Fluorescent Composite Cotton Fabric Modified with Crosslinked Chitosan for Theranostic Applications
Abstract
:1. Introduction
2. Materials and Methods
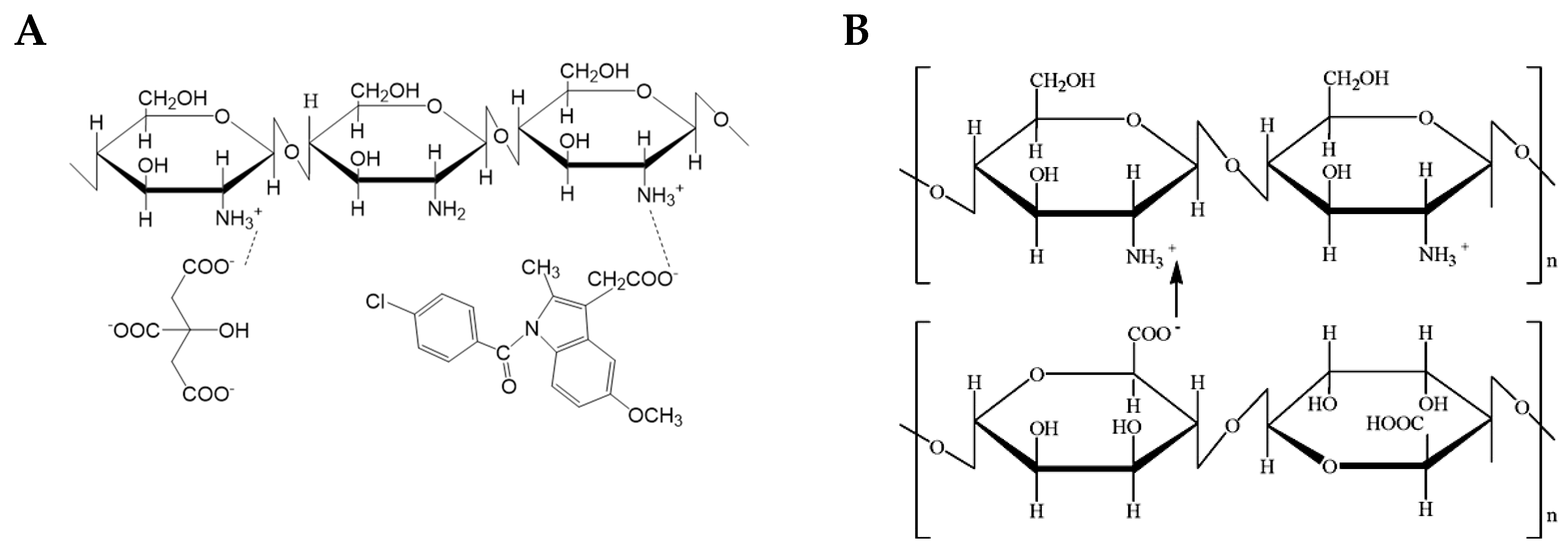
2.1. Modification of Chitosan with 4-Dimethylamino-1,8-naphtalimide-Ch-NI
2.2. Preparation of Composite Materials
2.3. CN Composite Material
2.4. CNI Composite Material
2.5. CNIA Composite Material
2.6. Characterization of the Composite Materials
2.7. Determination of the Degree of Gel Swelling
2.8. In Vitro Gel Erosion
2.9. Scanning Electron Microscope (SEM)
2.10. IR, 1H-NMR, and Fluorescence Analysis
2.11. Thermal Properties Measurement
2.12. In Vitro Release of Indomethacin from Composite Materials
2.13. Antibacterial Assay
3. Results and Discussion
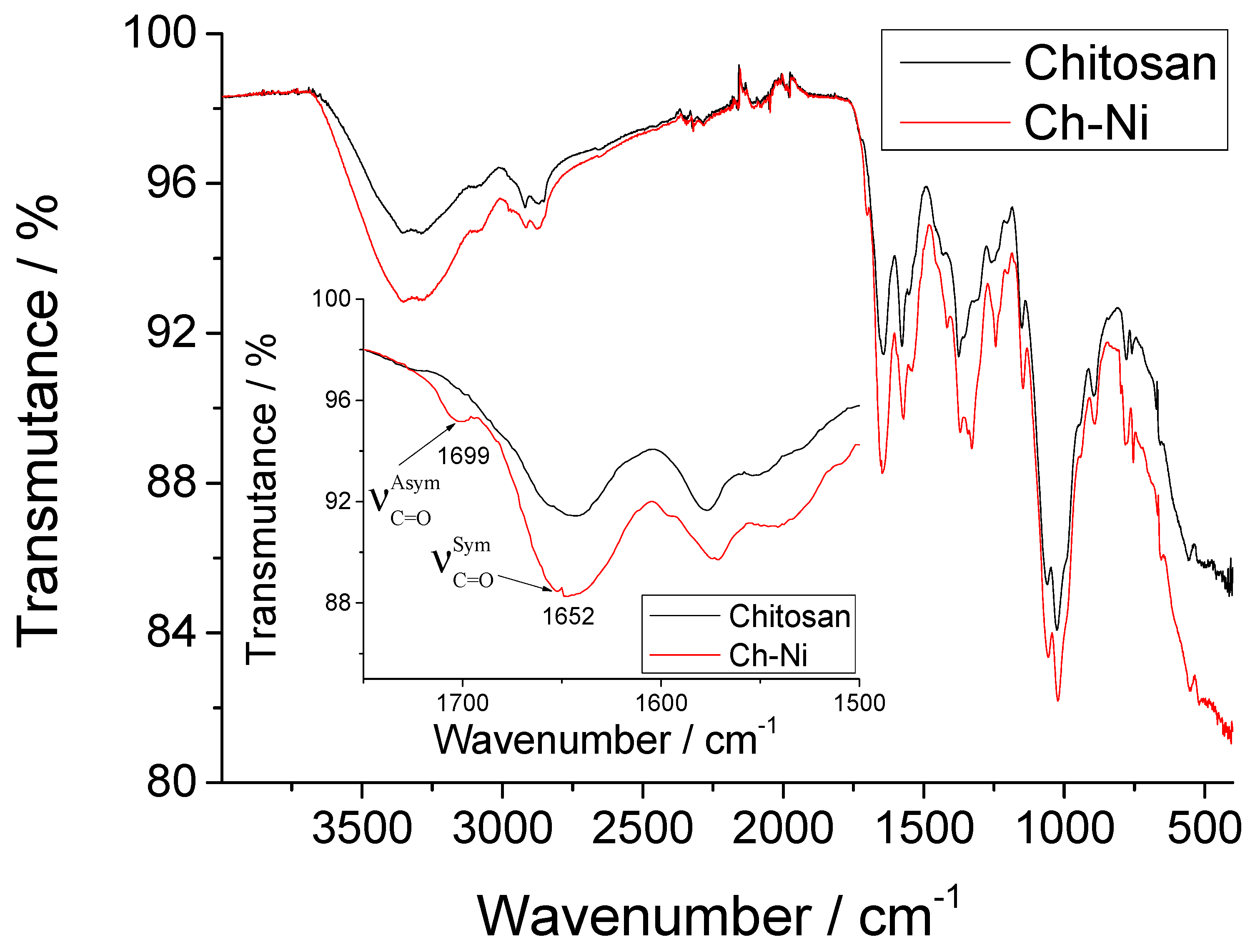
3.1. Synthesis and Characterization of Ch-NI
3.2. Thermogravimetric Analysis
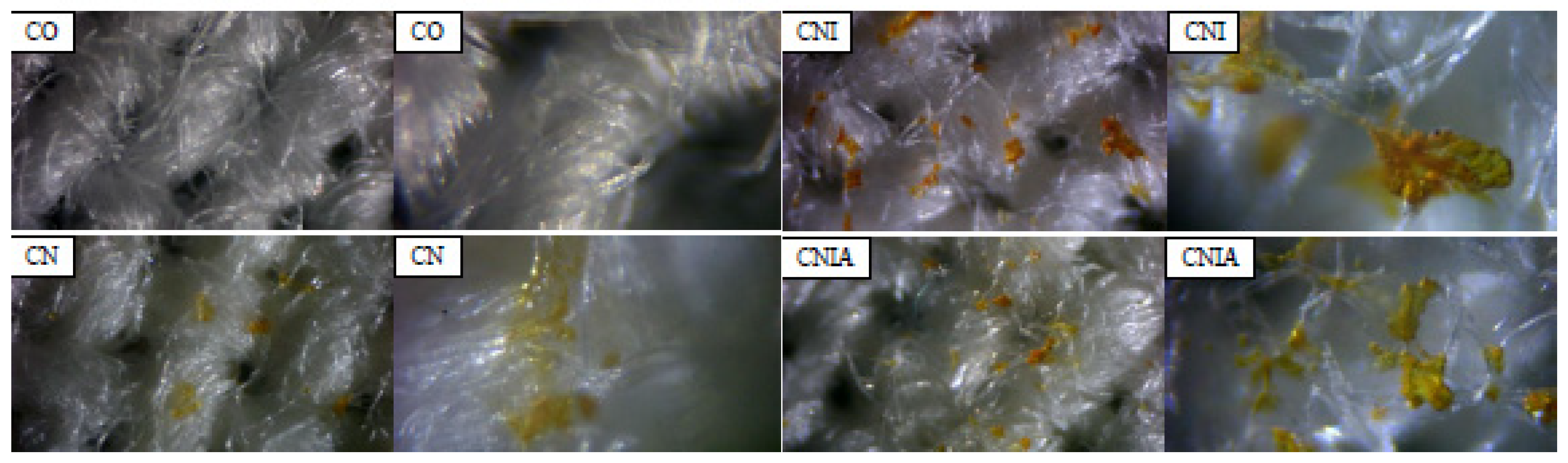
3.3. Optical Microscope Examination
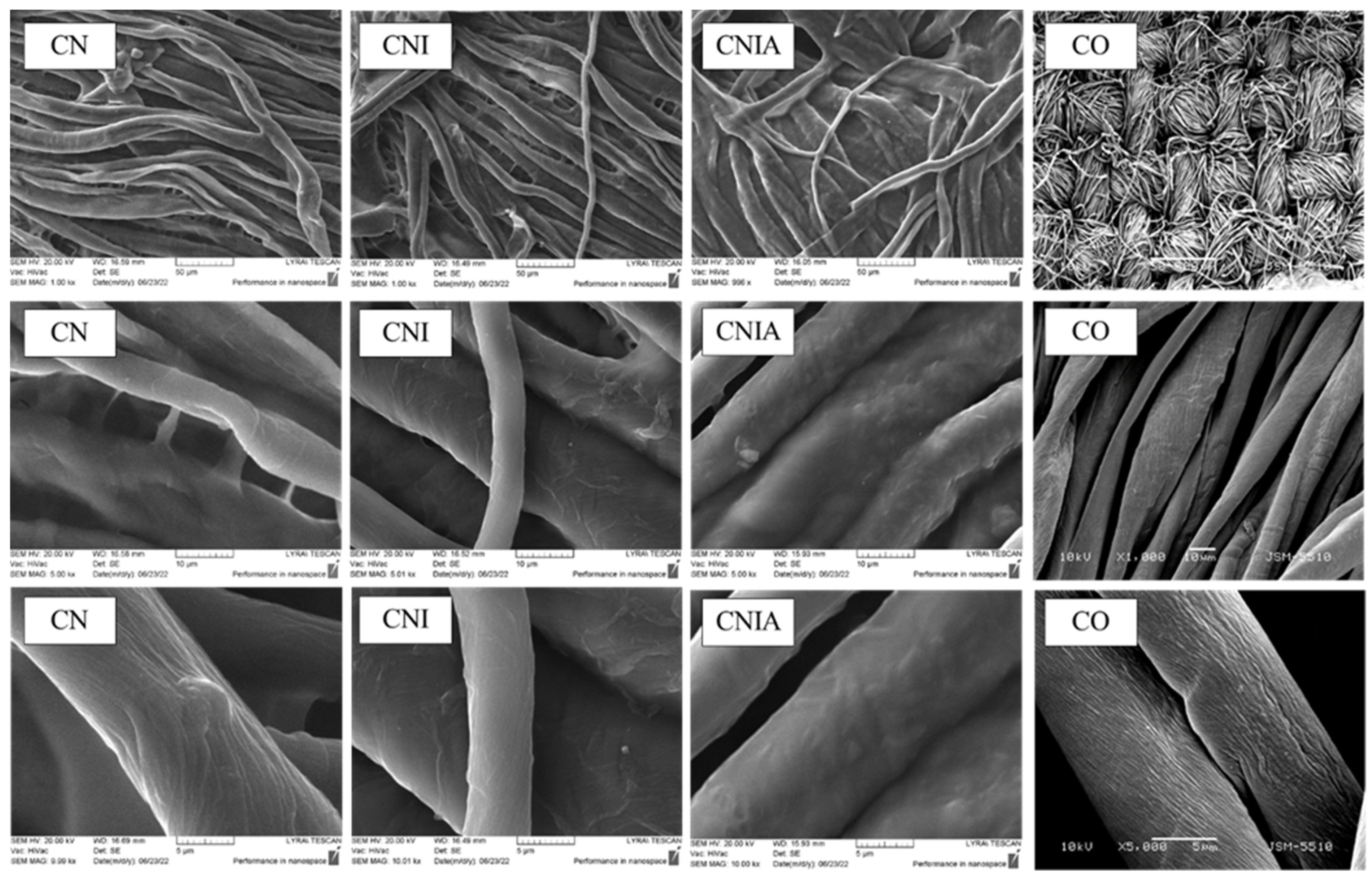
3.4. Scanning Microscope Examination
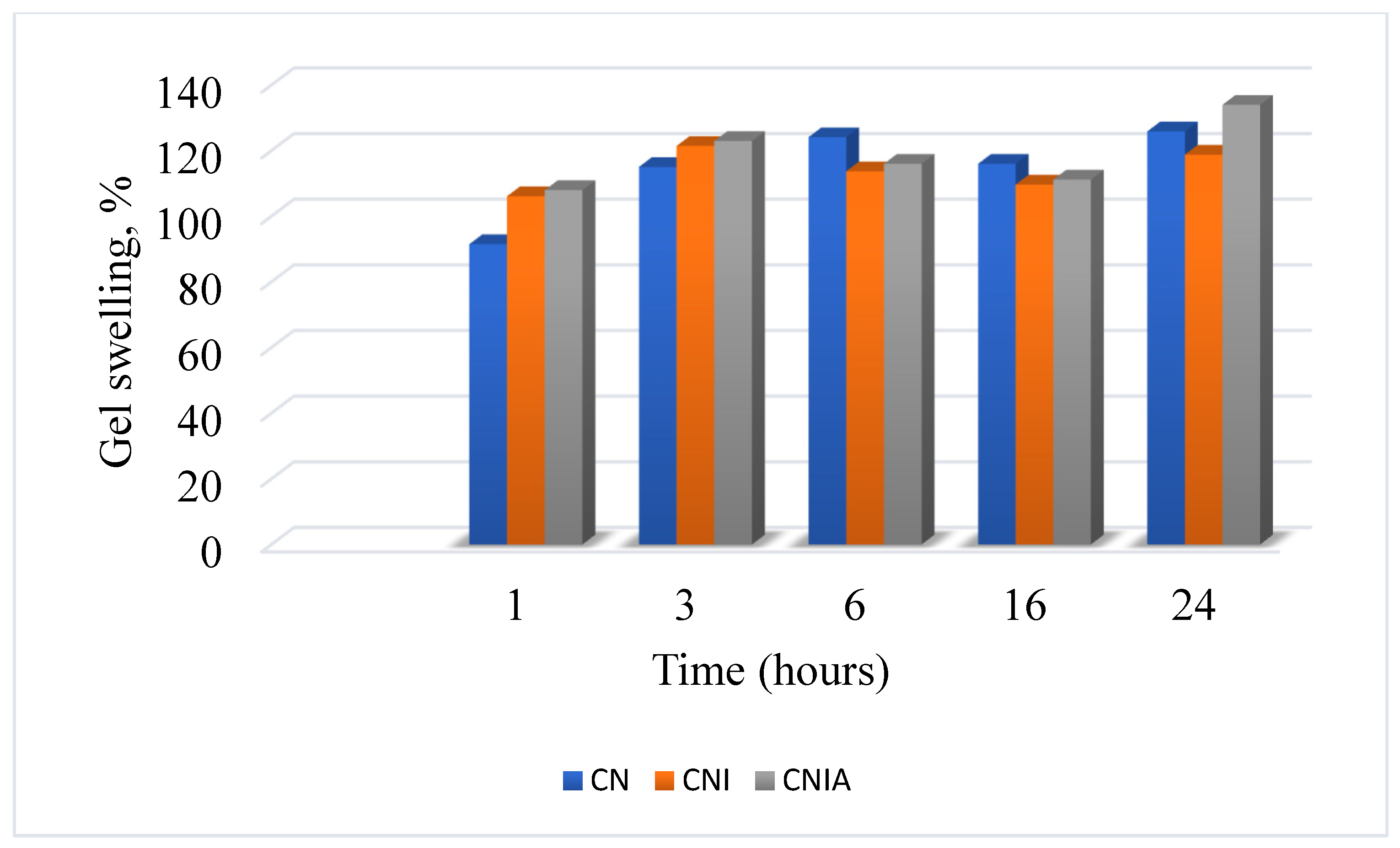
3.5. Determination of the Swelling Degree of the Material Coatings
3.6. Erosion of the Chitosan Gel
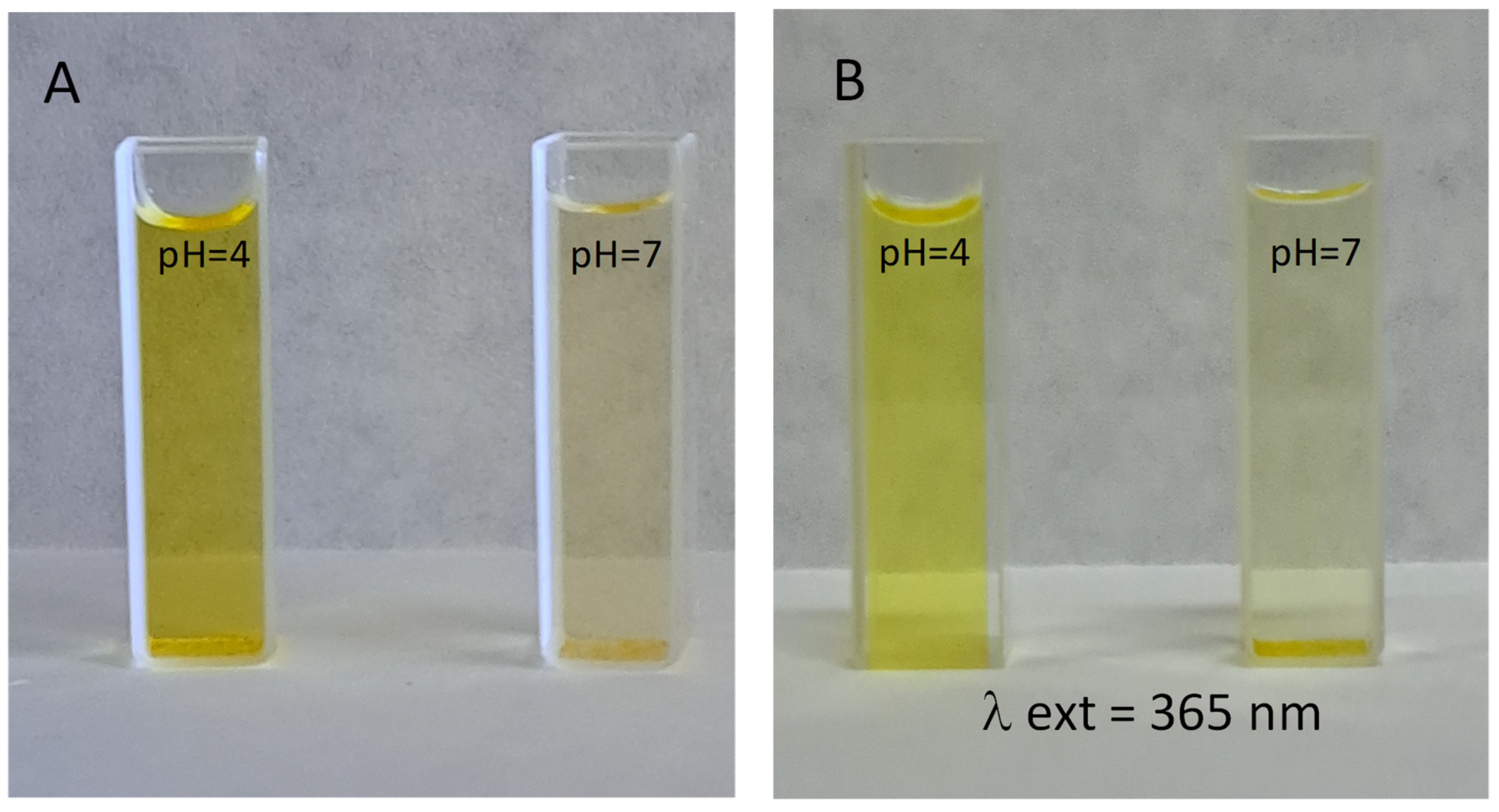
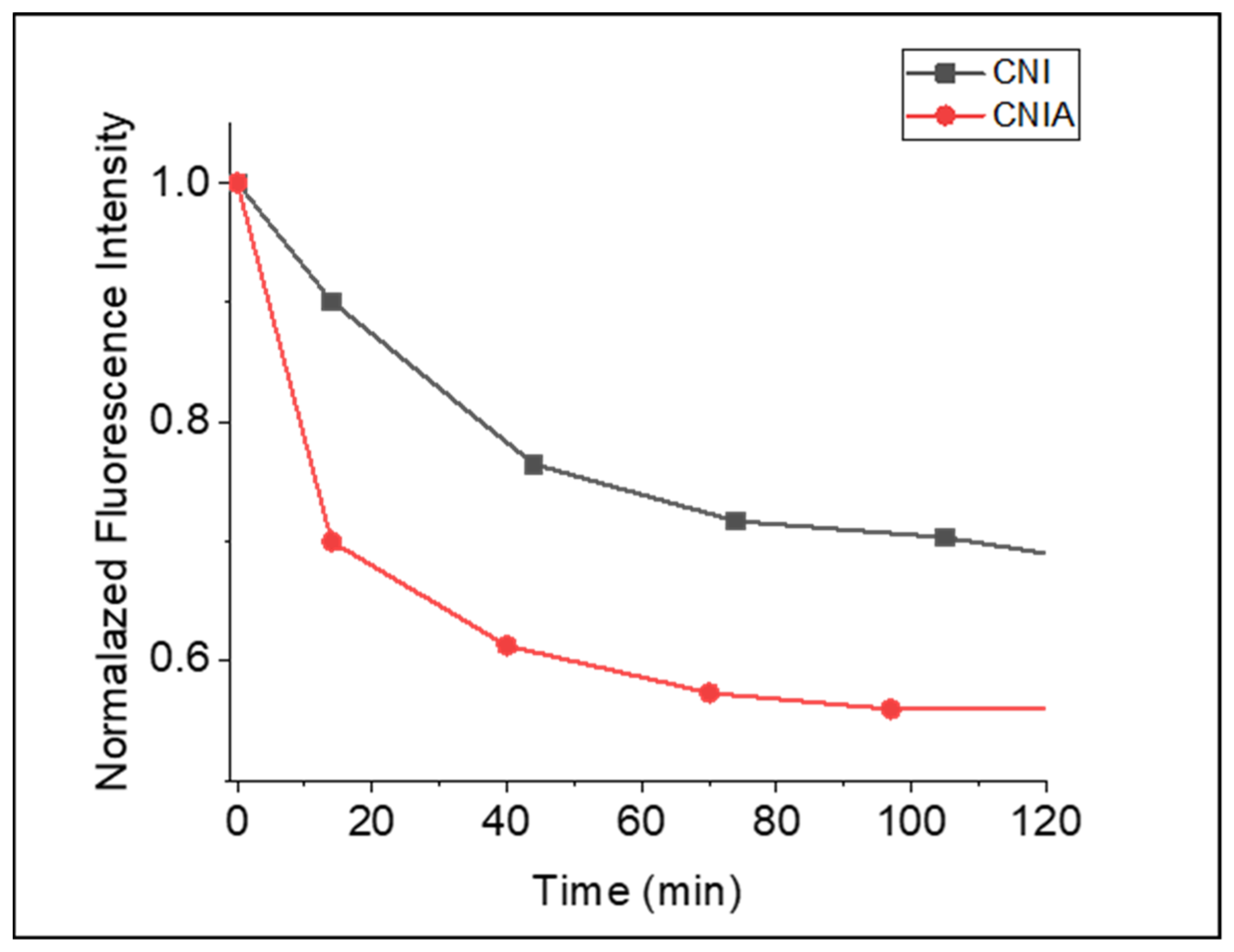
3.7. Fluorescence Analysis
3.8. Release of Indomethacin from CNI and CNIA Composites
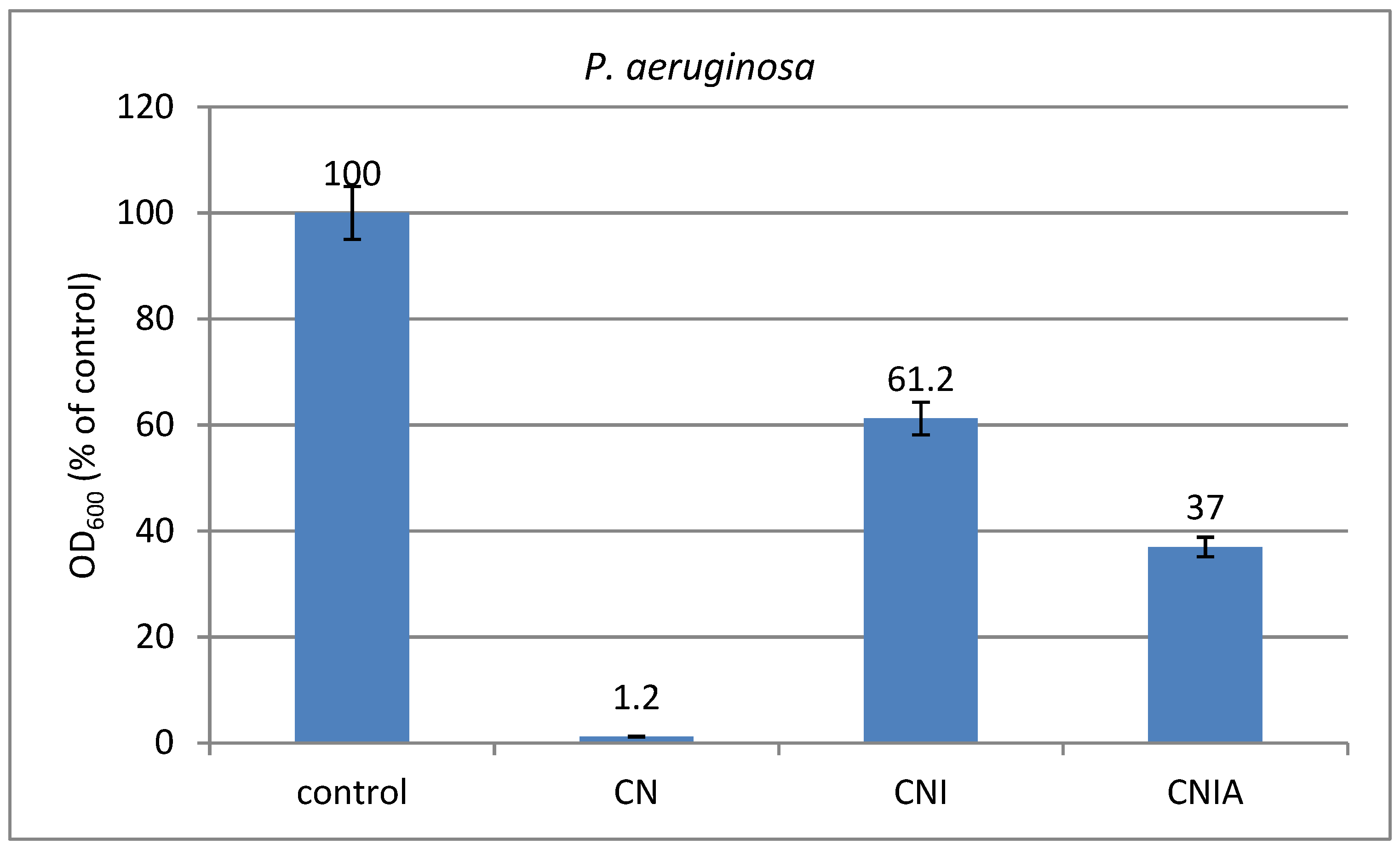
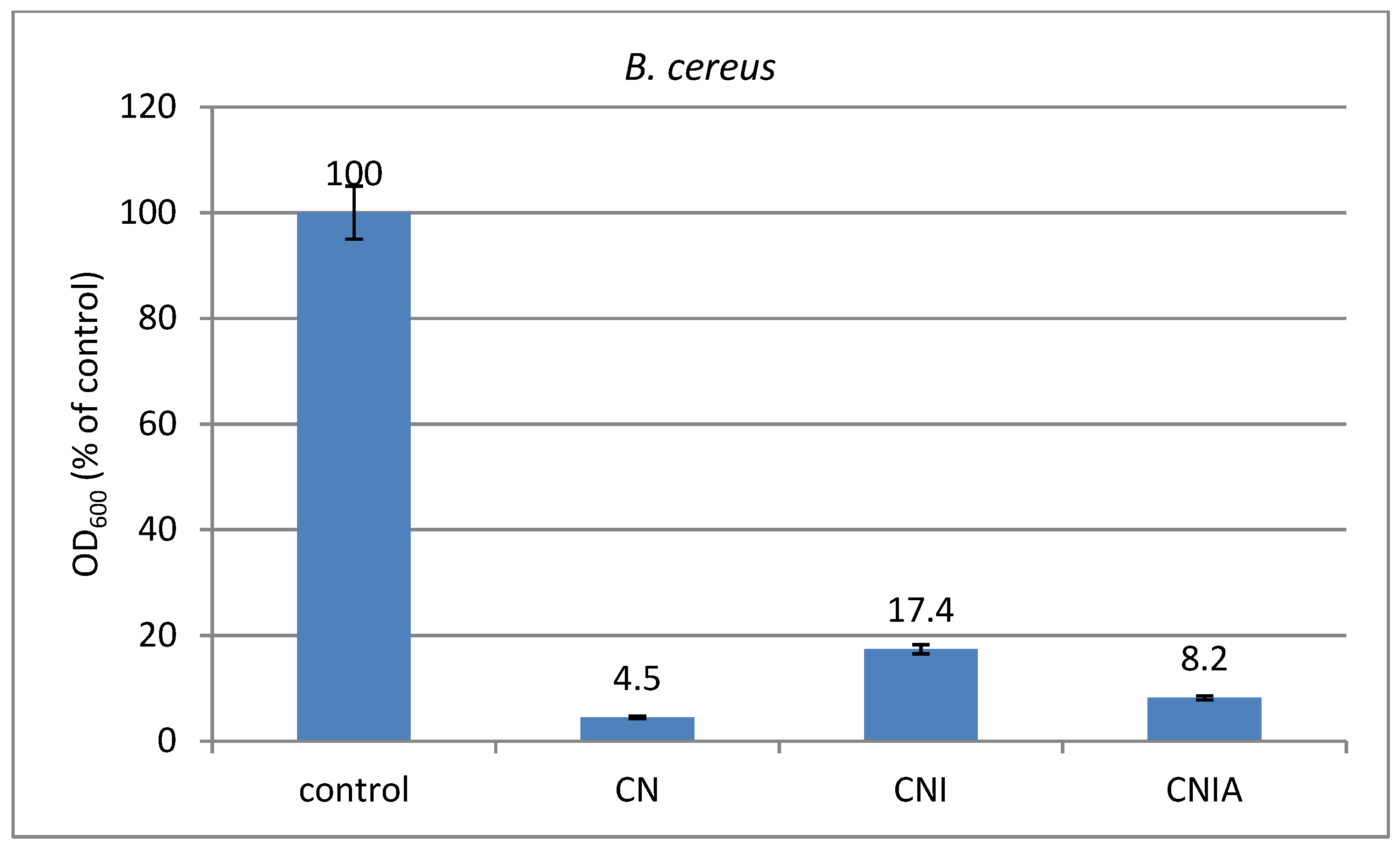
3.9. Antimicrobial Activity of Composite Materials against Bacterial Strains of P. aeruginosa and B. cereus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tehrany, P.M.; Rahmanian, P.; Rezaee, A.; Ranjbarpazuki, G.; Fard, F.S.; Asadollah Salmanpour, Y.M.; Zandieh, A.; Ranjbarpazuki, A.; Asghari, S.; Javani, N.; et al. Multifunctional and theranostic hydrogels for wound healing acceleration: An emphasis on diabetic-related chronic wounds. Environ. Res. 2023, 238, 117087. [Google Scholar] [CrossRef] [PubMed]
- Saberianpour, S.; Melotto, G.; Forss, R.; Redhead, L.; Elsom, J.; Terrazzini, N.; Sandeman, S.; Sarker, D.; Bucca, G.; Hesketh, A.; et al. Development of theranostic wound dressings: Harnessing the knowledge of biospecific interactions at the biomaterial interface to promote healing and identify biomarkers. Expert Rev. Med. Devices 2023, 20, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yang, C.; Chen, Y.; Liu, J.; Liu, Z.; Chen, H.-J. The trends in wound management: Sensing, therapeutic treatment, and theranostics. J. Sci. Adv. Mater. Devices 2023, 8, 100619. [Google Scholar] [CrossRef]
- Avossa, J.; Pota, G.; Vitiello, G.; Macagnano, A.; Zanfardino, A.; Di Napoli, M.; Pezzella, A.; D’Errico, G.; Varcamonti, M.; Luciani, G. Multifunctional mats by antimicrobial nanoparticles decoration for bioinspired smart wound dressing solutions. Mater. Sci. Eng. C 2021, 123, 111954. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guel, M.; Ávila-Orta, C.A.; Cabello-Alvarado, C.; Cadenas-Pliego, G.; Esparza-González, S.C.; Pérez-Alvarez, M.; Quiñones-Jurado, Z.V. Non-Woven Fabrics Based on Nanocomposite Nylon 6/ZnO Obtained by Ultrasound-Assisted Extrusion for Improved Antimicrobial and Adsorption Methylene Blue Dye Properties. Polymers 2021, 13, 1888. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Stoilova, O.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun Eco-Friendly Materials Based on Poly(3-hydroxybutyrate) (PHB) and TiO2 with Antifungal Activity Prospective for Esca Treatment. Polymers 2020, 12, 1384. [Google Scholar] [CrossRef]
- Georgieva, S.; Todorov, P.; Staneva, D.; Grozdanov, P.; Nikolova, I.; Grabchev, I. Metal–Peptide Complexes with Antimicrobial Potential for Cotton Fiber Protection. J. Funct. Biomater. 2023, 14, 106. [Google Scholar] [CrossRef]
- Staneva, D.; Said, A.I.; Vasileva-Tonkova, E.; Grabchev, I. Enhanced Photodynamic Efficacy Using 1,8-Naphthalimides: Potential Application in Antibacterial Photodynamic Therapy. Molecules 2022, 27, 5743. [Google Scholar] [CrossRef]
- Park, S.; Han, U.; Choi, D.; Hong, J. Layer-by-layer assembled polymeric thin films as prospective drug delivery carriers: Design and applications. Biomater. Res. 2018, 22, 29. [Google Scholar] [CrossRef]
- Li, W.; Lei, X.; Feng, H.; Li, B.; Kong, J.; Xing, M. Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics 2022, 14, 297. [Google Scholar] [CrossRef]
- Aubert-Viard, F.; Mogrovejo-Valdivia, A.; Tabary, N.; Maton, M.; Chai, F.; Neut, C.; Martel, B.; Blanchemain, N. Evaluation of antibacterial textile covered by layer-by-layer coating and loaded with chlorhexidine for wound dressing application. Mater. Sci. Eng. C 2019, 100, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Lengert, E.V.; Koltsov, S.I.; Li, J.; Ermakov, A.V.; Parakhonskiy, B.V.; Skorb, E.V.; Skirtach, A.G. Nanoparticles in Polyelectrolyte Multilayer Layer-by-Layer (LbL) Films and Capsules—Key Enabling Components of Hybrid Coatings. Coatings 2020, 10, 1131. [Google Scholar] [CrossRef]
- Hameed, A.Z.; Raj, S.A.; Kandasamy, J.; Baghdadi, M.A.; Shahzad, M.A. Chitosan: A Sustainable Material for Multifarious Applications. Polymers 2022, 14, 2335. [Google Scholar] [CrossRef]
- Inta, O.; Yoksan, R.; Limtrakul, J. Hydrophobically modified chitosan: A bio-based material for antimicrobial active film. Mater. Sci. Eng. C 2014, 42, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Hui, P.C. Review of Stimuli-Responsive Polymers in Drug Delivery and Textile Application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, G.S.; El-Banna, S.T.; El-Bahy, G.S.; Abdelrazek, E.M.; Kamal, M. Alginate coated chitosan nanogel for the controlled topical delivery of Silver sulfadiazine. Carbohydr. Polym. 2017, 177, 194–202. [Google Scholar] [CrossRef]
- Gontero, D.; Lessard-Viger, M.; Brouard, D.; Guillermo Bracamonte, A.; Boudreau, D.; Veglia, A.V. Smart multifunctional nanoparticles design as sensors and drug delivery systems based on supramolecular chemistry. Microchem. J. 2017, 130, 316–328. [Google Scholar] [CrossRef]
- Atanasova, D.; Staneva, D.; Grabchev, I. Modified with chitosan cotton fabric for control release of indomethacin. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1188, 012004. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; de Baynast, H.; Doco, T.; Michaud, P.; et al. Modification of Chitosan for the Generation of Functional Derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Bosch, P. Fluorescent Hydrogel–Textile Composite Material Synthesized by Photopolymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 838–847. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Herrera-Méndez, C.H.; Rodríguez-Núñez, J.R. An overview of the chemical modifications of chitosan and their advantages. Green Mater. 2018, 6, 131–142. [Google Scholar] [CrossRef]
- Munro, N.H.; Hanton, L.R.; Robinson, B.H.; Simpson, J. Synthesis and characterisation of fluorescent chitosan derivatives containing substituted naphthalimides. React. Funct. Polym. 2008, 68, 671–678. [Google Scholar] [CrossRef]
- Sarkar, K.; Kundu, P.P. PAMAM conjugated chitosan through naphthalimide moiety for enhanced gene transfection efficiency. Carbohydr. Polym. 2013, 98, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Koh, J. Synthesis, physiochemical and optical properties of chitosan based dye containing naphthalimide group. Carbohydr. Polym. 2013, 94, 221–228. [Google Scholar] [CrossRef]
- Meng, Z.; Yin, J.; Zhao, F.; Li, M.; Zhang, Y.; Liang, Y.; Wang, Z.; Yang, Y. An efficient chitosan-based naphthalimide-modified fluorescent sensor for rapid detection of 2,4-dinitrophenylhydrazine and its applications in environmental analysis. Eur. Polym. J. 2021, 158, 110705. [Google Scholar] [CrossRef]
- Meng, Z.; Li, X.; Liang, Y.; Gu, Y.; Xu, X.; Wang, Z.; Yang, Y.; Wang, S. An efficient chitosan-naphthalimide fluorescent probe for simultaneous detection and adsorption of Hg2+ and its application in seafood, water and soil environments. Int. J. Biol. Macromol. 2023, 247, 125807. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, Z.; Liang, Y.; Zhou, G.; Li, X.; Xu, X.; Yang, Y.; Wang, S. A naphthalimide functionalized chitosan-based fluorescent probe for specific detection and efficient adsorption of Cu2+. Int. J. Biol. Macromol. 2023, 239, 124261. [Google Scholar] [CrossRef]
- Dodangeh, M.; Grabchev, I.; Staneva, D.; Gharanjig, K. 1,8-Naphthalimide Derivatives as Dyes for Textile and Polymeric Materials: A Review. Fibers Polym. 2021, 22, 2368–2379. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J.; Song, W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int. J. Pharm. 2001, 212, 19–28. [Google Scholar] [CrossRef]
- Alexiou, M.S.; Tychopoulos, V.; Ghorbanian, S.; Tyman, J.H.P.; Brown, R.G.; Brittain, P. The UV-Visible Absorption and Fluorescence of some Substituted 1,8-Naphthalimides and Naphthalic Anhydrides. J. Chem. Soc. Perkin Ttrans. 1990, 2, 837–842. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Bojinov, V.; Guittonneau, S. Synthesis and Properties of Fluorescent 1,8-Naphthalimide Derivatives as dyes for Liquid Crystals. J. Mat. Chem. 2000, 10, 1291–1296. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef]
- Ofridam, F.; Tarhini, M.; Lebaz, N.; Gagnière, É.; Mangin, D.; Elaissari, A. pH-sensitive polymers: Classification and some fine potential applications. Polym. Adv. Technol. 2021, 32, 1455–1484. [Google Scholar] [CrossRef]
- Gomes, A.P.; Mano, J.F.; Queiroz, J.A.; Gouveia, I.C. Layer-by-layer deposition of antibacterial polyelectrolytes on cotton fibres. J. Polym. Environ. 2012, 20, 1084–1094. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Wang, X.; Liang, S.; Li, N.; An, H. Progress in research on natural cellulosic fibre modifications by polyelectrolytes. Carbohydr. Polym. 2022, 278, 118966. [Google Scholar] [CrossRef]
- Visan, A.I.; Popescu-Pelin, G.; Socol, G. Degradation Behavior of Polymers Used as Coating Materials for Drug Delivery—A Basic Review. Polymers 2021, 13, 1272. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Paracini, N.; Schneck, E.; Imberty, A.; Micciulla, S. Lipopolysaccharides at Solid and Liquid Interfaces: Models for Biophysical Studies of the Gram-negative Bacterial Outer Membrane. Adv. Colloid Interface Sci. 2022, 301, 102603. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staneva, D.; Atanasova, D.; Grabchev, I. Fluorescent Composite Cotton Fabric Modified with Crosslinked Chitosan for Theranostic Applications. Appl. Sci. 2023, 13, 12660. https://doi.org/10.3390/app132312660
Staneva D, Atanasova D, Grabchev I. Fluorescent Composite Cotton Fabric Modified with Crosslinked Chitosan for Theranostic Applications. Applied Sciences. 2023; 13(23):12660. https://doi.org/10.3390/app132312660
Chicago/Turabian StyleStaneva, Desislava, Daniela Atanasova, and Ivo Grabchev. 2023. "Fluorescent Composite Cotton Fabric Modified with Crosslinked Chitosan for Theranostic Applications" Applied Sciences 13, no. 23: 12660. https://doi.org/10.3390/app132312660




