Alterations in Surface Roughness and Chemical Characteristics of Sandblasted and Acid-Etched Titanium Implants after Irradiation with Different Diode Lasers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dental Laser
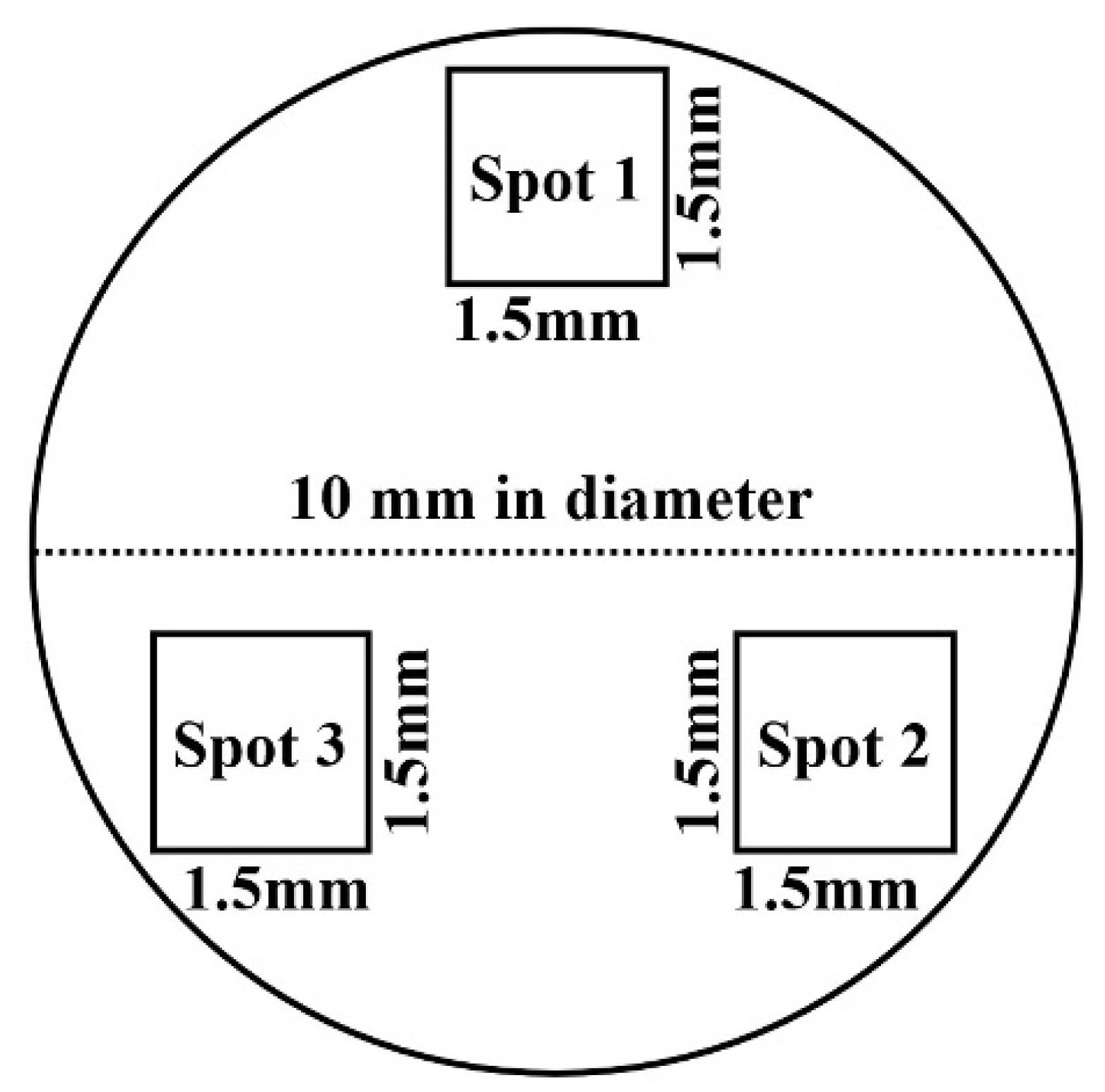
2.2. Preparation of Titanium Implants and Laser Irradiations
2.3. Surface Roughness
2.4. Energy-Dispersive Spectrometry
2.5. Statistical Analysis
3. Results
3.1. Surface Roughness
3.2. Atomic and Weight Percentage
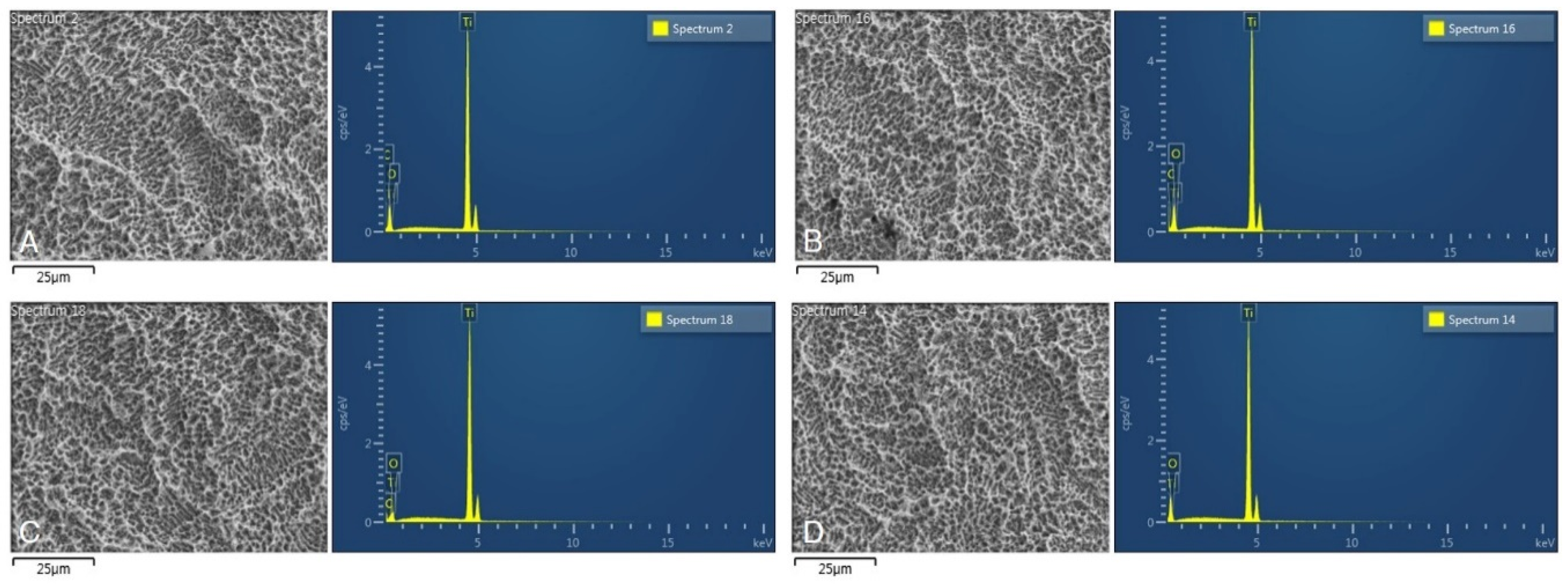
3.3. Scanning Electron Microscope Images and EDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shin, S.I.; Min, H.K.; Park, B.H.; Kwon, Y.H.; Park, J.B.; Herr, Y.; Heo, S.J.; Chung, J.H. The effect of Er:YAG laser irradiation on the scanning electron microscopic structure and surface roughness of various implant surfaces: An in vitro study. Lasers Med. Sci. 2011, 26, 767–776. [Google Scholar] [CrossRef]
- Gholami, G.A.; Karamlou, M.; Fekrazad, R.; Ghanavati, F.; Hakimiha, N.; Romanos, G. Comparison of the Effects of Er, Cr: YSGG Laser and Super-Saturated Citric Acid on the Debridement of Contaminated Implant Surfaces. J. Lasers Med. Sci. 2018, 9, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, S.; Bianchi, S.; Tomei, A.R.; Continenza, M.A.; Macchiarelli, G. Microbiological and SEM-EDS evaluation of titanium surfaces exposed to periodontal gel: In vitro study. Materials 2019, 12, 1448. [Google Scholar] [CrossRef] [Green Version]
- Deppe, H.; Horch, H.H.; Neff, A. Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: A 5-year clinical report. Int. J. Oral Maxillofac. Implants 2007, 22, 79–86. [Google Scholar]
- Rühling, A.; Kocher, T.; Kreusch, J.; Plagmann, H.C. Treatment of subgingival implant surfaces with Teflon-coated sonic and ultrasonic scaler tips and various implant curettes. An in vitro study. Clin. Oral Implants Res. 1994, 5, 19–29. [Google Scholar] [CrossRef]
- Shetty, B.; Dinesh, A.; Seshan, H. Comparitive effects of tetracyclines and citric acid on dentin root surface of periodontally involved human teeth: A scanning electron microscope study. J. Indian Soc. Periodontol. 2008, 12, 8–15. [Google Scholar] [CrossRef]
- Romanos, G.E.; Everts, H.; Nentwig, G.H. Effects of diode and Nd:YAG laser irradiation on titanium discs: A scanning electron microscope examination. J. Periodontol. 2000, 71, 810–815. [Google Scholar] [CrossRef]
- Fox, S.C.; Moriarty, J.D.; Kusy, R.P. The effects of scaling a titanium implant surface with metal and plastic instruments: An in vitro study. J. Periodontol. 1990, 61, 485–490. [Google Scholar] [CrossRef]
- Stubinger, S.; Etter, C.; Miskiewicz, M.; Homann, F.; Saldamli, B.; Wieland, M.; Sader, R. Surface alterations of polished and sandblasted and acid-etched titanium implants after Er:YAG, carbon dioxide, and diode laser irradiation. Int. J. Oral Maxillofac. Implants 2010, 25, 104–111. [Google Scholar]
- Moritz, A.; Schoop, U.; Goharkhay, K.; Schauer, P.; Doertbudak, O.; Wernisch, J.; Sperr, W. Treatment of periodontal pockets with a diode laser. Lasers Surg. Med. 1998, 22, 302–311. [Google Scholar] [CrossRef]
- Rios, F.G.; Viana, E.R.; Ribeiro, G.M.; González, J.C.; Abelenda, A.; Peruzzo, D.C. Temperature evaluation of dental implant surface irradiated with high-power diode laser. Lasers Med. Sci. 2016, 31, 1309–1316. [Google Scholar] [CrossRef]
- Tosun, E.; Tasar, F.; Strauss, R.; Kıvanc, D.G.; Ungor, C. Comparative evaluation of antimicrobial effects of Er:YAG, diode, and CO₂ lasers on titanium discs: An experimental study. J. Oral Maxillofac. Surg. 2012, 70, 1064–1069. [Google Scholar] [CrossRef]
- Block, C.M.; Mayo, J.A.; Evans, G.H. Effects of the Nd:YAG dental laser on plasma-sprayed and hydroxyapatite-coated titanium dental implants: Surface alteration and attempted sterilization. Int. J. Oral Maxillofac. Implants 1992, 7, 441–449. [Google Scholar]
- Torkzaban, P.; Kasraei, S.; Torabi, S.; Farhadian, M. Low-level laser therapy with 940 nm diode laser on stability of dental implants: A randomized controlled clinical trial. Lasers Med. Sci. 2018, 33, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Gutknecht, N.; Dieter, S.; Schwarz, F.; Crespi, R.; Sculean, A. Laser wavelengths and oral implantology. Lasers Med. Sci. 2009, 24, 961–970. [Google Scholar] [CrossRef]
- Ercan, E.; Arin, T.; Kara, L.; Çandirli, C.; Uysal, C. Effects of Er, Cr:YSGG laser irradiation on the surface characteristics of titanium discs: An in vitro study. Lasers Med. Sci. 2014, 29, 875–880. [Google Scholar] [CrossRef]
- Faggion, C.M. Laser Therapy as an Adjunct Treatment for Peri-Implant Mucositis and Peri-Implantitis Provides No Extra Benefit for Most Clinical Outcomes. J. Evid. Based Dent. Pract. 2019, 19, 203–206. [Google Scholar] [CrossRef]
- Geminiani, A.; Caton, J.G.; Romanos, G.E. Temperature change during non-contact diode laser irradiation of implant surfaces. Lasers Med. Sci. 2012, 27, 339–342. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D.M. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef]
- Lausmaa, J.; Kasemo, B. Surface spectroscopic characterization of titanium implant materials. Appl. Surf. Sci. 1990, 44, 133–146. [Google Scholar] [CrossRef]
- Shibli, J.A.; Marcantonio, E.; d’Avila, S.; Guastaldi, A.C.; Marcantonio, E. Analysis of failed commercially pure titanium dental implants: A scanning electron microscopy and energy-dispersive spectrometer x-ray study. J. Periodontol. 2005, 76, 1092–1099. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Choi, S.H.; Ryu, J.J.; Koh, S.Y.; Park, J.H.; Lee, I.S. The biocompatibility of SLA-treated titanium implants. Biomed. Mater. 2008, 3, 025011. [Google Scholar] [CrossRef]
| Laser Types | Output Power | Actual Power | Power Density (W/cm2) | Energy Density (kJ/cm2) |
|---|---|---|---|---|
| K2 mobile (λ = 980 nm) | 1.0 W | 1.1812 W | 969.2 | 14 |
| 2.0 W | 2.2378 W | 1905.2 | 28 | |
| 3.0 W | 3.4866 W | 2715 | 40 | |
| Epic 10 TM (λ = 940 nm) | 1.0 W | 0.7628 W | 607.4 | 9 |
| 2.0 W | 1.4548 W | 1157.4 | 17 | |
| 3.0 W | 2.2612 W | 1788 | 26 | |
| Saeshin (λ = 980 nm) | 1.0 W | 0.8604 W | 668.6 | 10 |
| 2.0 W | 1.6334 W | 1334.6 | 20 | |
| 3.0 W | 2.4698 W | 1957.8 | 29 |
| Output Power | Laser Types | Distance from Laser Irradiation | |||||
|---|---|---|---|---|---|---|---|
| 0 mm | 1 mm | 2 mm | 3 mm | 4 mm | 5 mm | ||
| Surface Temperature (Mean ± SD, °C) | |||||||
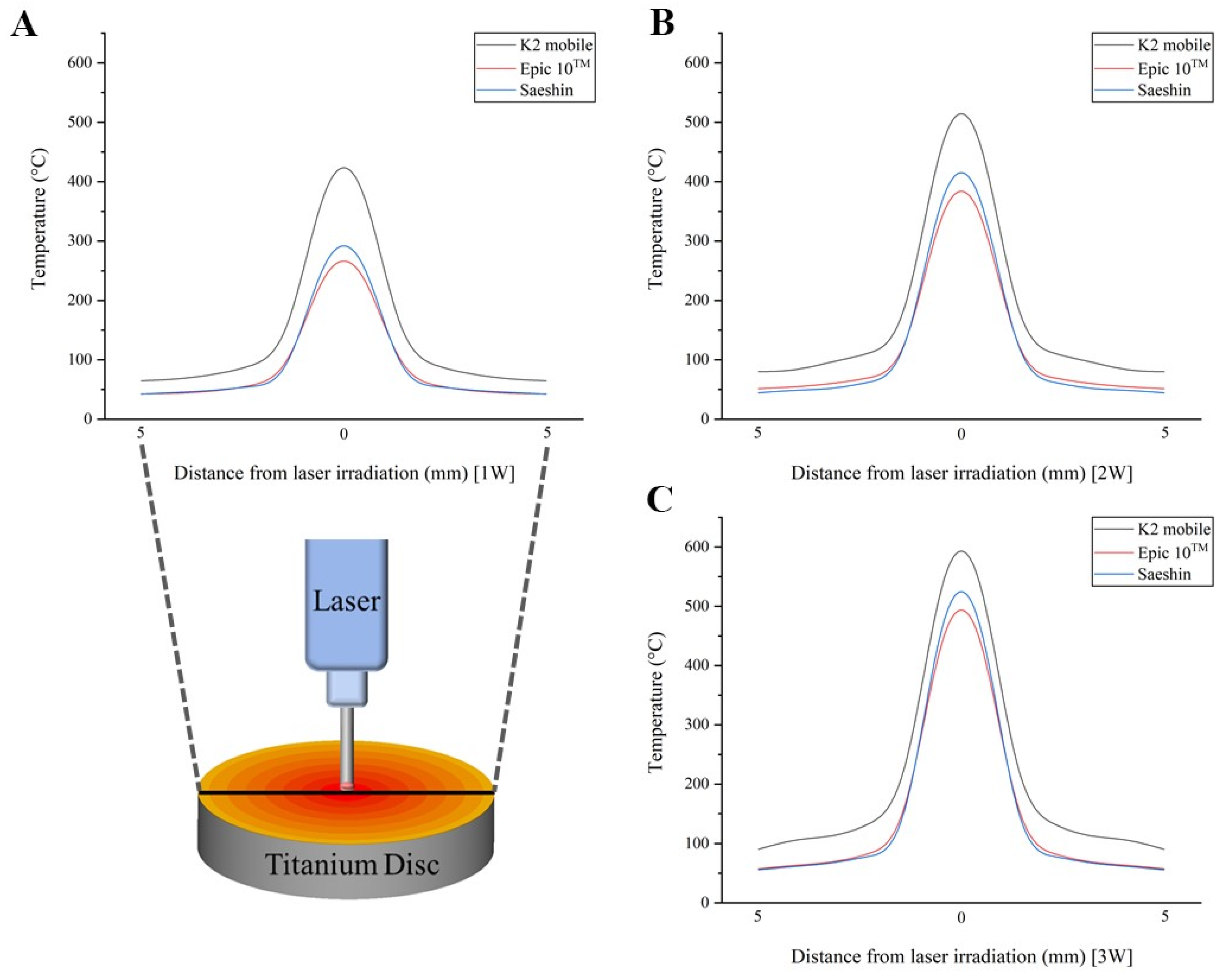
| 1.0 W | K2 mobile | 436.6 ± 18 a | 118.3 ± 24.3 a | 88.3 ± 6.2 a | 75.1 ± 5.7 a | 69.2 ± 5.2 a | 67.8 ± 4.3 a |
| Epic 10 TM | 301.7 ± 21.6 b | 61.8 ± 2.5 b | 55.2 ± 4.7 b | 48.5 ± 3.5 b | 45.1 ± 2.1 b | 42.4 ± 2.4 b | |
| Saeshin | 274.6 ± 11.3 b | 74.4 ± 15.3 b | 55.5 ± 3.9 b | 47.2 ± 3.5 b | 43.5 ± 3.3 b | 42.7 ± 2.7 b | |
| p | <0.001 § | <0.001 § | <0.001 § | <0.001 § | <0.001 § | <0.001 § | |
| 2.0 W | K2 mobile | 630.8 ± 24.8 a | 135.6 ± 18.3 a | 109.3 ± 4.9 a | 95.2 ± 4.7 a | 86 ± 2.6 a | 82.2 ± 2.8 a |
| Epic 10 TM | 429.7 ± 16.8 b | 77.5 ± 12.3 b | 61.8 ± 6.4 b | 50.7 ± 3.5 b | 49 ± 3.2 b | 44.7 ± 2.7 b | |
| Saeshin | 396.7 ± 15.5 b | 85.3 ± 11.5 b | 68.8 ± 3.1 b | 59.9 ± 2.9 b | 54.1 ± 1.6 b | 51.7 ± 1.7 b | |
| p | <0.001 § | <0.001 § | <0.001 § | <0.001 § | <0.001 § | <0.001 § | |
| 3.0 W | K2 mobile | 811.4 ± 23.2 a | 171.5 ± 24.8 a | 129.6 ± 12.2 a | 107.6 ± 3.3 a | 101.3 ± 4.2 a | 91.5 ± 3.5 a |
| Epic 10 TM | 543.3 ± 15.9 b | 91.6 ± 4.6 b | 78.4 ± 7.8 b | 66.5 ± 3.4 b | 61.6 ± 0.8 b | 55.9 ± 1.9 b | |
| Saeshin | 510.3 ± 14.5 b | 107.9 ± 15.6 b | 81.5 ± 7.6 b | 67.7 ± 2.1 b | 63.7 ± 2.6 b | 57.6 ± 2.2 b | |
| p | <0.001 § | <0.001 § | <0.001 § | <0.001 § | <0.001 § | <0.001 § | |
| No. of Disc | Group | Laser Types | Output Power | Ra Value (n = 3, µm) |
|---|---|---|---|---|
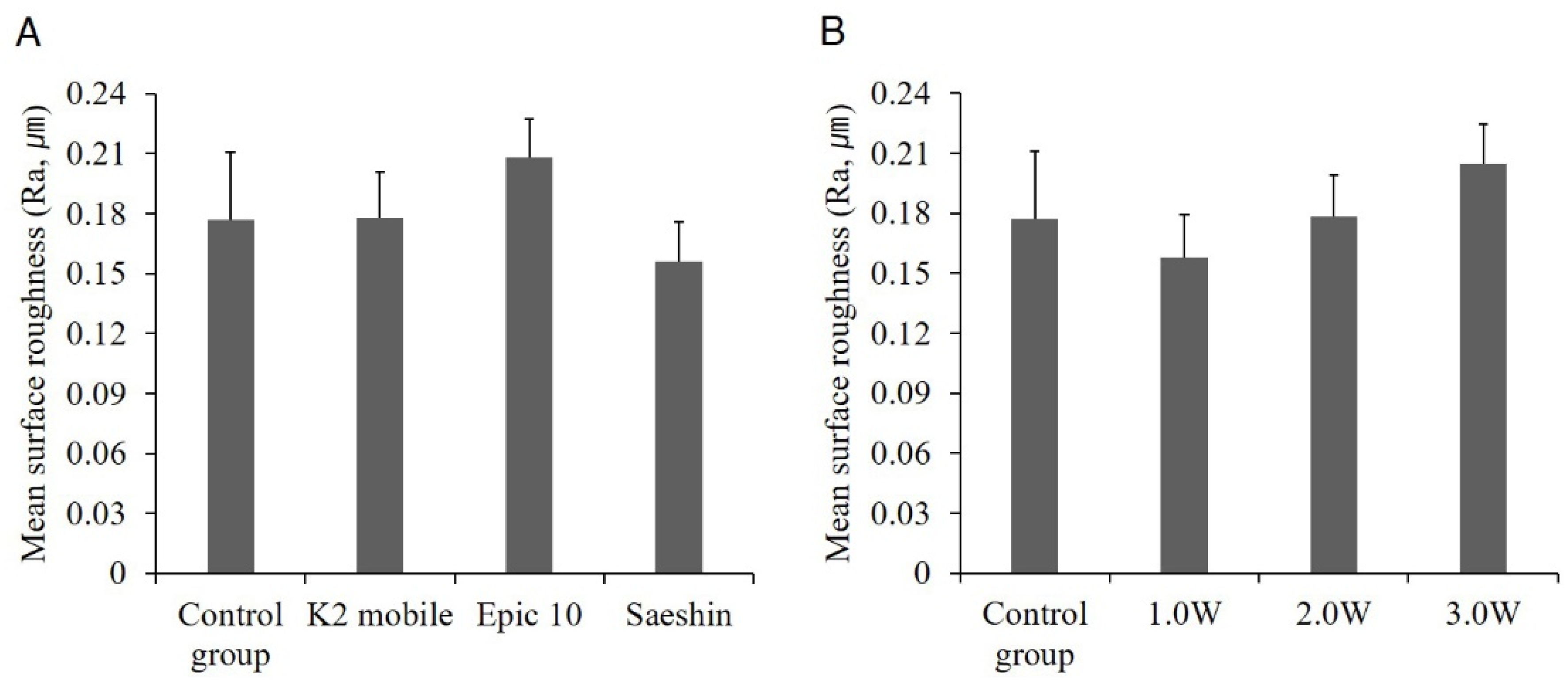
| No. 1 | Control group | No irradiation | 0.177 ± 0.018 | |
| No. 2 | Experimental Group 1 | K2 mobile (λ = 980 nm) | 1.0 W | 0.147 ± 0.007 |
| No. 3 | 2.0 W | 0.204 ± 0.029 | ||
| No. 4 | 3.0 W | 0.183 ± 0.073 | ||
| No. 5 | Experimental Group 2 | Epic 10TM (λ = 940 nm) | 1.0 W | 0.182 ± 0.070 |
| No. 6 | 2.0 W | 0.174 ± 0.043 | ||
| No. 7 | 3.0 W | 0.267 ± 0.095 | ||
| No. 8 | Experimental Group 3 | Saeshin (λ = 980 nm) | 1.0 W | 0.144 ± 0.067 |
| No. 9 | 2.0 W | 0.158 ± 0.056 | ||
| No. 10 | 3.0 W | 0.166 ± 0.040 | ||
| Group | Laser Types | Output Powers | Weight Percentage | Atomic Percentage | ||||
|---|---|---|---|---|---|---|---|---|
| C | O | Ti | C | O | Ti | |||
| Experimental Group 1 | K2 mobile | 1.0 W | 0 | 4.50 | 95.50 | 0 | 12.36 | 87.64 |
| 2.0 W | 0 | 3.50 | 96.50 | 0 | 9.0 | 90.20 | ||
| 3.0 W | 2.91 | 5.01 | 92.08 | 9.77 | 12.64 | 77.59 | ||
| Experimental Group 2 | Epic 10TM | 1.0 W | 2.10 | 4.24 | 93.65 | 7.31 | 11.08 | 81.62 |
| 2.0 W | 2.43 | 5.05 | 92.52 | 8.27 | 12.89 | 78.85 | ||
| 3.0 W | 2.17 | 4.91 | 92.92 | 7.43 | 12.64 | 79.92 | ||
| Experimental Group 3 | Saeshin | 1.0 W | 1.89 | 5.79 | 92.32 | 6.45 | 14.79 | 78.76 |
| 2.0 W | 0 | 4.11 | 95.89 | 0 | 11.38 | 88.62 | ||
| 3.0 W | 0 | 4.52 | 95.48 | 0 | 12.40 | 87.60 | ||
| Control Group | No irradiation | 2.03 | 4.33 | 93.65 | 7.05 | 11.29 | 81.66 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K.; Park, S.-Y.; Son, K.; Kim, Y.-G.; Yu, W.-J.; Lee, K.-B.; Lee, J.-M. Alterations in Surface Roughness and Chemical Characteristics of Sandblasted and Acid-Etched Titanium Implants after Irradiation with Different Diode Lasers. Appl. Sci. 2020, 10, 4167. https://doi.org/10.3390/app10124167
Kim H-K, Park S-Y, Son K, Kim Y-G, Yu W-J, Lee K-B, Lee J-M. Alterations in Surface Roughness and Chemical Characteristics of Sandblasted and Acid-Etched Titanium Implants after Irradiation with Different Diode Lasers. Applied Sciences. 2020; 10(12):4167. https://doi.org/10.3390/app10124167
Chicago/Turabian StyleKim, Hak-Ki, Su-Yeon Park, Keunbada Son, Yong-Gun Kim, Won-Jae Yu, Kyu-Bok Lee, and Jae-Mok Lee. 2020. "Alterations in Surface Roughness and Chemical Characteristics of Sandblasted and Acid-Etched Titanium Implants after Irradiation with Different Diode Lasers" Applied Sciences 10, no. 12: 4167. https://doi.org/10.3390/app10124167






