Theranostic Investigation of Gadolinium-159 for Hepatocellular Carcinoma: Monte Carlo Simulation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Absorbed Dose Calculation
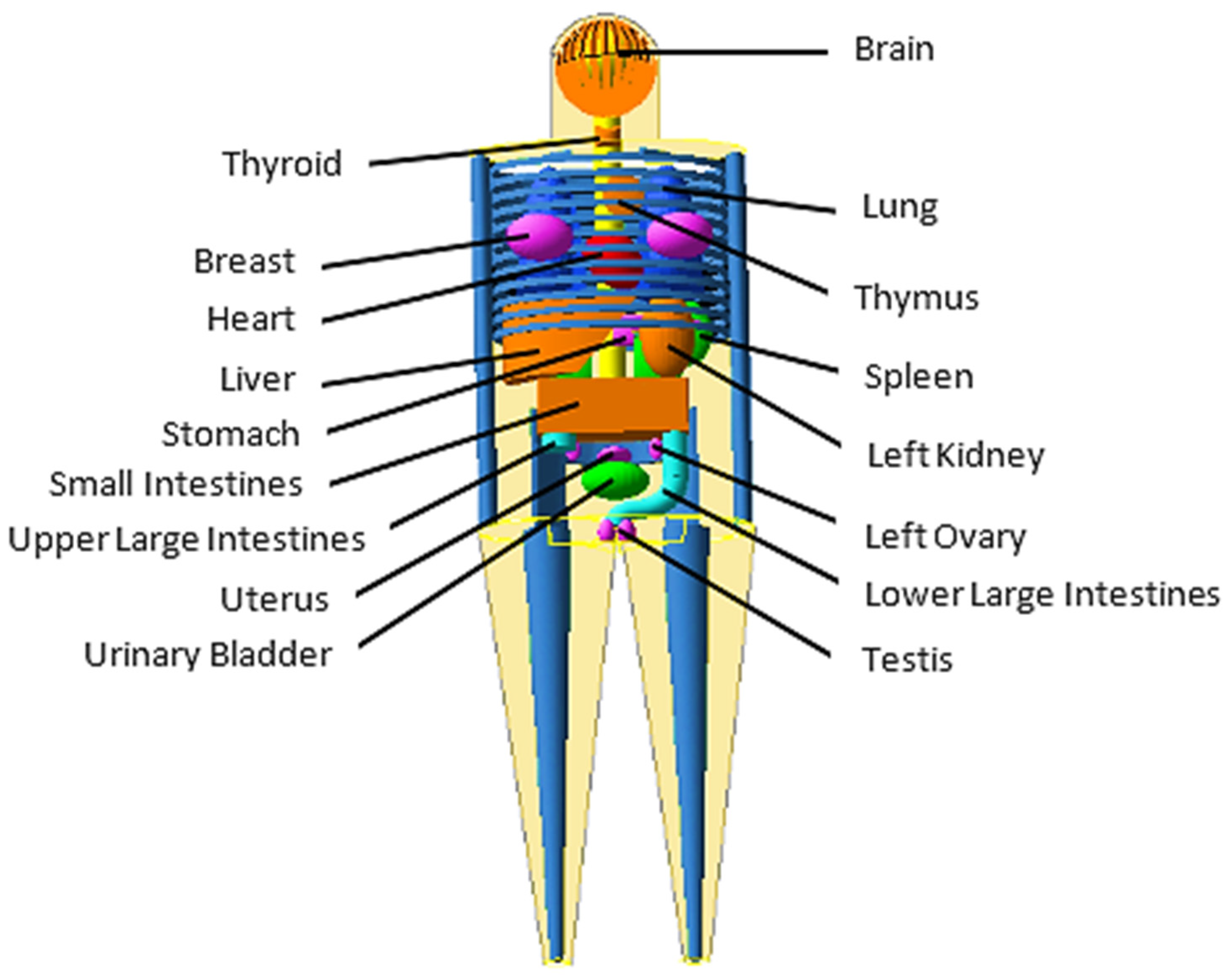
MIRD-5 Phantom Geant4 MC Simulations
2.2. Scintigraphic Imaging
2.2.1. GATE MC Simulation
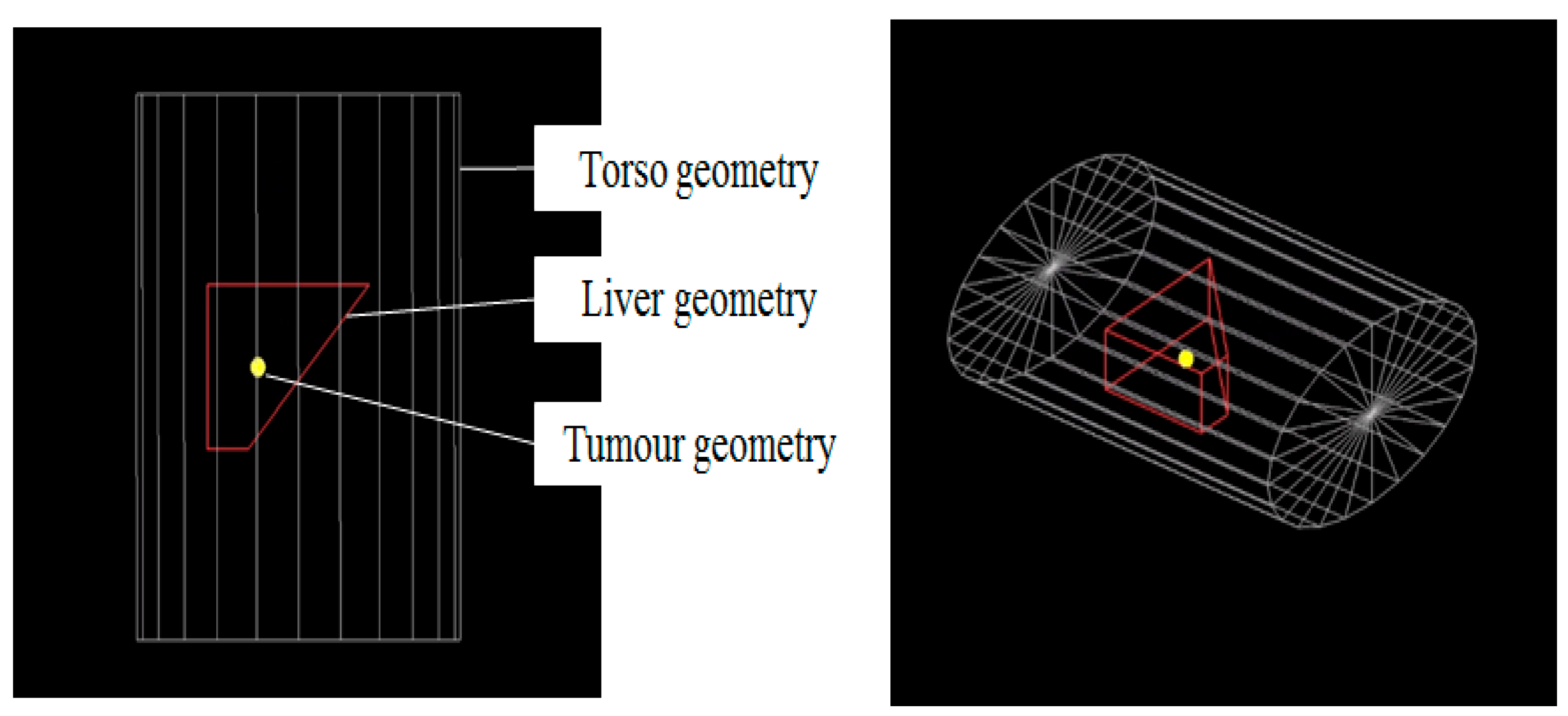
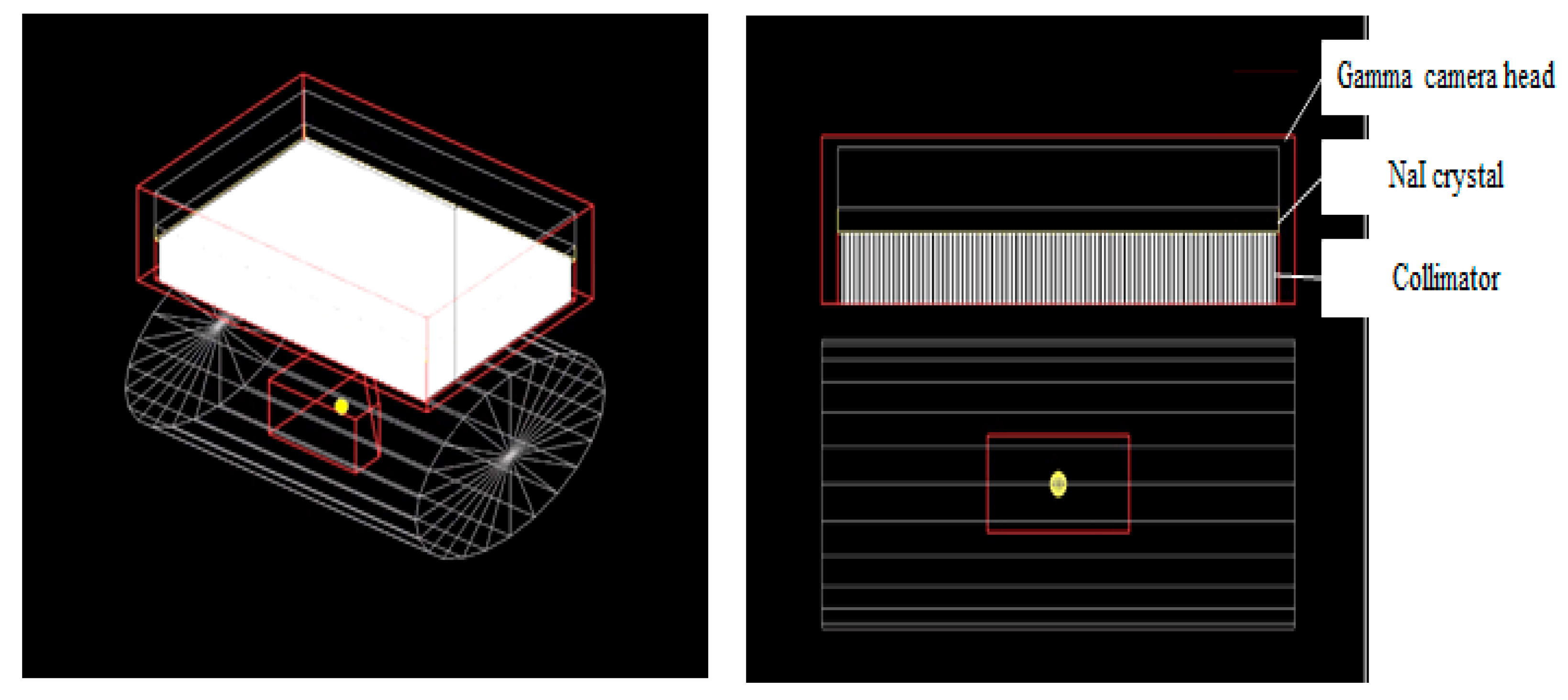
2.2.2. Geometry Setup
2.2.3. Image Acquisition
2.2.4. Image Quality Analysis Using ImageJ
3. Results and Discussion
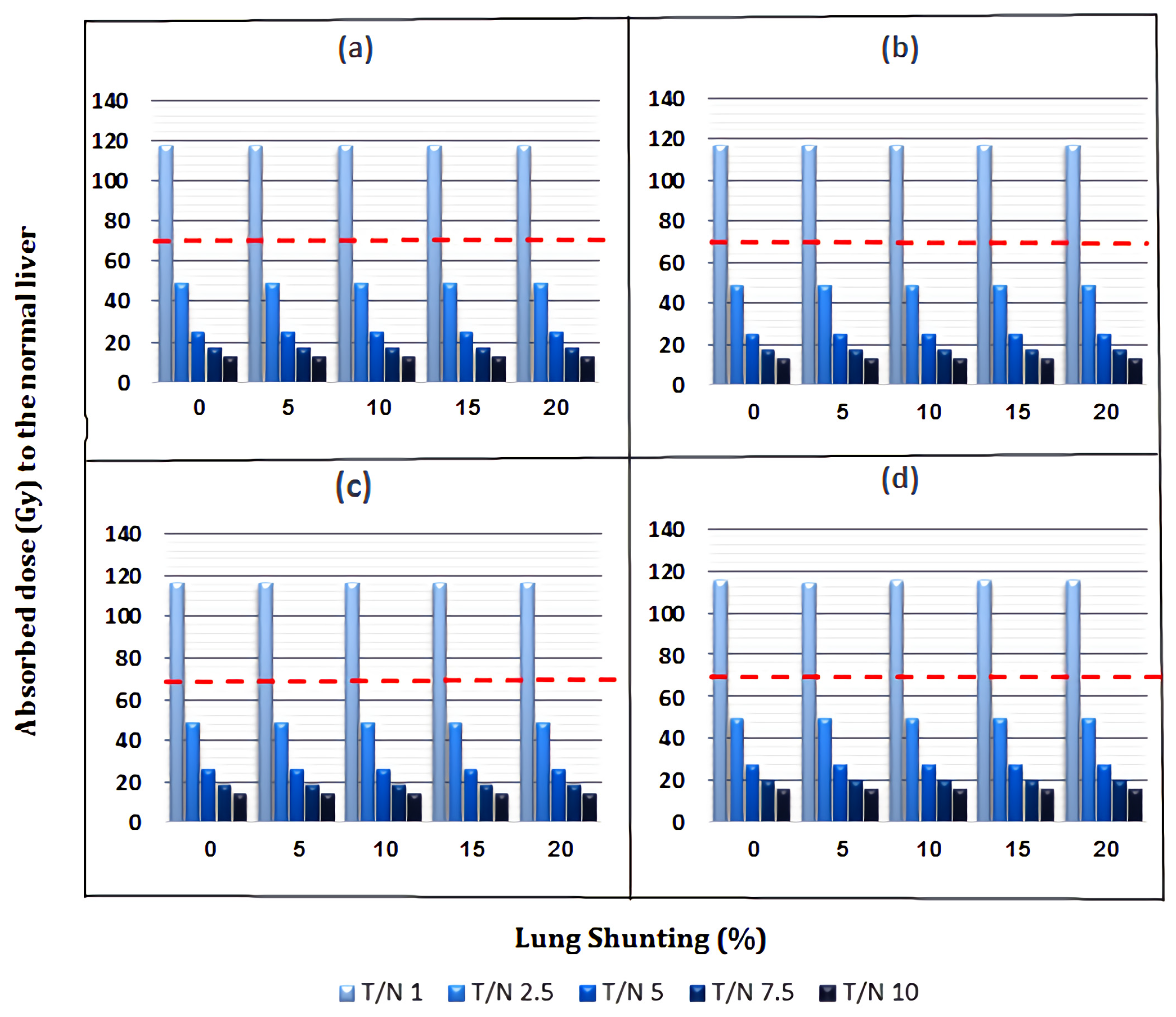
3.1. Absorbed Dose to Normal Liver
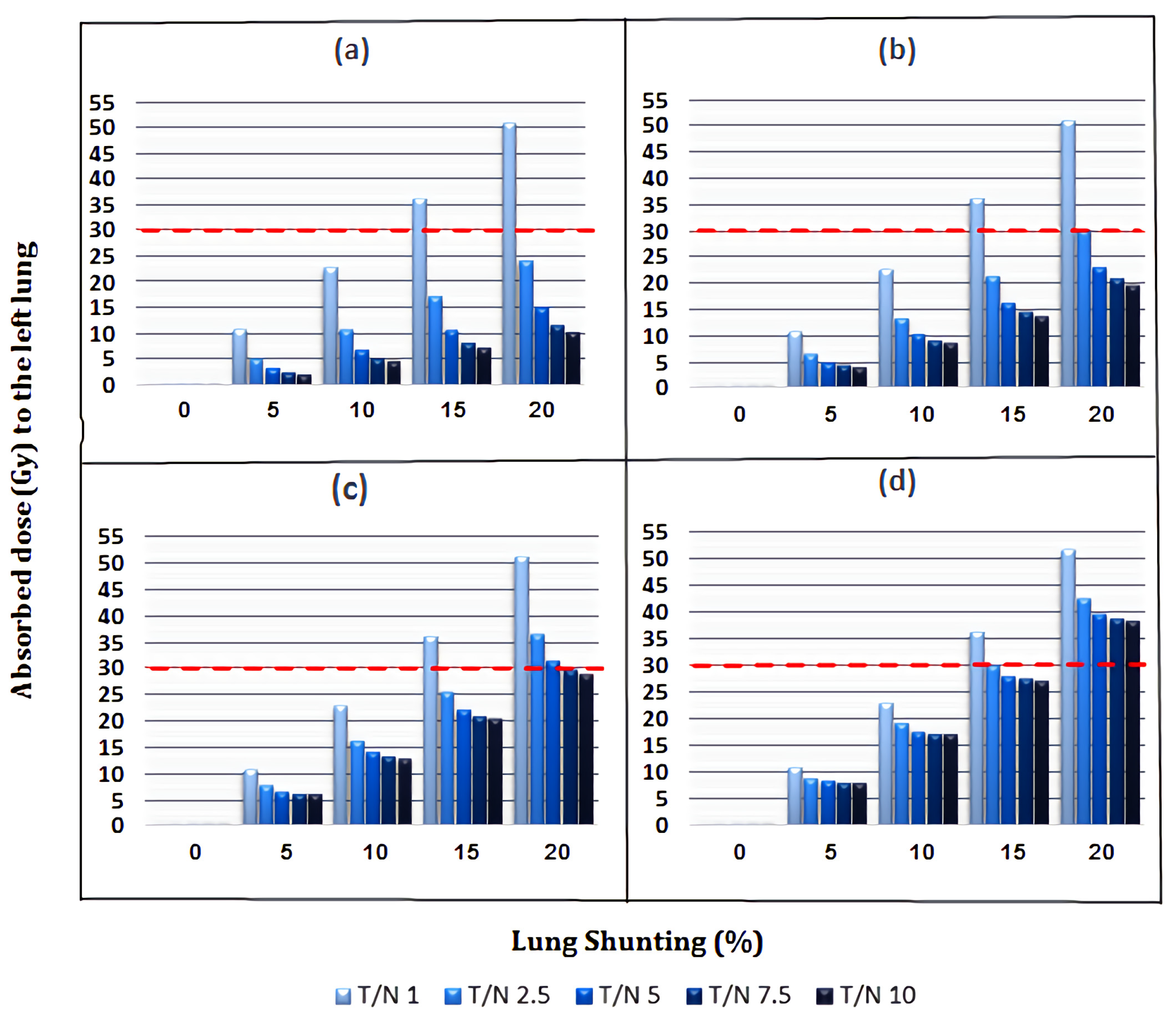
3.2. Absorbed Dose to Lungs
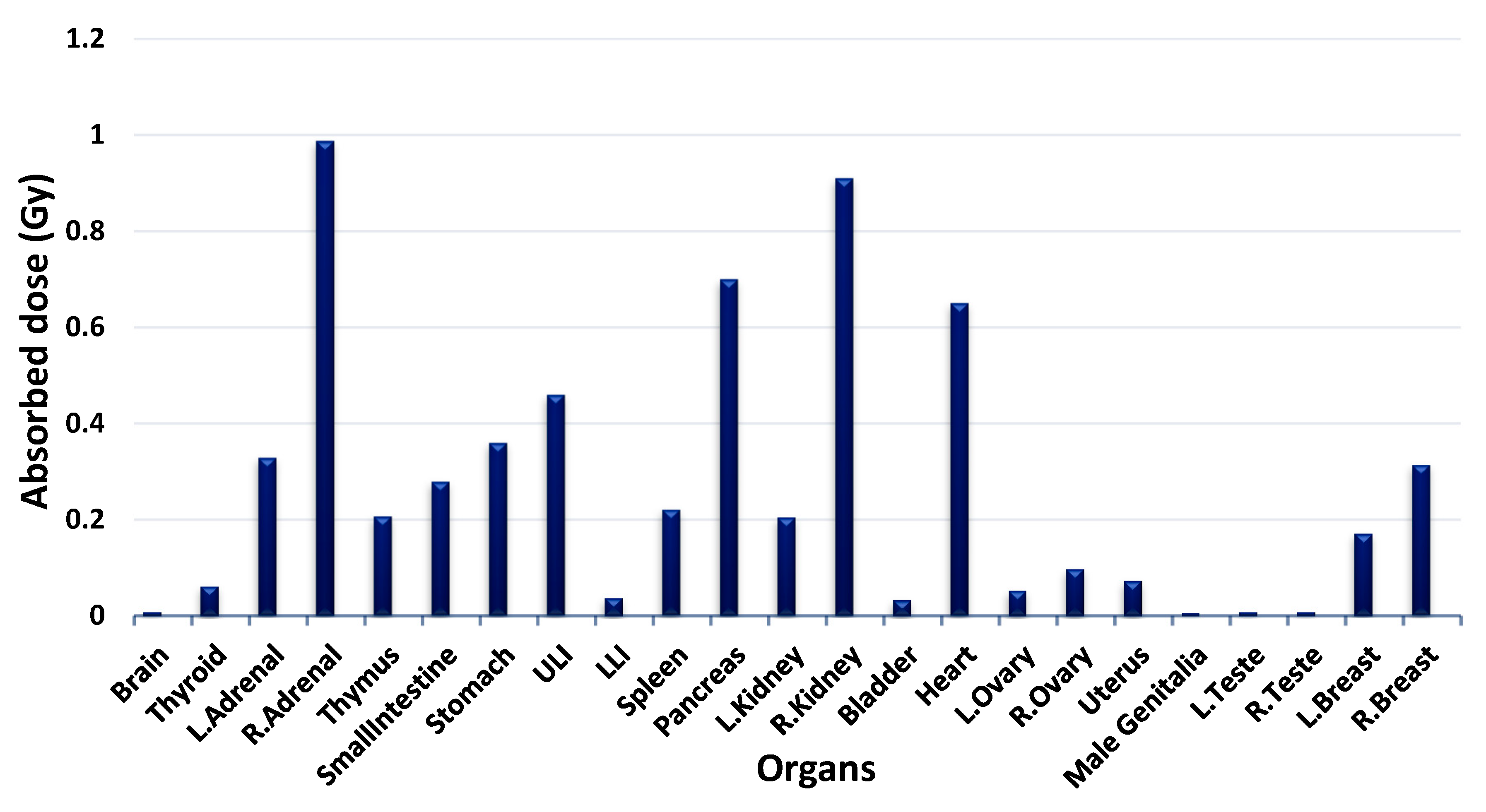
3.3. Absorbed Dose to Other Organs
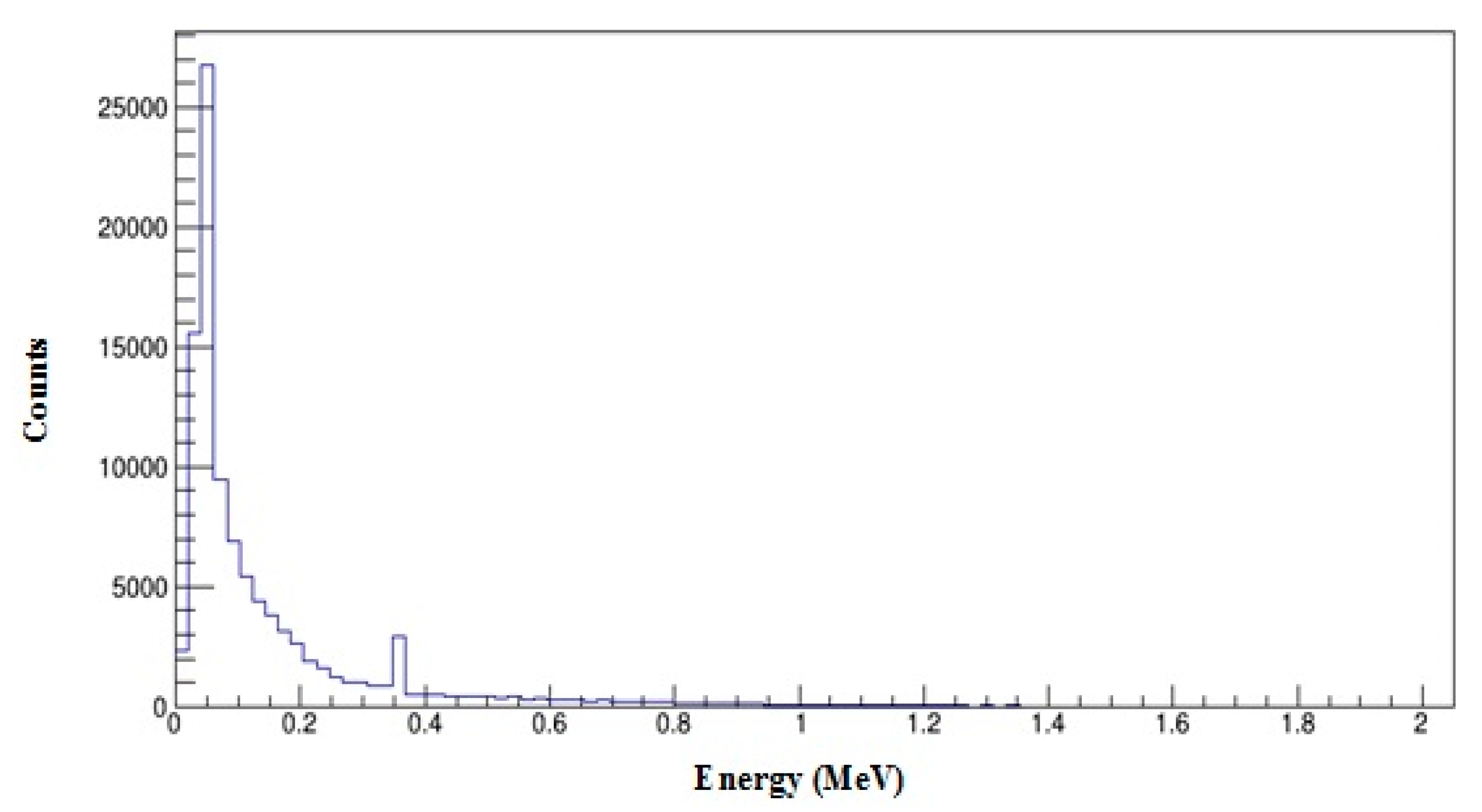
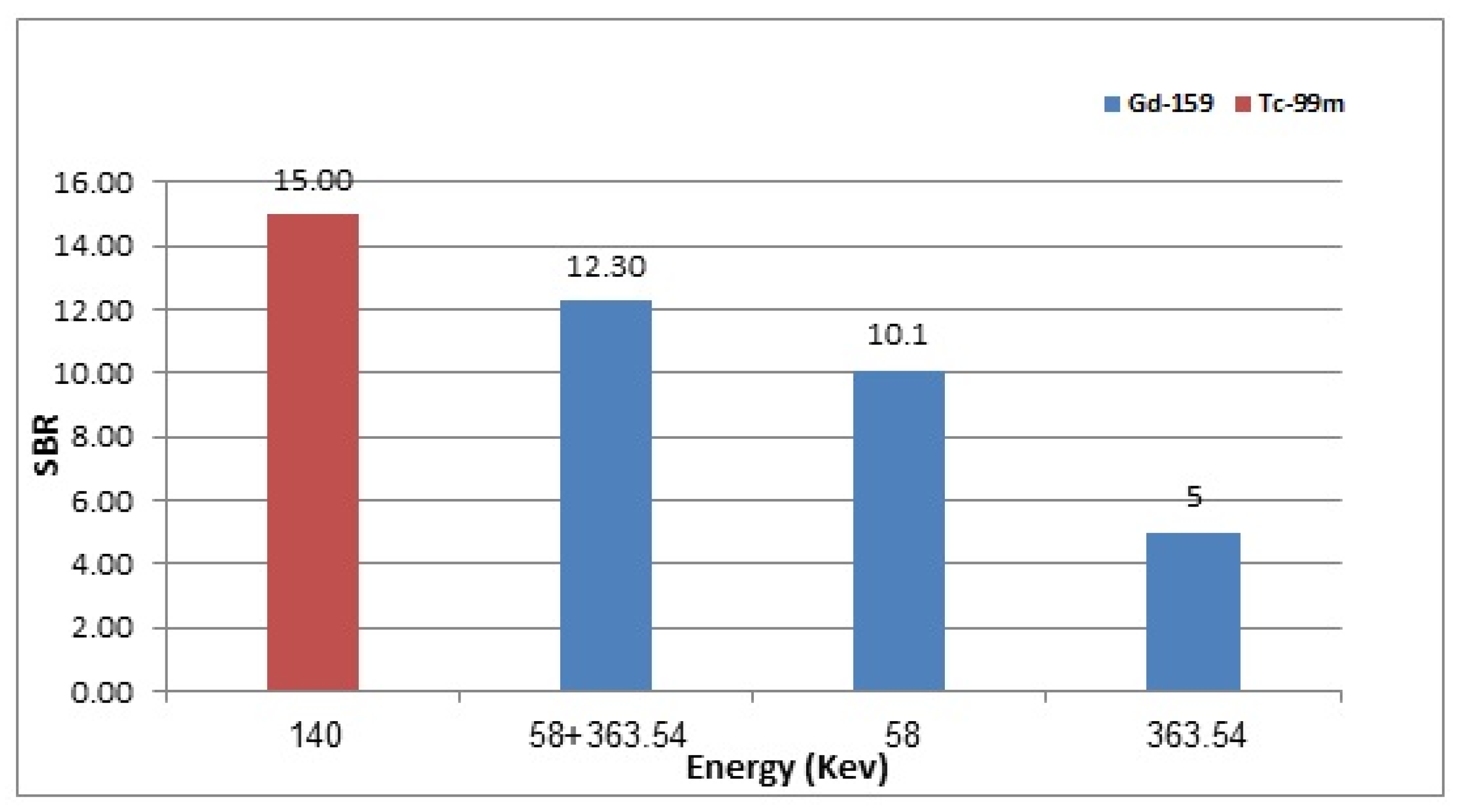
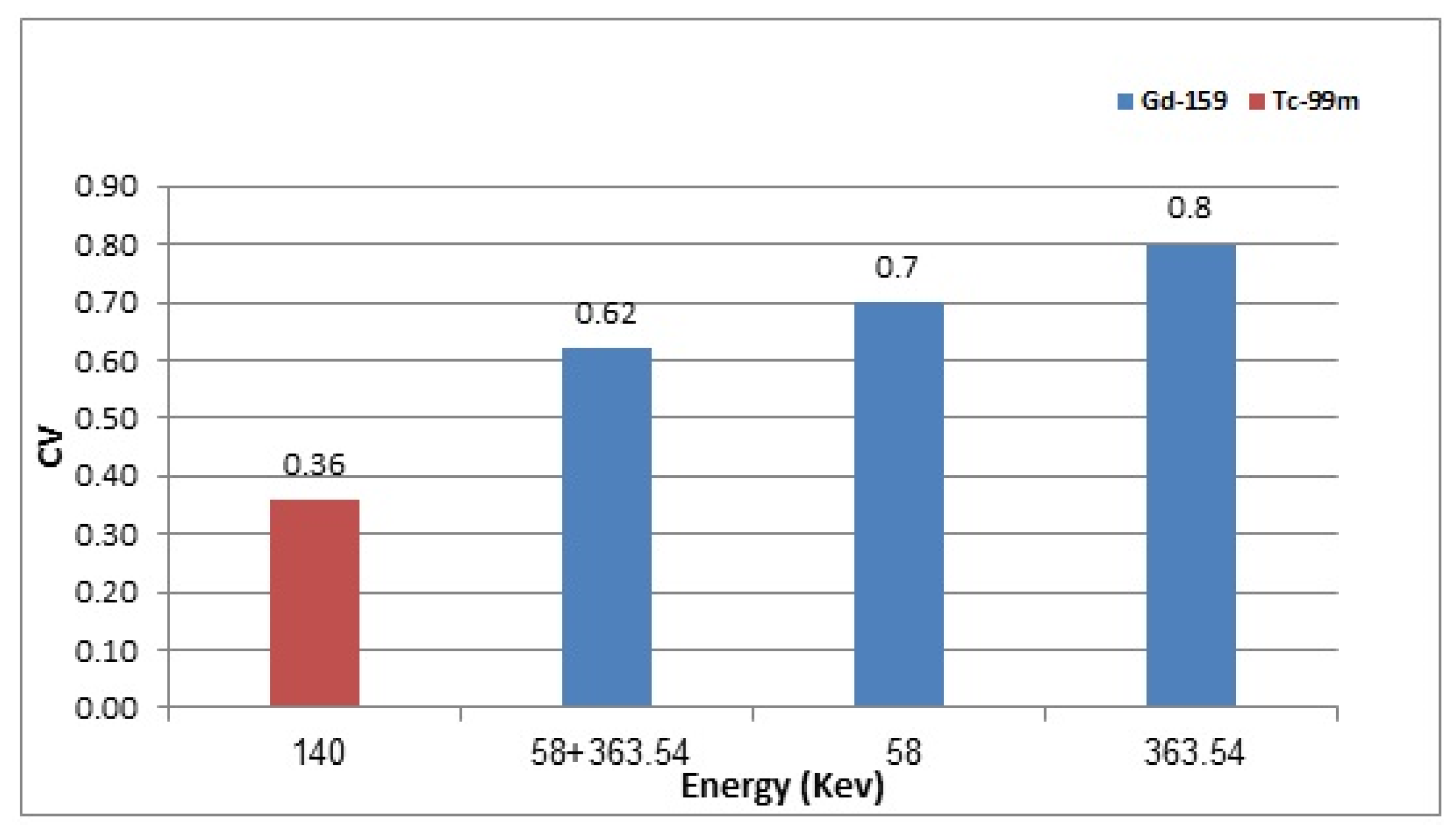
3.4. 159Gd vs. 99mTc Scintigraphic Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 159Gd | Gadolinium-159 |
| 90Y | Yttrium-90 |
| MC | Monte Carlo |
| HCC | Hepatocellular Carcinoma |
| TI | Tumour involvement |
| T/N | Tumour-to-normal liver uptake ratio |
| LS | Lung shunting |
| TARE | Transarterial radioembolization |
| OARs | Organs at risk |
| GATE | Geant4 Application for Tomographic Emission |
| Geant4 | GEometry ANd Tracking |
| 99mTc | Technetium-99m |
| 153Sm | Samarium-153 |
| 166Ho | Holmium-166 |
| 177Lu | Lutetium-177 |
| 188Re | Rhenium-188 |
| MRI | Magnetic resonance imaging |
| SPECT | Single-photon emission computed tomography |
| MIRD | Medical Internal Radiation Dose |
| HEGP | High-energy general-purpose |
| LEHR | Low-energy high-resolution |
| SBR | Signal to background ratio |
| CV | Coefficient of variation |
| ROI | Region of interest |
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. International Agency for Research on Cancer Cancer today; World Health Organization: Geneva, Switzerland, 2016.
- World Health Organization. Projections of Mortality and Causes of Death, 2016 to 2060; World Health Organization: Geneva, Switzerland, 2019. Available online: http://www.who.int/healthinfo/global_burden_disease/projections/en/ (accessed on 14 October 2018).
- Liu, Z.; Jiang, Y.; Yuan, H.; Fang, Q.; Cai, N.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019, 70, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Cidon, E.U. Systemic treatment of hepatocellular carcinoma: Past, present and future. World J. Hepatol. 2017, 9, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Gates, V.L.; Atassi, B.; Lewandowski, R.J.; Ryu, R.K.; Sato, K.T.; A Nemcek, A.; Omary, R.; Salem, R. Radioembolization with Yttrium-90 microspheres: Review of an emerging treatment for liver tumors. Future Oncol. 2007, 3, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Stabin, M. Nuclear medicine dosimetry. Phys. Med. Biol. 2006, 51, R187. [Google Scholar] [CrossRef]
- Gholami, S.; Longo, F.; Nedaie, H.A.; Berti, A.; Mousavi, M.; Meigooni, A.S. Application of Geant4 Monte Carlo simulation in dose calculations for small radiosurgical fields. Med. Dosim. 2018, 43, 214–223. [Google Scholar] [CrossRef]
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assié, K.; Autret, D.; Avner, S.; Barbier, R.; Bardies, M.; Bloomfield, P.M.; et al. GATE: A simulation toolkit for PET and SPECT. Phys. Med. Biol. 2004, 49, 4543–4561. [Google Scholar] [CrossRef]
- Shen, S.; DeNardo, G.L.; Yuan, A.; A DeNardo, D.; DeNardo, S.J. Planar gamma camera imaging and quantitation of yttrium-90 bremsstrahlung. J. Nucl. Med. 1994, 35, 1381–1389. [Google Scholar]
- Rong, X.; Du, Y.; Ljungberg, M.; Rault, E.; Vandenberghe, S.; Frey, E.C. Development and evaluation of an improved quantitative 90Y bremsstrahlung SPECT method. Med. Phys. 2012, 39, 2346–2358. [Google Scholar] [CrossRef] [Green Version]
- Wright, C.L.; Zhang, J.; Tweedle, M.F.; Knopp, M.V.; Hall, N.C. Theranostic imaging of Yttrium-90. BioMed Res. Int. 2015, 2015, 481279. [Google Scholar] [CrossRef] [Green Version]
- Hashikin, N.; Yeong, C.H.; Guatelli, S.; Abdullah, B.J.J.; Ng, K.H.; Malaroda, A.; Rosenfeld, A.B.; Perkins, A.C. Organ Doses from Hepatic Radioembolization with 90Y, 153Sm, 166Ho and 177Lu: A Monte Carlo Simulation Study Using Geant4. J. Phys. Conf. Ser. 2016, 694, 012059. [Google Scholar] [CrossRef]
- Bé, M.M.; Chechev, V.P. Recommended standards for gamma ray intensities. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2013, 728, 157–172. [Google Scholar] [CrossRef] [Green Version]
- Lau, W.-Y.; Kennedy, A.S.; Kim, Y.H.; Lai, H.K.; Lee, R.-C.; Leung, T.W.; Liu, C.-S.; Salem, R.; Sangro, B.; Shuter, B.; et al. Patient selection and activity planning guide for selective internal radiotherapy with yttrium-90 resin microspheres. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Snyder, W. Estimates of specific absorbed fractions for monoenergetic photon sources uniformly distributed in various organs of a heterogeneous phantom. MIRD Pam. 1978, 5, 10025874511. [Google Scholar]
- Cristy, M.; Eckerman, K. Specific Absorbed Fractions of Energy at Various Ages from Internal Photon Sources: 1, Methods; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1987. [Google Scholar]
- Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Dubois, P.A.; Asai, M.; Barrand, G.; Capra, R.; Chauvie, S.; Chytracek, R.; et al. Geant4 developments and applications. IEEE Trans. Nucl. Sci. 2006, 53, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. GEANT4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. A Accel. Spectrom. Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef] [Green Version]
- Chauvie, S.; Guatelli, S.; Ivanchenko, V.; Longo, F.; Mantero, A.; Mascialino, B.; Nieminen, P.; Pandola, L.; Parlati, S.; Peralta, L.; et al. Geant4 Low Energy Electromagnetic Physics. In Proceedings of the IEEE Symposium Conference Record Nuclear Science, Rome, Italy, 16–22 October 2004. [Google Scholar]
- Jan, S.; Benoit, D.; Becheva, E.; Carlier, T.; Cassol, F.; Descourt, P.; Frisson, T.; Grevillot, L.; Guigues, L.; Maigne, L.; et al. GATE V6: A major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys. Med. Biol. 2011, 56, 881–901. [Google Scholar] [CrossRef]
- Konik, A.; Madsen, M.T.; Sunderland, J.J. GATE simulations of small animal SPECT for determination of scatter fraction as a function of object size. IEEE Trans. Nucl. Sci. 2012, 59, 1887–1891. [Google Scholar] [CrossRef]
- Stute, S.; Carlier, T.; Cristina, K.; Noblet, C.; Martineau, A.; Hutton, B.; Barnden, L.; Buvat, I. Monte Carlo simulations of clinical PET and SPECT scans: Impact of the input data on the simulated images. Phys. Med. Biol. 2011, 56, 6441–6457. [Google Scholar] [CrossRef] [Green Version]
- Lazaro, D.; Buvat, I.; Loudos, G.; Strul, D.; Santin, G.; Giokaris, N.; Donnarieix, D.; Maigne, L.; Spanoudaki, V.; Styliaris, S.; et al. Validation of the GATE Monte Carlo simulation platform for modelling a CsI (Tl) scintillation camera dedicated to small-animal imaging. Phys. Med. Biol. 2004, 49, 271. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://github.com/OpenGATE/GateContrib (accessed on 16 August 2022).
- Huey, O.S.; See, Y.J.; Nabila, S.; Ping, H.S.; Suzanah, I. Collimator and energy window optimization for practical imaging protocol and quantification of Yttrium-90 bremsstrahlung spect/ct: A phantom study. Radiat. Phys. Chem. 2021, 178, 109080. [Google Scholar] [CrossRef]
- Sirtex Medical Inc. Sirtex Medical Training Manual. Training Program: Physicians and Institutions; SIRTeX Medical Limited: Woburn, MA, USA, 2015. [Google Scholar]
- Garin, E.; Rolland, Y.; Laffont, S.; Edeline, J. Clinical impact of 99m Tc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with 90 Y-loaded microspheres. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Lau, W.Y.; Leung, T.W.T.; Chan, M.; Johnson, P.J.; Li, A.K.C. Clinical evaluation of the partition model for estimating radiation doses from yttrium-90 microspheres in the treatment of hepatic cancer. Eur. J. Nucl. Med. 1997, 24, 293–298. [Google Scholar] [PubMed]
- Memon, K.; Lewandowski, R.J.; Kulik, L.; Riaz, A.; Mulcahy, M.F.; Salem, R. Radioembolization for Primary and Metastatic Liver Cancer. In Seminars in Radiation Oncology; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Sarfaraz, M.; Kennedy, A.S.; Cao, Z.J.; Sackett, G.D.; Yu, C.X.; Lodge, M.A.; Murthy, R.; Line, B.R.; Van Echo, D.A. Physical aspects of yttrium-90 microsphere therapy for nonresectable hepatic tumors. Med. Phys. 2003, 30, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.-P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Helmberger, T.; Golfieri, R.; Pech, M.; Pfammatter, T.; Arnold, D.; Cianni, R.; Maleux, G.; Munneke, G.; Pellerin, O.; Peynircioglu, B.; et al. Clinical application of trans-arterial radioembolization in hepatic malignancies in europe: First results from the prospective multicentre observational study CIRSE registry for SIR-spheres therapy (CIRT). Cardiovasc. Interv. Radiol. 2021, 44, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Gulec, S.A.; Mesoloras, G.; Stabin, M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: The MIRD equations for dose calculations. J. Nucl. Med. 2006, 47, 1209–1211. [Google Scholar]
- Kao, Y.H.; Magsombol, B.M.; Toh, Y.; Tay, K.H.; Chow, P.K.; Goh, A.S.W.; Ng, D.C.E. Personalized predictive lung dosimetry by technetium-99m macroaggregated albumin SPECT/CT for yttrium-90 radioembolization. EJNMMI Res. 2014, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Marcié, S.; Gerard, J.; Dejean, C.; Feuillade, J.; Gautier, M.; Montagné, L.; Fuentes, C.; Hannoun-Levi, J. The inverse square law: A basic principle in brachytherapy. Cancer Radiothér. 2022, 26, 1075–1077. [Google Scholar] [CrossRef]
- Turner, J.; Claringbold, P.G.; Klemp, P.F.; Cameron, P.J.; A Martindale, A.; Glancy, R.J.; E Norman, P.; Hetherington, E.L.; Najdovski, L.; Lambrecht, R.M. 166Ho-microsphere liver radiotherapy: A preclinical SPECT dosimetry study in the pig. Nucl. Med. Commun. 1994, 15, 545–553. [Google Scholar] [CrossRef]
- Soares, D.C.F.; de Oliveira, M.C.; dos Santos, R.G.; Andrade, M.S.; Vilela, J.M.C.; Cardoso, V.N.; Ramaldes, G.A. Liposomes radiolabeled with 159Gd-DTPA-BMA: Preparation, physicochemical characterization, release profile and in vitro cytotoxic evaluation. Eur. J. Pharm. Sci. 2011, 42, 462–469. [Google Scholar] [CrossRef]
- Soares, D.C.F.; de Oliveira, M.C.; de Barros, A.L.B.; Cardoso, V.N.; Ramaldes, G.A. Liposomes radiolabeled with 159Gd: In vitro antitumoral activity, biodistribution study and scintigraphic image in Ehrlich tumor bearing mice. Eur. J. Pharm. Sci. 2011, 43, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Galvão, I.; Neves, M.; Santos, R. Evaluation of Gd and Gd 159 as New Approaches for Cancer Treatment. In Proceedings of the International Nuclear Atlantic Conference, Belo Horizonte, Brazil, 24–28 October 2011. [Google Scholar]
- Cipreste, M.F.; Peres, A.M.; Cotta, A.A.; Aragón, F.H.; Antunes, A.D.M.; Leal, A.S.; Macedo, W.A.; de Sousa, E.M. Synthesis and characterization of 159Gd-doped hydroxyapatite nanorods for bioapplications as theranostic systems. Mater. Chem. Phys. 2016, 181, 301–311. [Google Scholar] [CrossRef]
- Braat, A.J.; Huijbregts, J.E.; Molenaar, I.Q.; Rinkes, I.H.M.B.; van den Bosch, M.A.A.J.; Lam, M.G.E.H. Hepatic radioembolization as a bridge to liver surgery. Front. Oncol. 2014, 4, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewaraja, Y.K.; Ljungberg, M.; Koral, K.F. Accuracy of 131I tumor quantification in radioimmunotherapy using SPECT imaging with an ultra-high-energy collimator: Monte Carlo study. J. Nucl. Med. 2000, 41, 1760–1767. [Google Scholar]
- Perez-Garcia, H.; Barquero, R. The HURRA filter: An easy method to eliminate collimator artifacts in high-energy gamma camera images. Rev. Española Med. Nucl. Imagen Mol. 2017, 36, 27–36. [Google Scholar] [CrossRef]
- Bouzekraoui, Y.; Bentayeb, F.; Asmi, H.; Bonutti, F. Determination of the energy windows for the triple energy window scatter correction method in gadolinium-159 single photon emission computed tomography using Monte Carlo simulation. Iran. J. Med. Phys. 2019, 16, 405–409. [Google Scholar]





| Radioisotope | Collimator | Length (mm) | Septal Thickness (mm) | Hole Diameter (mm) |
|---|---|---|---|---|
| 159Gd | High-energy general purpose (HEGP) | 60 | 2 | 4 |
| 99mTc | Low-energy high-resolution (LEHR) | 24.05 | 0.160 | 1.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, A.S.; Abdul Hadi, M.F.R.; Ashour, N.I.; Hashikin, N.A.A. Theranostic Investigation of Gadolinium-159 for Hepatocellular Carcinoma: Monte Carlo Simulation Study. Appl. Sci. 2022, 12, 12396. https://doi.org/10.3390/app122312396
Musa AS, Abdul Hadi MFR, Ashour NI, Hashikin NAA. Theranostic Investigation of Gadolinium-159 for Hepatocellular Carcinoma: Monte Carlo Simulation Study. Applied Sciences. 2022; 12(23):12396. https://doi.org/10.3390/app122312396
Chicago/Turabian StyleMusa, Ahmed Sadeq, Muhammad Fahmi Rizal Abdul Hadi, Nabeel Ibrahim Ashour, and Nurul Ab. Aziz Hashikin. 2022. "Theranostic Investigation of Gadolinium-159 for Hepatocellular Carcinoma: Monte Carlo Simulation Study" Applied Sciences 12, no. 23: 12396. https://doi.org/10.3390/app122312396





