formulation, In Vitro and In Silico Evaluations of Anise (Pimpinella anisum L.) Essential Oil Emulgel with Improved Antimicrobial Effects. Gels 2023, 9, 111. [Google Scholar] [CrossRef]
- Velev, O.; Gurkov, T.; Chakarova, S.; Dimitrova, B.; Ivanov, I.; Borwankar, R. Experimental investigations on model emulsion systems stabilized with non-ionic surfactant blends. Colloids Surf. A Physicochem. Eng. Asp. 1994, 83, 43–55. [Google Scholar] [CrossRef]
- Huibers, P.D.T.; Shah, D.O. Evidence for Synergism in Nonionic Surfactant Mixtures: Enhancement of Solubilization in Water-in-Oil Microemulsions. Langmuir 1997, 13, 5762–5765. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, L.; Dai, T.; Chen, J.; Liu, W.; Liu, C.; Chen, M.; Liang, R. Emulsifying and emulsion stabilization mechanism of pectin from Nicandra physaloides (Linn.) Gaertn seeds: Comparison with apple and citrus pectin. Food Hydrocoll. 2022, 130, 107674. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Guerra-Rosas, M.; Morales-Castro, J.; Salvia-Trujillo, L.; Martín-Belloso, O. Influence of essential oils and pectin on nanoemulsion formulation: A ternary phase experimental approach. Food Hydrocoll. 2018, 81, 209–219. [Google Scholar] [CrossRef]
- Huang, S.; Wang, H.; Wang, S.; Sha, X.; Chen, N.; Hu, Y.; Tu, Z. Pectin Stabilized Fish Gelatin Emulsions: Physical Stability, Rheological, and Interaction Properties. Front. Nutr. 2022, 9, 961875. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gabriele, D.; Seta, L.; Baldino, N.; de Cindio, B.; Marino, R. Rheological investigation of pectin-based emulsion gels for pharmaceutical and cosmetic uses. Rheol. Acta 2015, 54, 41–52. [Google Scholar] [CrossRef]
- Hou, J.; Guo, J.; Wang, J.; Yang, X. Effect of interfacial composition and crumbliness on aroma release in soy protein/sugar beet pectin mixed emulsion gels. J. Sci. Food Agric. 2016, 96, 4449–4456. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, S.; Lian, Y.; Yang, Y.; Tian, G.; Zhao, C.; Gao, W.; Zheng, J. Effects of hydrosoluble calcium ions and organic acids on citrus oil emulsions stabilized with citrus pectin. Food Hydrocoll. 2020, 100, 105413. [Google Scholar] [CrossRef]
- Jiang, W.-X.; Qi, J.-R.; Liao, J.-S.; Yang, X.-Q. Acid/ethanol induced pectin gelling and its application in emulsion gel. Food Hydrocoll. 2021, 118, 106774. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, J.; Wang, Y.; Ye, X.; Chen, S.; Pan, H.; Chen, J. Fabrication of rhamnogalacturonan-I enriched pectin-based emulsion gels for protection and sustained release of curcumin. Food Hydrocoll. 2022, 128, 107592. [Google Scholar] [CrossRef]
- Santos, J.; Alfaro-Rodríguez, M.-C.; Vega, L.; Muñoz, J. Relationship between HLB Number and Predominant Destabilization Process in Microfluidized Nanoemulsions Formulated with Lemon Essential Oil. Appl. Sci. 2023, 13, 5208. [Google Scholar] [CrossRef]
- Lorenzo, G.; Zaritzky, N.; Califano, A. Rheological analysis of emulsion-filled gels based on high acyl gellan gum. Food Hydrocoll. 2013, 30, 672–680. [Google Scholar] [CrossRef]
- Isusi, G.S.; Bindereif, B.; Karbstein, H.; van der Schaaf, U. Polymer or microgel particle: Differences in emulsifying properties of pectin as microgel or as individual polymer chains. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124793. [Google Scholar] [CrossRef]
- Shahrizan, M.S.M.; Aziz, Z.H.A.; Katas, H. Fluid gels: A systematic review towards their application in pharmaceutical dosage forms and drug delivery systems. J. Drug Deliv. Sci. Technol. 2022, 67, 102947. [Google Scholar] [CrossRef]
- García, M.C.; Alfaro, M.C.; Calero, N.; Muñoz, J. Influence of gellan gum concentration on the dynamic viscoelasticity and transient flow of fluid gels. Biochem. Eng. J. 2011, 55, 73–81. [Google Scholar] [CrossRef]
- Gabriele, A.; Spyropoulos, F.; Norton, I. Kinetic study of fluid gel formation and viscoelastic response with kappa-carrageenan. Food Hydrocoll. 2009, 23, 2054–2061. [Google Scholar] [CrossRef]





| Emulgels | Oil (wt%) | Emulsifier (wt%) | Pectin (wt%) | CaCl2 (wt%) | Buffer + Water (wt%) |
|---|---|---|---|---|---|
| 75P/25E | 3.75 | 3.75 | 0.225 | 0.225 | 92.05 |
| 50P/50E | 7.50 | 7.50 | 0.150 | 0.150 | 84.70 |
| 25P/75E | 11.25 | 11.25 | 0.075 | 0.075 | 77.35 |
| Emulgel | Day | K (Pa·sn) | n | R2 |
|---|---|---|---|---|
| 25P/75E | 1 | 0.007 ± 2 × 10−4 | 0.92 ± 10 × 10−3 | 0.84 |
| 6 | 0.007 ± 1 × 10−4 | 0.97 ± 4 × 10−3 | 0.82 | |
| 15 | 0.014 ± 3 × 10−4 | 0.92 ± 5 × 10−3 | 0.95 | |
| 30 | 0.039 ± 4 × 10−4 | 0.81 ± 3 × 10−3 | 1.00 | |
| 50P/50E | 1 | 0.006 ± 2 × 10−4 | 0.84 ± 18 × 10−3 | 0.91 |
| 6 | 0.006 ± 3 × 10−4 | 0,87 ± 19 × 10−3 | 0.82 | |
| 15 | 0.008 ± 3 × 10−4 | 0.84 ± 12 × 10−3 | 0.93 | |
| 30 | 0.579 ± 324 × 10−4 | 0.03 ± 21 × 10−3 | 0.93 | |
| 75P/25E | 1 | 0.018 ± 20 × 10−4 | 0.580 ± 2 × 10−4 | 0.91 |
| 6 | 1.249 ± 310 × 10−4 | 0.002 ± 2 × 10−4 | 1.00 | |
| 15 | 2.861 ± 320 × 10−4 | 5 × 10−15 ± 0 | 1.00 | |
| 30 | 4.951 ± 109 × 10−3 | 9 × 10−16 ± 0 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, J.; Alfaro-Rodríguez, M.-C.; Prieto-Vargas, P.; Lobo, C.; Garcia, M.C. Preparation of Nanoemulgels Containing Lemon Essential Oil and Pectin: Physical Stability and Rheological Properties. Appl. Sci. 2023, 13, 12662. https://doi.org/10.3390/app132312662
Muñoz J, Alfaro-Rodríguez M-C, Prieto-Vargas P, Lobo C, Garcia MC. Preparation of Nanoemulgels Containing Lemon Essential Oil and Pectin: Physical Stability and Rheological Properties. Applied Sciences. 2023; 13(23):12662. https://doi.org/10.3390/app132312662
Chicago/Turabian StyleMuñoz, José, María-Carmen Alfaro-Rodríguez, Paula Prieto-Vargas, Carlos Lobo, and María Carmen Garcia. 2023. "Preparation of Nanoemulgels Containing Lemon Essential Oil and Pectin: Physical Stability and Rheological Properties" Applied Sciences 13, no. 23: 12662. https://doi.org/10.3390/app132312662






