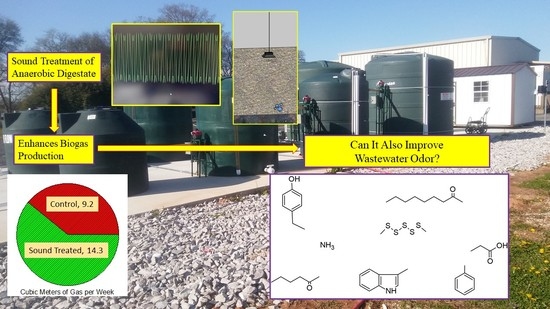
Acoustic Stimulation of Anaerobic Digestion: Effects on Biogas Production and Wastewater Malodors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Digester Description
2.2. Volatile Compound Extraction and Quantification
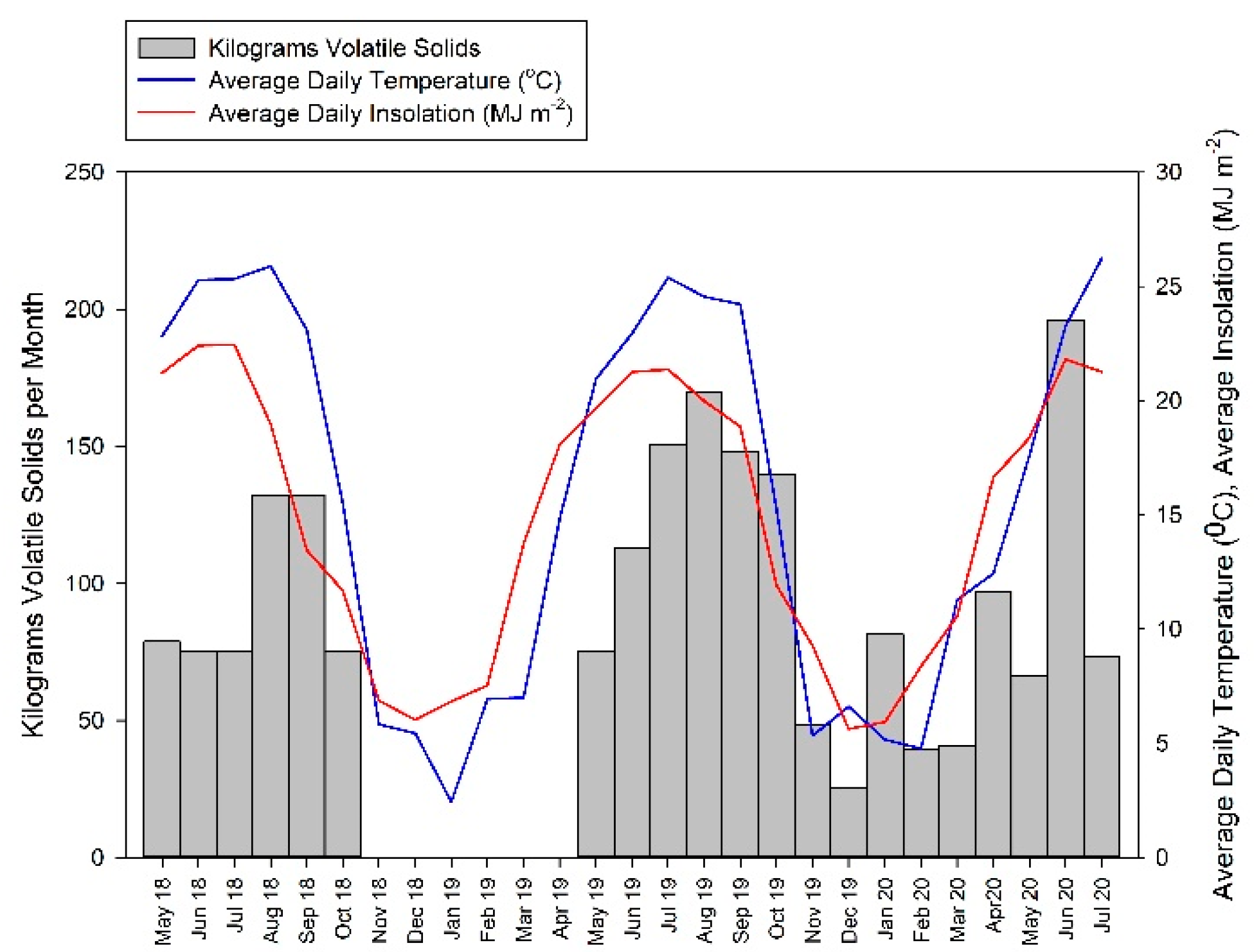
3. Results
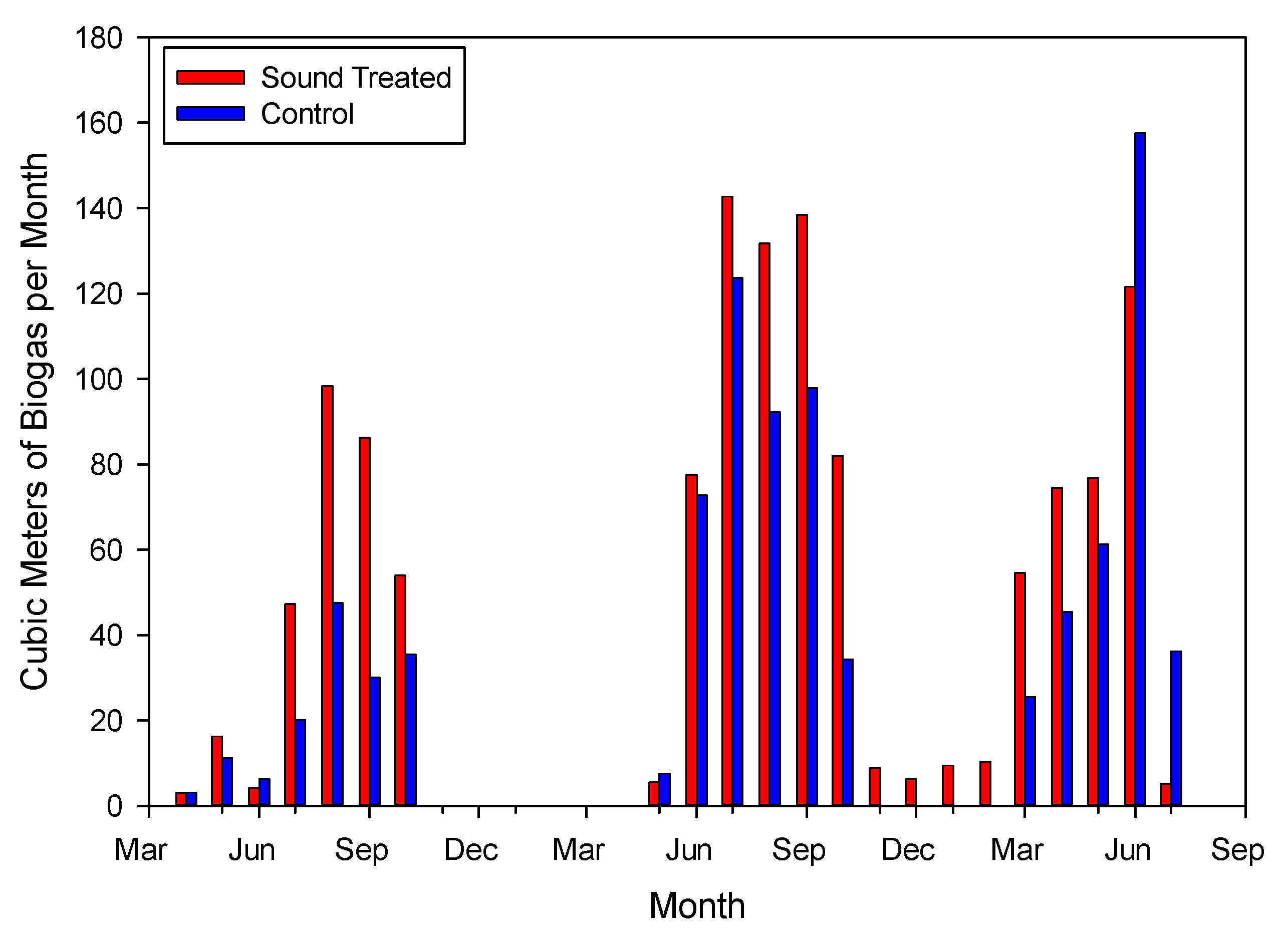
3.1. Biogas Production
3.2. Digestate Malodors
3.3. Secondary Ketones


3.4. Aromatic Malodorants





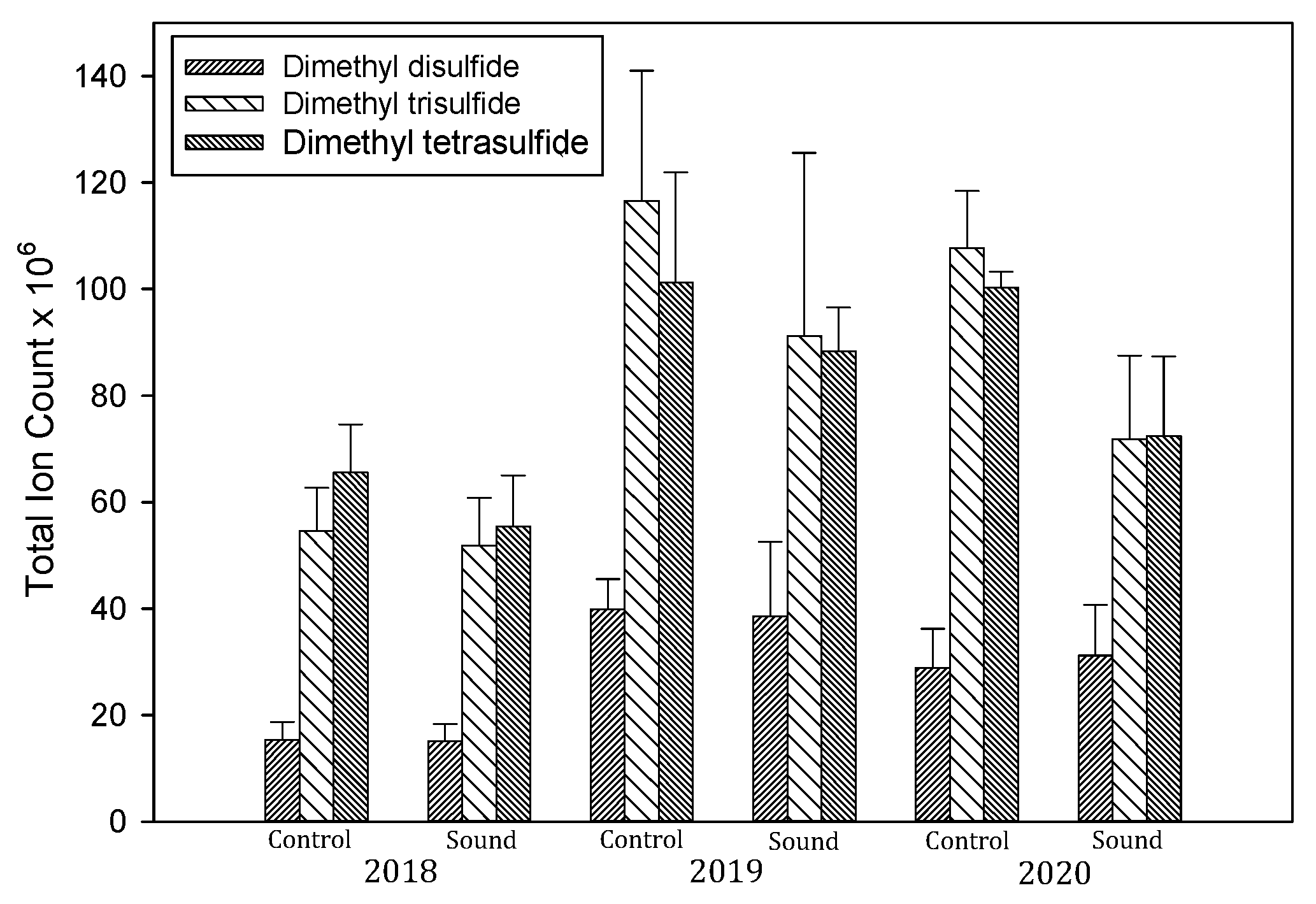
3.5. Volatile Sulfur Compounds
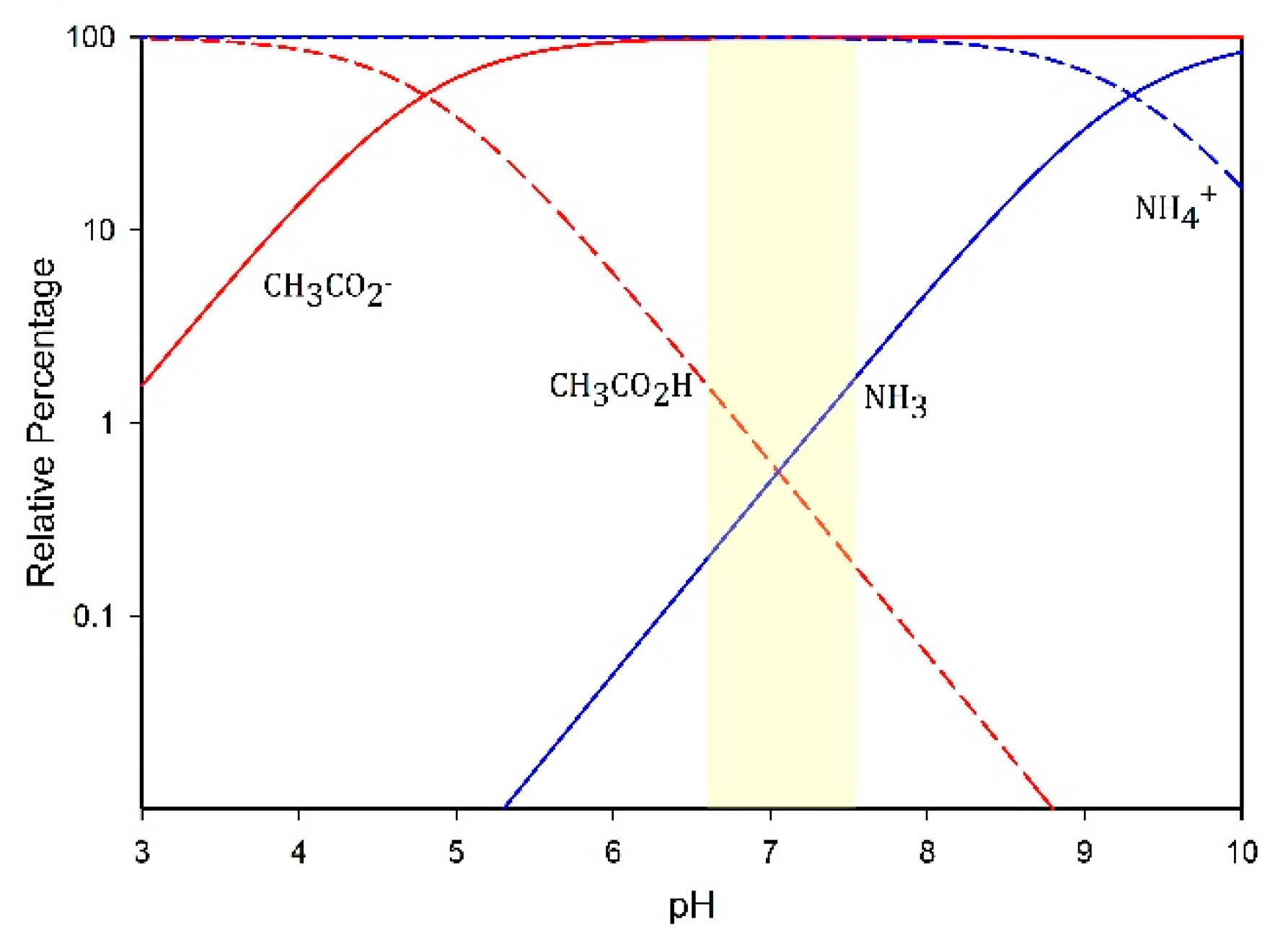
3.6. Volatile Fatty Acids and Ammonium
3.7. Sonic Treatment of Anaerobic Digestate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lafratta, M.; Thorpe, R.B.; Ouki, S.K.; Shana, A.; Germain, E.; Willcocks, M.; Lee, J. Dynamic biogas production from anaerobic digestion of sewage sludge for on-demand electricity generation. Bioresour. Technol. 2020, 310, 123415. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, R.; Cox, H.H.J.; Oh, S.; Fan, S.; Kearney, R.J.; Abkian, V.; Haug, R.T. Thermophilic-Anaerobic Digestion to Produce Class A Biosolids: Initial Full-Scale Studies at Hyperion Treatment Plant. Water Environ Res. 2006, 78, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Orzi, V.; Scagglia, B.; Lonati, S.; Riva, C.; Boccasile, G.; Alborali, G.L.; Adani, F. The role of biological processes in reducing both odor impact and pathogen content during mesophilic anaerobic digestion. Sci. Total Environ. 2015, 526, 116–126. [Google Scholar] [CrossRef]
- Welsh, F.W.; Schulte, D.D.; Kroeker, E.J.; Lapp, H.M. The Effect of anaerobic digestion upon swine manure odors. Can. Agric. Eng. 1977, 19, 122–126. [Google Scholar]
- Wilson, C.A. The Effect of Steady-State Digestion Temperature on The Performance, Stability, and Biosolids Odor Production Associated with Thermophilic Anaerobic Digestion. Master’s Thesis, Virginia Polytechnic Institute, Blacksburg, VA, USA, 8 November 2006. [Google Scholar]
- United States Environmental Protection Agency. Biosolids Technology Fact Sheet. Multi-Stage Anaerobic Digestion. Available online: https://www.epa.gov/sites/default/files/2018-11/documents/multistage-anaerobic-digestion-factsheet.pdf (accessed on 2 August 2022).
- Loughrin, J.; Antle, S.; Sistani, K.; Lovanh, N. In situ acoustic treatment of anaerobic digesters to improve biogas yields. Environments 2020, 7, 11. [Google Scholar] [CrossRef]
- Loughrin, J.; Antle, S.; Simmons, J.; Sistani, K.; Lovanh, N. In situ sonification of anaerobic digestion: Extended evaluation of performance in a temperate climate. Energies 2020, 13, 5349. [Google Scholar] [CrossRef]
- ChemIDPlus Advanced. Available online: https://chem.nlm.nih.gov/chemidplus/ (accessed on 28 June 2022).
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef]
- Chappuis, C.F.-J.; Niclass, Y.; Vuilleumier, C.; Starkenmann, C. Quantitative headspace analysis of selected odorants from latrines in Africa and India. Envrion. Sci. Technol. 2015, 49, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Jeon, E.-C.; Son, H.-K.; Sa, J.-H. Emission characteristics and factors of selected odorous compounds at a wastewater treatment plant. Sensors 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Ouaabou, R.; Ennahli, S.; Nabil, B.; Hssaini, L.; Hanine, H.; Hernández, F.; Ouhammou, M.; Mahrouz, M. Multivariate cherry quality assessment using morphological, biochemical and volatile compound traits. Int. J. Fruit Sci. 2020, 20, S1328–S1347. [Google Scholar] [CrossRef]
- Do, J.Y.; Salunkhe, D.K.; Olson, L.E. Isolation, identification and comparison of the volatiles of peach fruit as related to harvest maturity and artificial ripening. J. Food Sci. 1969, 34, 618–621. [Google Scholar] [CrossRef]
- Nishimura, O.; Yamaguchi, K.; Mihara, S.; Shibamoto, T. Volatile constituents of guava fruits (Psidium guajava L.) and canned puree. J. Agric. Food Chem. 1989, 37, 1139–1142. [Google Scholar] [CrossRef]
- Guterman, I.; Masci, T.; Chen, X.; Negre, F.; Pichersky, E.; Dudareva, N.; Weiss, D.; Vainstein, A. Generation of phenylpropanoid pathway-derived volatiles in transgenic plants: Rose alcohol acetyltransferase produces phenylethyl acetate and benzyl acetate in petunia flowers. Plant Mol. Biol. 2006, 60, 555–563. [Google Scholar] [CrossRef]
- Bera, P.; Kotamreddy, N.R.; Samanta, T.; Maiti, S.; Mitra, A. Inter-specific variation in headspace scent volatiles composition of four commercially cultivated jasmine flowers. Nat. Prod. Res. 2015, 29, 1328–1335. [Google Scholar] [CrossRef]
- Wilson, R.A.; Tfaily, M.M.; Rich, V.I.; Keller, J.K.; Bridgham, S.D.; Zalman, C.M.; Meredith, L.; Hanson, P.J.; Hines, M.; Pfeifer-Meister, L.; et al. Hydrogenation of organic matter as a terminal electron sink sustains high CO2: CH4 production ratios during anaerobic decomposition. Org. Geochem. 2017, 112, 22–32. [Google Scholar] [CrossRef]
- Mosandi, A.; Heusinger, G.; Gessner, M. Analytical and sensory differentiation of 1-octen-3-ol enantiomers. J. Agric. Food Chem. 1986, 34, 119–122. [Google Scholar] [CrossRef]
- Goloshchapova, K.; Stehling, S.; Heydeck, D.; Blum, M.; Kuhn, H. Functional characterization of a novel arachidonic acid 12S-lipoxygenase in the halotolerant bacterium Myxococcus fulvus exhibiting complex social living patterns. Microbiol. 2019, 8, e00775. [Google Scholar] [CrossRef]
- Schulz, H. Beta oxidation of fatty acids. Biochim. Biophys. Acta 1991, 1081, 109–120. [Google Scholar] [CrossRef]
- Goh, E.-B.B.; Baidoo, E.E.K.; Keasling, J.D.; Beller, H.R. Engineering of bacterial methyl ketone synthesis for biofuels. Appl. Environ. Microbiol. 2012, 78, 70–80. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, X.; Li, Y.; Wang, P.; Niu, S.; Zhang, K.; Huang, X. Biosynthesis of the nematode attractant 2-heptanone and Its co-evolution between the pathogenic bacterium Bacillus nematocida and non-pathogenic bacterium Bacillus subtilis. Front. Microbiol. 2019, 10, 1489. [Google Scholar] [CrossRef]
- Cometto-Muñiz, J.E.; Abraham, M.H.J. Olfactory psychometric functions for homologous 2-ketones. Behav. Brain Res. 2009, 201, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Zargar, K.; Saville, R.; Phelan, R.M.; Tringe, S.G.; Petzold, C.J.; Keasling, J.D.; Beller, H.R. In vitro characterization of phenylacetate decarboxylase, a novel enzyme catalyzing toluene biosynthesis in an anaerobic microbial community. Sci. Rep. 2016, 6, 31362. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wei, Y.; Liu, X.; Zhou, Y.; Jiang, L.; Yin, J.; Wang, F.; Hu, Y.; Urs, A.N.N.; Liu, Y.; et al. Indoleacetate decarboxylase is a glycyl radical enzyme catalysing the formation of malodorant skatole. Nat. Comm. 2018, 9, 4224. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Sato, T.; Nomoto, K.; Tsuji, H. Identification of phenol- and p-cresol-producing intestinal bacteria by using media supplemented with tyrosine and its metabolites. FEMS Microb. Ecol. 2018, 94, fiy125. [Google Scholar] [CrossRef]
- Backman, L.R.F.; Funk, M.A.; Dawson, C.D.; Drennan, C.L. New tricks for the glycyl radical enzyme family. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 674–695. [Google Scholar] [CrossRef]
- Louie, G.V.; Bowman, M.E.; Moffitt, M.C.; Baiga, T.J.; Moore, B.S.; Noel, J.P. Structural Determinants and Modulation of substrate specificity in phenylalanine-tyrosine ammonia-lyases. Chem. Biol. 2006, 13, 1327–1338. [Google Scholar] [CrossRef]
- Burns, R.O.; DeMoss, R.D. Properties of tryptophanase from Escherichia coli. Biochem. Biophys. Acta 1962, 65, 233–244. [Google Scholar] [CrossRef]
- Couto, J.A.; Campos, F.M.; Figueiredo, A.R.; Hogg, T.A. Ability of lactic acid bacteria to produce volatile phenols. Am. J. Enol. Vitic. 2006, 57, 166–171. [Google Scholar]
- Santamaría, L.; Reverón, I.; de Felipe, F.L.; de las Rivas, B.; Muñoz, R. Ethylphenol formation by Lactobacillus plantarum: Identification of the enzyme involved in the reduction of vinylphenols. Appl. Environ. Microbiol. 2018, 84, e0164-18. [Google Scholar] [CrossRef]
- Ma, Q.; Meng, N.; Li, Y.; Wang, J. Occurrence, impacts, and microbial transformation of 3-methylindole (skatole): A critical review. J. Hazard. Mater. 2021, 416, 126181. [Google Scholar] [CrossRef]
- Suflita, J.M.; Liang, L.-N.; Saxena, A. The anaerobic biodegradation of o-, m- and p-cresol by sulfate-reducing bacterial enrichment cultures obtained from a shallow anoxic aquifer. J. Ind. Microbiol. 1989, 4, 255–266. [Google Scholar] [CrossRef]
- Sharma, N.; Doerner, K.C.; Alok, P.C.; Choudhary, M. Skatole remediation potential of Rhodopseudomonas palustris WKU-KDNS3 isolated from an animal waste lagoon. Lett. Appl. Microbiol. 2014, 60, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Kohda, C.; Ando, T.; Nakai, Y. Isolation and characterization of anaerobic indole- and skatole-degrading bacteria from composting animal wastes. J. Gen. Appl. Microbiol. 1997, 43, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-D.; Berry, D.F. Degradation of substituted indoles by an indole-degrading methanogenic consortium. Appl. Environ. Microbiol. 1991, 57, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Takeuchi, N. Measurement of odor threshold by triangle odor bag method. Odor Meas. Rev. 2003, 118, 118–127. [Google Scholar]
- Williams, B.T.; Martínez, A.B.; Cowles, K.; Bermejo, A.; Curson, A.R.J.; Zhang, Y.; Newton-Payne, S.; Hind, A.J.; Li, C.-Y.; Rivera, P.P.L.; et al. Bacteria are important dimethylsulfoniopropionate producers in coastal sediments. Nat. Microbiol. 2019, 4, 1815–1825. [Google Scholar] [CrossRef]
- van Leerdam, R.C.; van den Bosch, P.L.F.; Lens, P.N.L.; Janssen, A.J.H. Reactions between methanethiol and biologically produced sulfur. Environ. Sci. Technol. 2011, 45, 1320–1326. [Google Scholar] [CrossRef]
- Joycelyn, P.C. Chemical reactions of thiols. In Biocehmistry of the SH Group; Watts, D.C., Ed.; Academic Press: London, UK, 1972; pp. 85–87. [Google Scholar]
- Pickering, I.J.; George, G.N.; Yu, E.Y.; Brune, D.C.; Tuschak, C.; Overmann, J.; Beatty, J.T.; Prince, R.C. Analysis of sulfur biochemistry of sulfur bacteria using x-ray absorption spectroscopy. Biochemistry 2001, 40, 8138–8145. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Energetics and application of heterotrophy in acetogenic bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef]
- Kouzuma, A.; Tsutsuumi, N.; Ishii, S.; Ueno, Y.; Abe, T.; Watanabe, K. Non-autotrophic methanogens dominate in anaerobic digesters. Sci. Rep. 2018, 7, 1510. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Chen, Z.; Hu, H.; Deng, C.; Wang, X. The benefits of autotrophic nitrogen removal from high concentration of urea wastewater through a process of urea hydrolysis and partial nitritation in sequencing batch reactor. J. Environ. Manag. 2021, 292, 112762. [Google Scholar] [CrossRef] [PubMed]
- Brennen, C.E. Cavitation and Bubble Dynamics, 1st ed.; Cambridge University Press: New York, NY, USA, 2014; p. 268. [Google Scholar]
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Wat. Res. 2001, 25, 2003–2009. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.-L.; Appels, L.; Dewil, R. Ultrasonic treatment of waste sludge: A review on mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1220–1288. [Google Scholar] [CrossRef]
- Zawieja, I.; Wlodarczyk, R.; Kowalczyk, M. Biogas generation from sonicated excess sludge. Water 2019, 11, 2127. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zhang, B.; Tao, J.; Li, Q.; Lv, B. Optimization of energy consumption of the ultrasonic pretreatment on sludge disintegration. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2nd International Conference on Manufacturing Technology, Materials and Chemical Engineering, Wuhan, China, 14–16 June 2019; IOP Publishing: Bristol, UK, 2019; p. 012198. [Google Scholar] [CrossRef]
- Alagöz, B.A.; Yenigün, O.; Erfinçler, A. Ultrasound assisted biogas production from co-digestion of wastewater sludges and agricultural wastes: Comparison with microwave pre-treatment. Ultason. Sonochem. 2017, 40, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Stroot, P.G.; McMahon, K.D.; Mackie, R.I.; Raskin, L. Anaerobic codigestion of municipal solid waste biosolids under various mixing conditions, I. Digester performance. Water Res. 2001, 35, 1804–1816. [Google Scholar] [CrossRef]
- Wu, B. Integration of mixing, heat transfer, and biochemical reaction kinetics in anaerobic methane fermentation. Biotechnol. Bioeng. 2012, 109, 2864–2874. [Google Scholar] [CrossRef]
| Compound | Molecular Weight (g mol−1) | Boiling Point (°C) | Log P |
|---|---|---|---|
| Dimethyl disulfide | 94.19 | 109.8 | 1.77 |
| Toluene | 92.14 | 110.6 | 2.73 |
| 2-Hexanone | 100.16 | 127.6 | 1.38 |
| 2-Heptanone | 114.18 | 151.0 | 1.98 |
| Benzaldehyde | 106.12 | 178.1 | 1.64 |
| Oct-1-en-3-ol | 128.21 | 175.0 | 2.60 |
| Phenol | 94.11 | 181.8 | 1.46 |
| Para-Cresol | 108.14 | 201.9 | 1.94 |
| 2-Nonanone | 142.24 | 195.3 | 3.14 |
| Benzyl acetate | 150.18 | 213.0 | 1.96 |
| Para-Ethylphenol | 122.17 | 217.9 | 2.58 |
| Indole | 117.15 | 254.0 | 2.14 |
| Skatole | 131.18 | 266.0 | 2.60 |
| 2-Tridecanone | 198.35 | 263.0 | 4.68 |
| Treatment | ||
|---|---|---|
| Compound | Control | Sound-Treated |
| Concentration (Milligrams per Liter) | ||
| Dimethyl disulfide | 171 ± 33.1 | 173 ± 29.6 |
| Toluene | 4.61 ± 2.1 | 6.0 ± 1.93 |
| Phenol | 97.5 ± 17 | 70.4 ± 12.4 |
| para-Cresol | 460 ± 116 | 435 ± 128 |
| para-Ethylphenol | 49.4 ± 25.3 | 50.9 ± 25.4 |
| Indole | 141 ± 30.2 | 104 ± 22.8 |
| Skatole | 116 ± 51.6 | 111 ± 17.2 |
| Concentration (Micrograms per Liter) | ||
| 2-Hexanone | 951 ± 349 | 581 ± 254 |
| 2-Heptanone | 835 ± 240 | 567 ± 161 |
| 2-Nonanone | 320 ± 119 | 347 ± 91 |
| 2-Tridecanone | 469 ± 333 | 717 ± 297 |
| 1-Octen-3-ol | 620 ± 379 | 452 ± 298 |
| Benzaldehyde | 41 ± 25 | 29 ± 14 |
| Benzyl acetate | 19 ± 12 | 32 ± 14 |
| Total identified | 1043 | 953 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loughrin, J.; Silva, P.; Lovanh, N.; Sistani, K. Acoustic Stimulation of Anaerobic Digestion: Effects on Biogas Production and Wastewater Malodors. Environments 2022, 9, 102. https://doi.org/10.3390/environments9080102
Loughrin J, Silva P, Lovanh N, Sistani K. Acoustic Stimulation of Anaerobic Digestion: Effects on Biogas Production and Wastewater Malodors. Environments. 2022; 9(8):102. https://doi.org/10.3390/environments9080102
Chicago/Turabian StyleLoughrin, John, Philip Silva, Nanh Lovanh, and Karamat Sistani. 2022. "Acoustic Stimulation of Anaerobic Digestion: Effects on Biogas Production and Wastewater Malodors" Environments 9, no. 8: 102. https://doi.org/10.3390/environments9080102