Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia)
Abstract
:1. Introduction
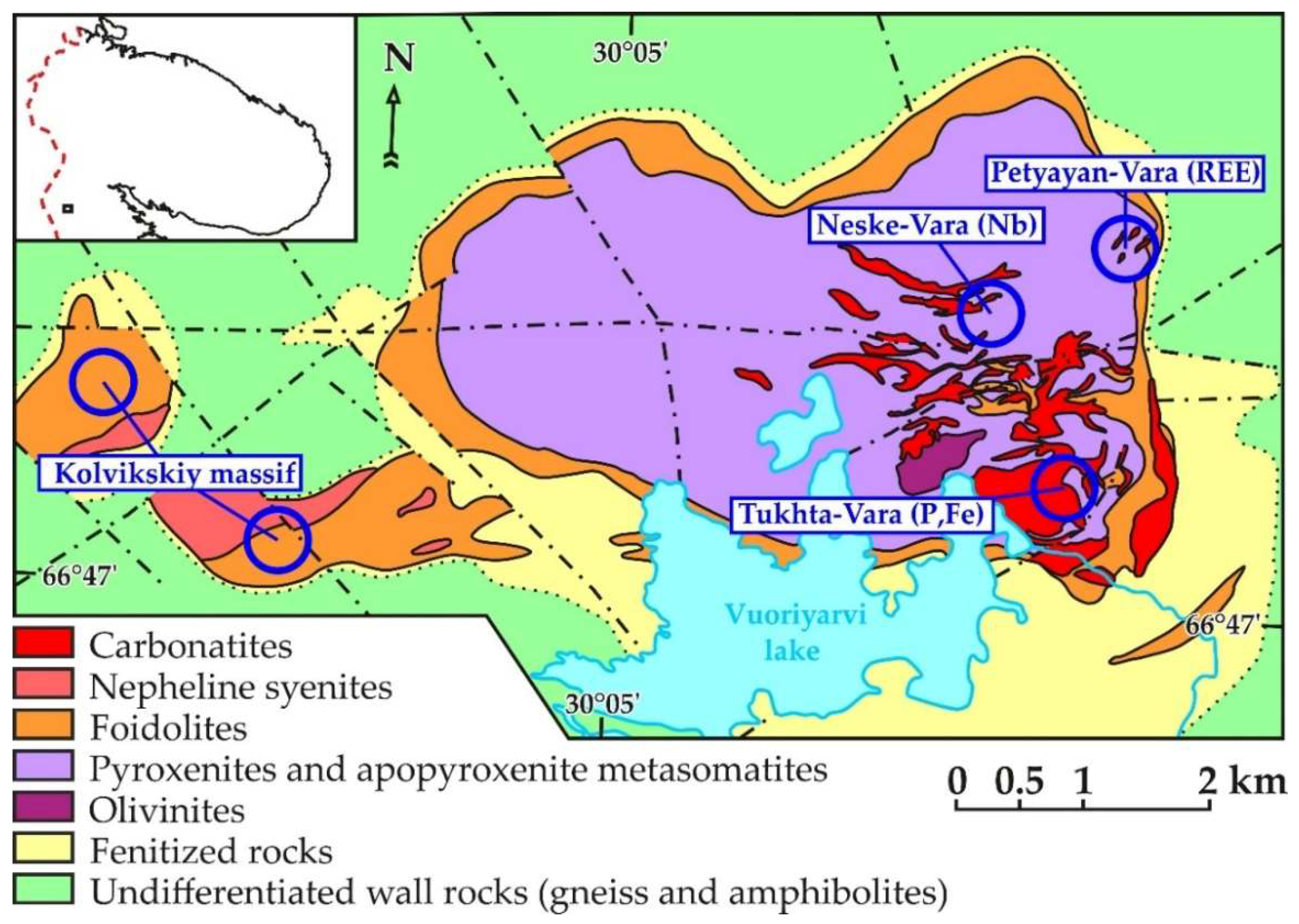
2. Geological Setting of the Petyayan-Vara Rocks
- (1)
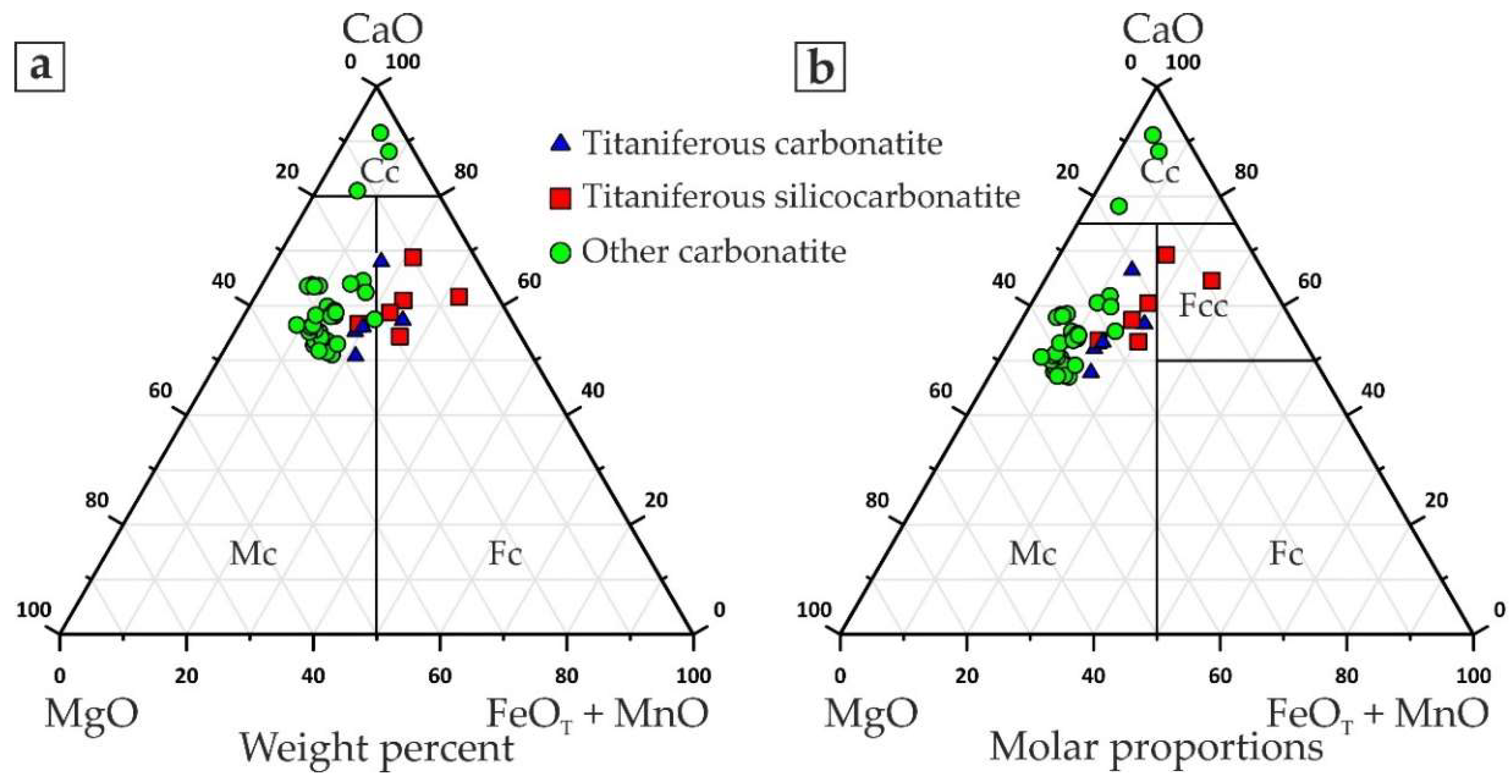
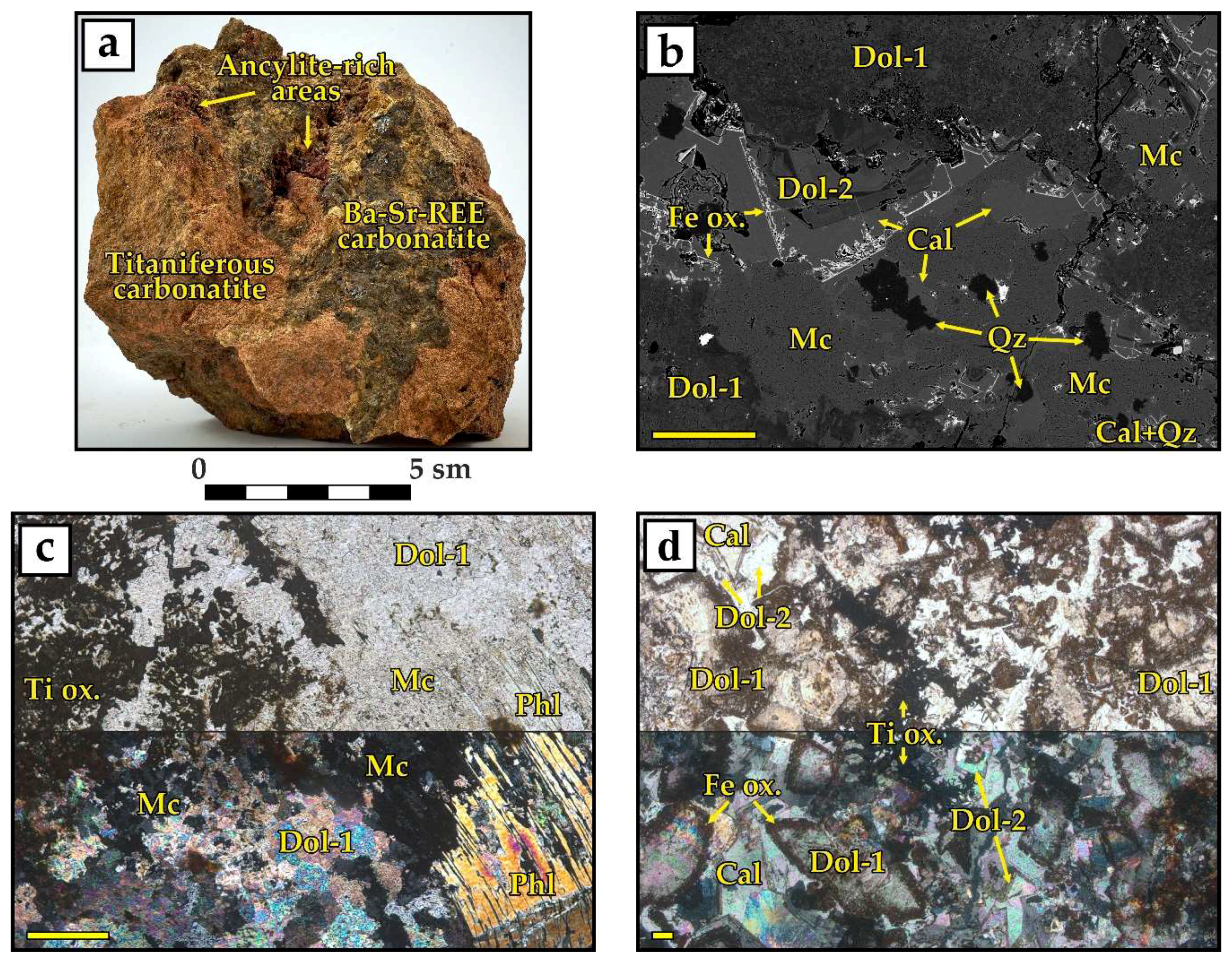
- Fine-grained dolomitic carbonatites and silicocarbonatites with microcline and/or phlogopite (hereinafter called titaniferous carbonatites), locally enriched with apatite (carbonatites with K-Al-Si-Ti-Fe ± P specialization);
- (2)
- Medium-grained dolomitic carbonatites with barite, strontianite, and ancylite-(Ce) (Ce,La)Sr(CO3)2(OH)×H2O ± bastnaesite-(Ce) (Ce,La)(CO3)F (carbonatites with Ba-REE-Sr specialization); and
- (3)
- Breccias of dolomitic carbonatites with cement consisting of fine-grained quartz and hydroxylbastnaesite-(Ce) (Ce,La)(CO3)(OH) (carbonatites with REE-Si specialization).
3. Research Material and Analytical Methods
4. Results
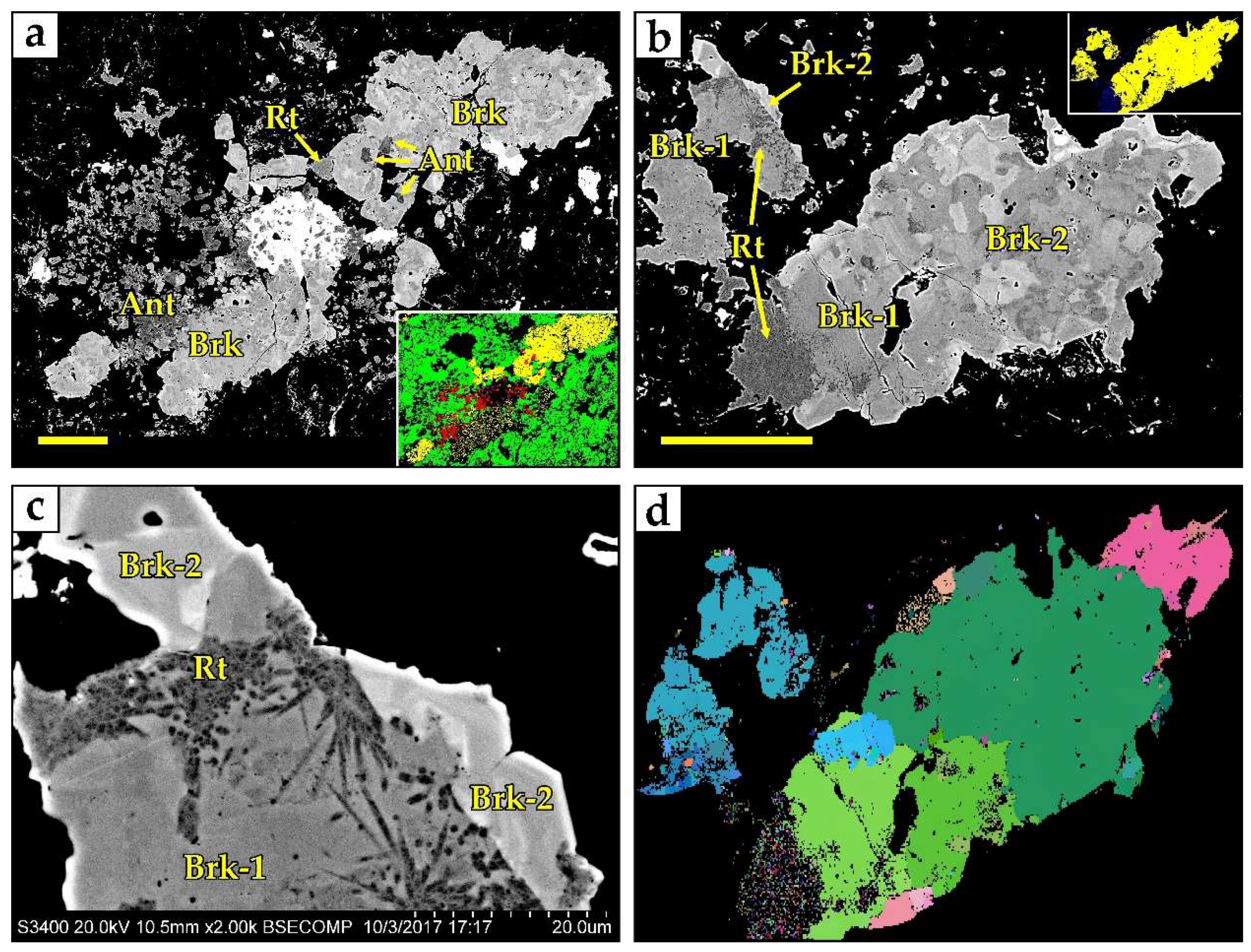
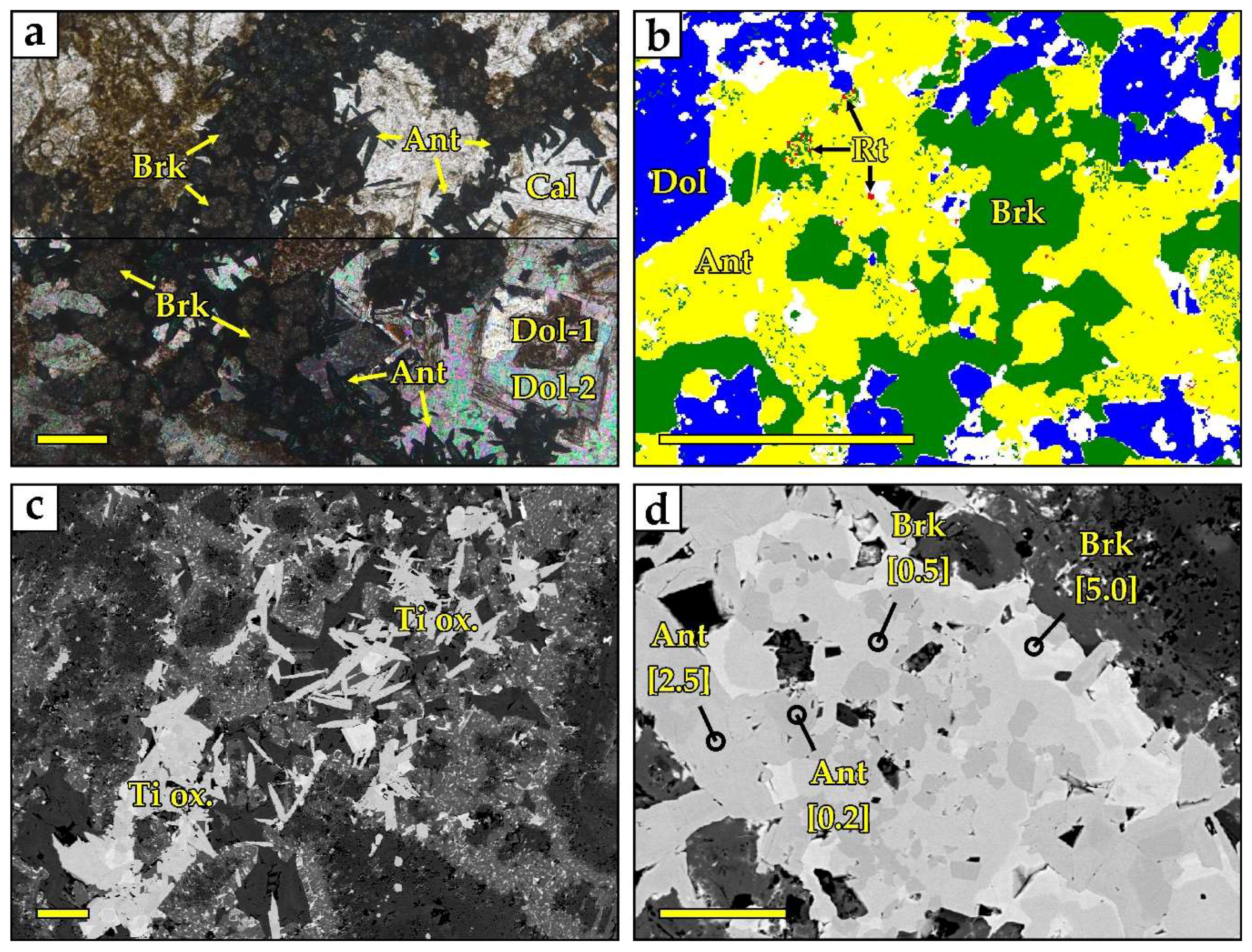
4.1. Titanium Minerals
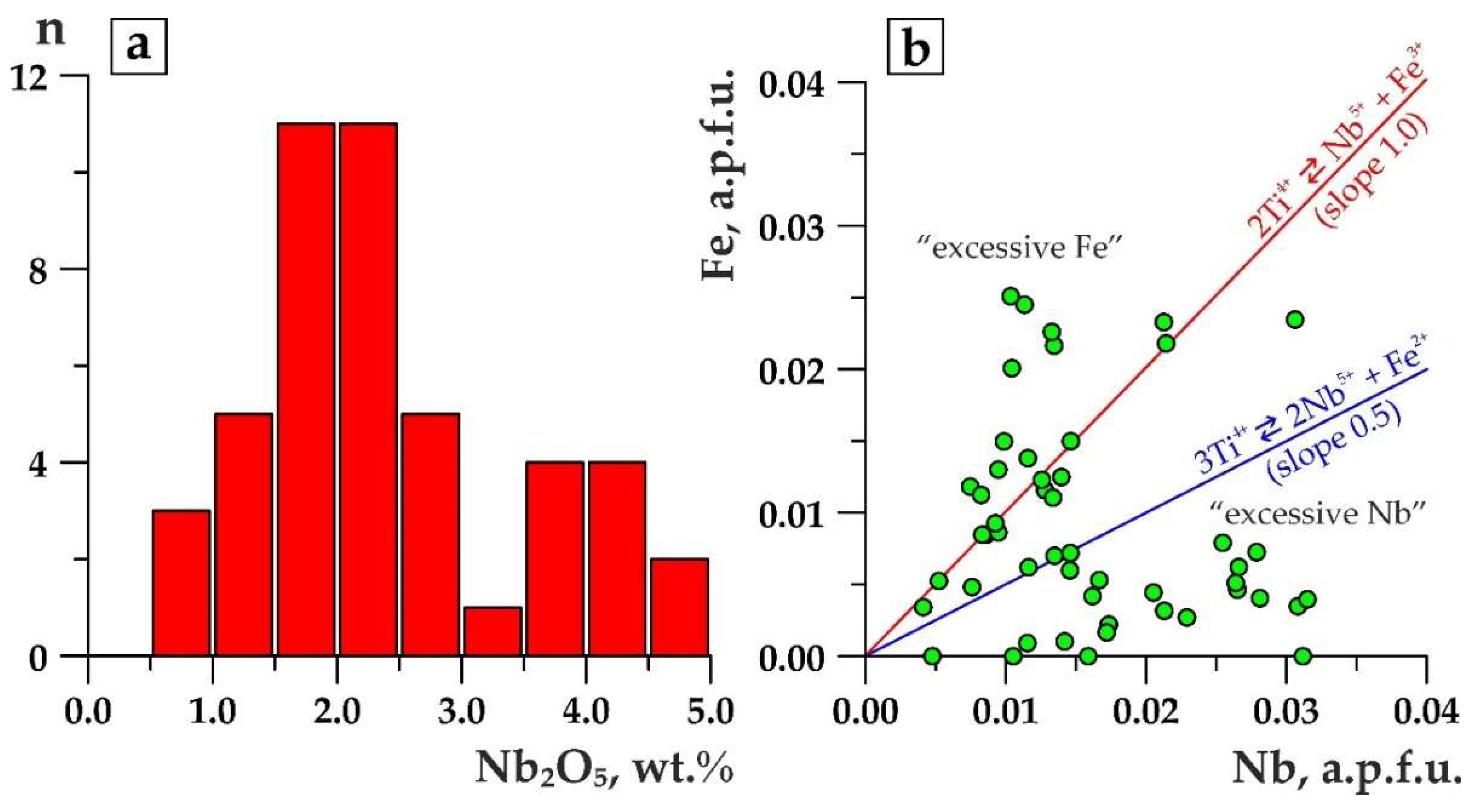
4.2. Niobium Minerals
5. Discussion
- (1)
- (2)
- Fluorcalciopyrochlore is naturally substituted by varieties, whereas in the A-site (1) Ca and Na contents decrease, (2) cations become minor up to xenopyrochlores (А-vacancy > 1 a.p.f.u), and (3) Ba, Sr, REE, Th, U, Pb, and H2O become the dominant cations. In the B-site, a decreasing trend in Nb content isomorphically substituted by Ti and Fe occurs. Y-site is characterized by F decreasing until it disappears, and OH-groups occur in this position. Different authors have linked this chemical specific feature of pyrochlores with magmatic [51], hydrothemal [27,28,31,49,52,53,54], or supergene [55,56] processes. The “hydrothermal” point of view prevails.
- (3)
- In pyrochlores that are most reasonably referred to supergenous [17,49,56,57], the same chemical features are the most manifested. Ba, Sr, REE, Th, U, Pb, H2O, and rarely K remain the dominant cations in the A-site, but in general, the occupancy rate of this position drops to the lowest level, down to zero. Ca and/or Na tend to disappear. No fluorine-varieties are detected among supergenous pyrochlore. They differ from “hydrothermal” pyrochlores with the B-site completely filled with niobium. With a vacant A-site, it makes supergenous pyrochlores become the richest in niobium: 75 wt % vs. 50 wt % in other varieties.
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Maitre, R.W.; Streckeisen, A.; Zanettin, B.; Le Bas, M.J.; Bonin, B.; Bateman, P.; Bellieni, G.; Dudek, A.; Efremova, S.; Keller, J.; et al. Igneous Rocks: A Classification and Glossary of Terms. Recommendations of the International Union of Geological Sciences Subcommission on the Systematics of Igneous Rocks, 2nd ed.; Le Maitre, R.W., Ed.; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780521619486. [Google Scholar]
- Woolley, A.R.; Kempe, D.R.C. Carbonatites: Nomenclature, average chemical compositions, and element distribution. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 1–14. [Google Scholar]
- Jones, A.P.; Genge, M.; Carmody, L. Carbonate Melts and Carbonatites. Rev. Mineral. Geochem. 2013, 75, 289–322. [Google Scholar] [CrossRef] [Green Version]
- Le Bas, M.J. Ferrocarbonatites: Geochemistry and magma-fluid state. Mem. Geol. Soc. India 1999, 43, 785–802. [Google Scholar]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A Detailed Assessment of Global Rare Earth Element Resources: Opportunities and Challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef]
- Doroshkevich, A.G.; Viladkar, S.G.; Ripp, G.S.; Burtseva, M.V. Hydrothermal REE mineralization in the Amba Dongar carbonatite complex, Gujarat, India. Can. Mineral. 2009, 47, 1105–1116. [Google Scholar] [CrossRef]
- Trofanenko, J.; Williams-Jones, A.E.; Simandl, G.J.; Migdisov, A.A. The Nature and Origin of the REE Mineralization in the Wicheeda Carbonatite, British Columbia, Canada. Econ. Geol. 2016, 111, 199–223. [Google Scholar] [CrossRef] [Green Version]
- Ngwenya, B.T. Hydrothermal rare earth mineralisation in carbonatites of the Tundulu complex, Malawi: Processes at the fluid/rock interface. Geochim. Cosmochim. Acta 1994, 58, 2061–2072. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Styles, M.T.; Appleton, J.D.; Gunn, G.; Wall, F. Evidence for dissolution-reprecipitation of apatite and preferential LREE mobility in carbonatite-derived late-stage hydrothermal processes. Am. Mineral. 2016, 101, 596–611. [Google Scholar] [CrossRef]
- Cooper, A.F.; Collins, A.K.; Palin, J.M.; Spratt, J. Mineralogical evolution and REE mobility during crystallisation of ancylite-bearing ferrocarbonatite, Haast River, New Zealand. Lithos 2015, 216–217, 324–337. [Google Scholar] [CrossRef]
- Duraiswami, R.; Shaikh, T. Fluid-rock interaction in the Kangankunde Carbonatite Complex, Malawi: SEM based evidence for late stage pervasive hydrothermal mineralisation. Open Geosci. 2014, 6. [Google Scholar] [CrossRef]
- Moore, M.; Chakhmouradian, A.R.; Mariano, A.N.; Sidhu, R. Evolution of rare-earth mineralization in the Bear Lodge carbonatite, Wyoming: Mineralogical and isotopic evidence. Ore Geol. Rev. 2015, 64, 499–521. [Google Scholar] [CrossRef]
- Prokopyev, I.R.; Borisenko, A.S.; Borovikov, A.A.; Pavlova, G.G. Origin of REE-rich ferrocarbonatites in southern Siberia (Russia): Implications based on melt and fluid inclusions. Mineral. Petrol. 2016, 110, 845–859. [Google Scholar] [CrossRef]
- Nadeau, O.; Cayer, A.; Pelletier, M.; Stevenson, R.; Jébrak, M. The Paleoproterozoic Montviel carbonatite-hosted REE–Nb deposit, Abitibi, Canada: Geology, mineralogy, geochemistry and genesis. Ore Geol. Rev. 2015, 67, 314–335. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Wall, F.; Bas, M.J. Le REE-Sr-Ba minerals from the Khibina carbonatites, Kola Peninsula, Russia: Their mineralogy, paragenesis and evolution. Mineral. Mag. 1998, 62, 225–250. [Google Scholar] [CrossRef]
- Mitchell, R.H. Primary and secondary niobium mineral deposits associated with carbonatites. Ore Geol. Rev. 2015, 64, 626–641. [Google Scholar] [CrossRef]
- Mitchell, R.H. Carbonatites and Carbonatites and Carbonatites. Can. Mineral. 2005, 43, 2049–2068. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R. High-field-strength elements in carbonatitic rocks: Geochemistry, crystal chemistry and significance for constraining the sources of carbonatites. Chem. Geol. 2006, 235, 138–160. [Google Scholar] [CrossRef]
- Fryklund, V.C.; Harner, R.S.; Kaiser, E.P. Niobium (Columbium) and titanium at Magnet Cove and Potash Sulphur Springs, Arkansas. Geol. Surv. Bull. 1954, 1015B, 23–56. [Google Scholar]
- Flohr, M.J.K. Titanium, vanadium, and niobium mineralization and alkali metasomatism from the Magnet Cove complex, Arkansas. Econ. Geol. 1994, 89, 105–130. [Google Scholar] [CrossRef]
- Verwoerd, W.J.; Viljoen, E.A.; Chevallier, L. Rare metal mineralization at the Salpeterkop carbonatite complex, Western Cape Province, South Africa. J. Afr. Earth Sci. 1995, 21, 171–186. [Google Scholar] [CrossRef]
- Werner, M.; Cook, N.J. Nb-rich brookite from Gross Brukkaros, Namibia: Substitution mechanisms and Fe2+/Fe3+ ratios. Mineral. Mag. 2001, 65, 437–440. [Google Scholar] [CrossRef]
- Kapustin, I.L. Mineralogy of Carbonatites; Amerind Publishing Company Pvt. Ltd.: New Dehli, India, 1980. [Google Scholar]
- Giovannini, A.L.; Bastos Neto, A.C.; Porto, C.G.; Pereira, V.P.; Takehara, L.; Barbanson, L.; Bastos, P.H.S. Mineralogy and geochemistry of laterites from the Morro dos Seis Lagos Nb (Ti, REE) deposit (Amazonas, Brazil). Ore Geol. Rev. 2017, 88, 461–480. [Google Scholar] [CrossRef]
- Howard, J.M. Brookite, Rutile Paramorphs after Brookite, and Rutile Twins from Magnet Cove, Arkansas. Rocks Miner. 1999, 74, 92–102. [Google Scholar] [CrossRef]
- Khromova, E.A.; Doroshkevich, A.G.; Sharygin, V.V.; Izbrodin, L.A. Compositional Evolution of Pyrochlore-Group Minerals in Carbonatites of the Belaya Zima Pluton, Eastern Sayan. Geol. Ore Depos. 2017, 59, 752–764. [Google Scholar] [CrossRef]
- Melgarejo, J.C.; Costanzo, A.; Bambi, A.C.J.M.; Gonçalves, A.O.; Neto, A.B. Subsolidus processes as a key factor on the distribution of Nb species in plutonic carbonatites: The Tchivira case, Angola. Lithos 2012, 152, 187–201. [Google Scholar] [CrossRef]
- Torro, L.; Villanova, C.; Castillo, M.; Campeny, M.; Gonçalves, A.O.; Melgarejo, J.C. Niobium and rare earth minerals from the Virulundo carbonatite, Namibe, Angola. Mineral. Mag. 2012, 76, 393–409. [Google Scholar] [CrossRef] [Green Version]
- Kapustin, Y.L. Mineralogy of the Weathering Crust of Carbonatites; Nedra: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Tremblay, J.; Bédard, L.P.; Matton, G. Columbitization of fluorcalciopyrochlore by hydrothermalism at the Saint-Honoré alkaline complex, Québec (Canada): New insights on halite in carbonatites. Ore Geol. Rev. 2017, 91, 695–707. [Google Scholar] [CrossRef]
- Downes, H.; Balaganskaya, E.; Beard, A.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef] [Green Version]
- Afanasyev, B.V. Mineral Resources of the Alkaline–Ultramafic Massifs of the Kola Peninsula; Roza Vetrov: St. Petersburg, Russia, 2011. (In Russian) [Google Scholar]
- Gittins, J.; Harmer, R.E. What is ferrocarbonatite? A revised classification. J. Afr. Earth Sci. 1997, 25, 159–168. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (Pbca, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Kravchenko-Berezhnoy, R.A.; Medvedeva, E.M.; Pakhomovsky, Y.A.; Polezhaeva, L.I.; Rezhenova, S.A. Combined usage of microprobe MS-46 and computer ‘Nairi-2’. In Instrumental Methods of Mineral Studies and Usage of Electronic Computing Devices; Kravchenko-Berezhnoy, R.A., Ed.; Kola Branch USSR Academy of Sciences: Apatity, Russia, 1976; pp. 46–69. [Google Scholar]
- Atencio, D.; Gieré, R.; Andrade, M.B.; Christy, A.G.; Kartashov, P.M. The pyrochlore supergroup of minerals: Nomenclature. Can. Mineral. 2010, 48, 673–678. [Google Scholar] [CrossRef]
- Meinhold, G. Rutile and its applications in earth sciences. Earth-Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Bonazzi, P.; Bindi, L.; Zoppi, M.; Capitani, G.C.; Olmi, F. Single-crystal diffraction and transmission electron microscopy studies of ‘silicified’ pyrochlore from Narssarssuk, Julianehaab district, Greenland. Am. Mineral. 2006, 91, 794–801. [Google Scholar] [CrossRef]
- Plavsa, D.; Reddy, S.M.; Agangi, A.; Clark, C.; Kylander-Clark, A.; Tiddy, C.J. Microstructural, trace element and geochronological characterization of TiO2 polymorphs and implications for mineral exploration. Chem. Geol. 2018, 476, 130–149. [Google Scholar] [CrossRef]
- Jiao, Y.; Chen, F.; Zhang, L.; Zhou, E.; Zhang, J. Hydrothermal synthesis of anatase and brookite nanotubes with superior photocatalytic and Li+insertion/extraction performances. Catal. Commun. 2014, 47, 32–35. [Google Scholar] [CrossRef]
- Leal, J.H.; Cantu, Y.; Gonzalez, D.F.; Parsons, J.G. Brookite and anatase nanomaterial polymorphs of TiO2 synthesized from TiCl3. Inorg. Chem. Commun. 2017, 84, 28–32. [Google Scholar] [CrossRef]
- Dachille, F.; Simons, P.Y.; Roy, R. Pressure-Temperatue studies of anatase, brookite, rutile and TiO2-II. Am. Mineral. 1968, 53, 1929–1939. [Google Scholar]
- Huberty, J.; Xu, H. Kinetics study on phase transformation from titania polymorph brookite to rutile. J. Solid State Chem. 2008, 181, 508–514. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Boniface, N. Crystal chemistry of pyrochlore from the Mesozoic Panda Hill carbonatite deposit, western Tanzania. J. Afr. Earth Sci. 2017, 126, 33–44. [Google Scholar] [CrossRef]
- Zurevinski, S.E.; Mitchell, R.H. Extreme compositional variation of pyrochlore-group minerals at the Oka carbonatite complex, Quebec: Evidence of magma mixing? Can. Mineral. 2004, 42, 1159–1168. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Doroshkevich, A.G. Mineralogy of secondary olivine-hosted inclusions in calcite carbonatites of the Belaya Zima alkaline complex, Eastern Sayan, Russia: Evidence for late-magmatic Na-Ca-rich carbonate composition. J. Geol. Soc. India 2017, 90, 524–530. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. Lueshite, pyrochlore and monazite-(Ce) from apatite-dolomite carbonatite, Lesnaya Varaka complex, Kola Peninsula, Russia. Mineral. Mag. 1998, 62, 769–782. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Sorokhtina, N.V.; Kononkova, N.N.; Klimovich, I.V. Uranium and thorium in carbonatitic minerals from the Guli massif, Polar Siberia. Geochem. Int. 2013, 51, 767–776. [Google Scholar] [CrossRef]
- Bambi, A.C.J.M.; Costanzo, A.; Gonçalves, A.O.; Melgarejo, J.C. Tracing the chemical evolution of primary pyrochlore from plutonic to volcanic carbonatites: The role of fluorine. Mineral. Mag. 2012, 76, 377–392. [Google Scholar] [CrossRef]
- Lumpkin, G.R.; Ewing, R.C. Geochemical alteration of pyrochlore group minerals; pyrochlore subgroup. Am. Mineral. 1995, 80, 732–743. [Google Scholar] [CrossRef]
- Zheng, L.; Gu, X.; Zhang, Y. Pyrochlore Chemistry from the Bonga Carbonatite-type Nb Deposit, Huila Province, Angola: Implications for Magmatic-Hydrothermal Processes of Carbonatite. Acta Geol. Sin. Engl. Ed. 2014, 88, 487–488. [Google Scholar] [CrossRef]
- Chebotarev, D.A.; Doroshkevich, A.G.; Klemd, R.; Karmanov, N.S. Evolution of Nb-mineralization in the Chuktukon carbonatite massif, Chadobets upland (Krasnoyarsk Territory, Russia). Period. Mineral. 2017, 86, 99–118. [Google Scholar] [CrossRef]
- Jo, A.; Bambi, C.M.; Costanzo, A.; Melgarejo, J.C.; Gon, A.O.; Alfonso, P.; Neto, A.B.; Manuel, J. Evolution of Pyrochlore in Carbonatites: The Angola Case. Rev. Soc. Esp. Mineral. 2008, 9, 43–44. [Google Scholar]
- Wall, F.; Williams, C.T.; Woolley, A.R.; Nasraoui, M. Pyrochlore from Weathered Carbonatite at Lueshe, Zaire. Mineral. Mag. 1996, 60, 731–750. [Google Scholar] [CrossRef]
- Williams, C.; Wall, F.; Woolley, A.; Phillipo, S. Compositional variation in pyrochlore from the Bingo carbonatite, Zaïre. J. Afr. Earth Sci. 1997, 25, 137–145. [Google Scholar] [CrossRef]
- Viladkar, S.G.; Ghose, I. U-rich pyrochlore in carbonatite of Newania, Rajasthan. Neues Jahrb. Mineral. Monatshefte 2002, 2002, 97–106. [Google Scholar] [CrossRef]
- Viladkar, S.G.; Bismayer, U.; Zietlow, P. Metamict U-rich pyrochlore of Newania carbonatite, Udaipur, Rajasthan. J. Geol. Soc. India 2017, 89, 133–138. [Google Scholar] [CrossRef]
- Viladkar, S.G.; Bismayer, U. U-rich pyrochlore from sevathur carbonatites, Tamil Nadu. J. Geol. Soc. India 2014, 83, 175–182. [Google Scholar] [CrossRef]
- Guowu, L.; Guangming, Y.; Fude, L.; Ming, X.; Xiangkun, G.; Baoming, P.; de Fourestier, J. Fluorcalciopyrochlore, A New Mineral Species From Bayan Obo, Inner Mongolia, P.R. China. Can. Mineral. 2016, 54, 1285–1291. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Kressall, R.D.; Crozier, J.; Pisiak, L.K.; Sidhu, R.; Yang, P. Carbonatite-hosted niobium deposit at Aley, northern British Columbia (Canada): Mineralogy, geochemistry and petrogenesis. Ore Geol. Rev. 2015, 64, 642–666. [Google Scholar] [CrossRef]
- Lazareva, E.V.; Zhmodik, S.M.; Dobretsov, N.L.; Tolstov, A.V.; Shcherbov, B.L.; Karmanov, N.S.; Gerasimov, E.Y.; Bryanskaya, A.V. Main minerals of abnormally high-grade ores of the Tomtor deposit (Arctic Siberia). Russ. Geol. Geophys. 2015, 56, 844–873. [Google Scholar] [CrossRef]
- Dowman, E.; Wall, F.; Treloar, P.J.; Rankin, A.H. Rare-earth mobility as a result of multiple phases of fluid activity in fenite around the Chilwa Island Carbonatite, Malawi. Mineral. Mag. 2017, 81, 1367–1395. [Google Scholar] [CrossRef]
- Van Baalen, M.R. Titanium mobility in metamorphic systems: A review. Chem. Geol. 1993, 110, 233–249. [Google Scholar] [CrossRef]
- Voncken, J.H.L. The Rare Earth Elements; SpringerBriefs in Earth Sciences; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-26807-1. [Google Scholar]
- Mariano, A.N.; Mariano, A. Rare Earth Mining and Exploration in North America. Elements 2012, 8, 369–376. [Google Scholar] [CrossRef]
- Andersen, A.K.; Clark, J.G.; Larson, P.B.; Neill, O.K. Mineral chemistry and petrogenesis of a HFSE(+HREE) occurrence, peripheral to carbonatites of the Bear Lodge alkaline complex, Wyoming. Am. Mineral. 2016, 101, 1604–1623. [Google Scholar] [CrossRef]
- Hammerli, J.; Rubenach, M. The Role of Halogens during Regional and Contact Metamorphism. In The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes; Harlov, D.E., Aranovich, L., Eds.; Springer Geochemistry; Springer International Publishing: Cham, Switzerland, 2018; pp. 649–712. ISBN 978-3-319-61665-0. [Google Scholar]
- Gieré, R. Hydrothermal mobility of Ti, Zr and REE: Examples from the Bergell and Adamello contact aureoles (Italy). Terra Nova 1990, 2, 60–67. [Google Scholar] [CrossRef]
- Timofeev, A.; Migdisov, A.A.; Williams-Jones, A.E. An experimental study of the solubility and speciation of niobium in fluoride-bearing aqueous solutions at elevated temperature. Geochim. Cosmochim. Acta 2015, 158, 103–111. [Google Scholar] [CrossRef]

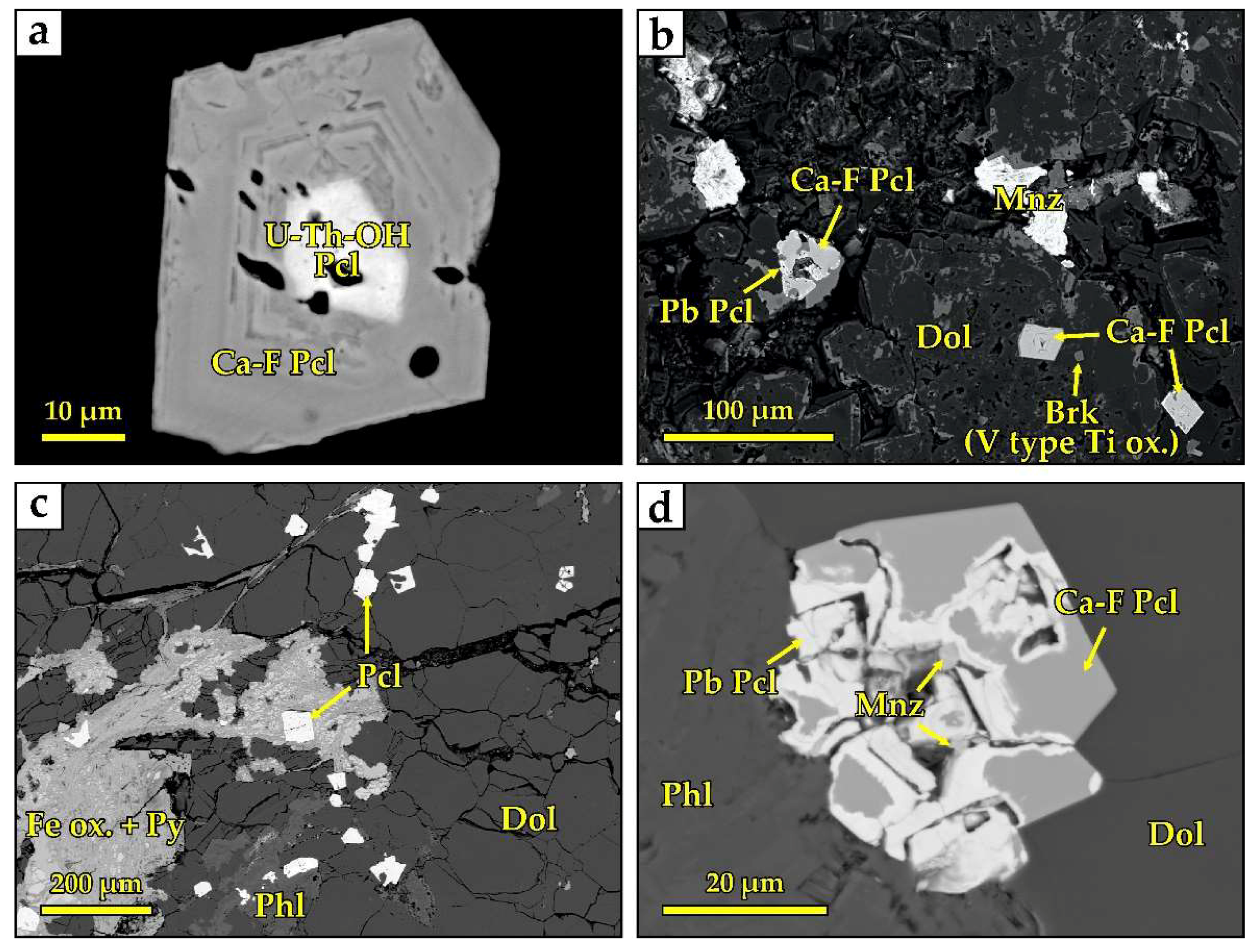
| Components | U-Th (1) 1 | Ca-F (1) | Ca-F (2) | Ca-F (2) | Pb (1) | Pb (1) | Pb (2) | Pb (2) |
|---|---|---|---|---|---|---|---|---|
| BaO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| K2O | b.d.l. | b.d.l. | 0.06 | 0.04 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Na2O | b.d.l. | 4.66 | 6.84 | 6.73 | 0.36 | b.d.l. | b.d.l. | b.d.l. |
| SrO | 4.09 | 1.41 | 1.19 | 1.25 | 1.42 | 1.80 | 2.00 | 3.13 |
| CaO | 5.88 | 15.48 | 16.49 | 16.25 | 4.02 | 1.05 | 1.17 | 1.51 |
| PbO | b.d.l. | b.d.l. | b.d.l. | b.d.l. | 32.14 | 31.10 | 26.81 | 25.41 |
| UO2 | 3.18 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| ThO | 9.71 | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Y2O3 | b.d.l. | 0.72 | 0.43 | 0.42 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| La2O3 | 0.42 | b.d.l. | 0.40 | 0.51 | b.d.l. | 2.20 | 2.92 | 2.55 |
| Ce2O3 | 2.47 | 0.66 | 0.74 | 0.93 | 0.54 | 4.21 | 5.75 | 5.29 |
| Nd2O3 | 0.44 | b.d.l. | 0.19 | 0.20 | b.d.l. | 0.33 | 0.51 | 0.21 |
| Sm2O3 | b.d.l. | b.d.l. | 0.20 | 0.22 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| SiO2 | 3.42 | b.d.l. | 0.12 | 0.26 | 0.47 | 0.16 | 0.26 | 0.60 |
| TiO2 | 8.94 | 4.95 | 4.74 | 5.12 | 2.72 | 3.33 | 3.21 | 2.22 |
| Fe2O3 | 3.24 | 0.39 | 0.11 | 0.17 | 0.71 | 0.57 | 0.73 | 0.61 |
| ZrO2 | b.d.l. | b.d.l. | 0.30 | 0.48 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Nb2O5 | 41.74 | 57.49 | 64.39 | 64.60 | 42.00 | 44.09 | 46.11 | 48.73 |
| Ta2O5 | b.d.l. | b.d.l. | 0.17 | 0.10 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| F | b.d.l. | 3.80 | 2.84 | 2.85 | b.d.l. | b.d.l. | b.d.l. | b.d.l. |
| Total | 83.53 | 89.56 | 99.21 | 100.13 | 84.38 | 88.84 | 89.47 | 90.26 |
| F=O | 0.00 | 1.60 | 1.20 | 1.20 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 83.53 | 87.96 | 98.01 | 98.93 | 84.38 | 88.84 | 89.47 | 90.26 |
| Formula calculated to 2 B-site cations | ||||||||
| Ba | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Na | 0.00 | 0.60 | 0.80 | 0.77 | 0.06 | 0.00 | 0.00 | 0.00 |
| Sr | 0.15 | 0.05 | 0.04 | 0.04 | 0.07 | 0.09 | 0.10 | 0.15 |
| Ca | 0.40 | 1.10 | 1.07 | 1.03 | 0.39 | 0.10 | 0.10 | 0.13 |
| Pb | 0.00 | 0.00 | 0.00 | 0.00 | 0.78 | 0.73 | 0.60 | 0.55 |
| U | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Th | 0.14 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Y | 0.00 | 0.03 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| La | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.07 | 0.09 | 0.08 |
| Ce | 0.06 | 0.02 | 0.02 | 0.02 | 0.02 | 0.13 | 0.17 | 0.16 |
| Nd | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 |
| Sm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Σ cat (A) | 0.81 | 1.80 | 1.96 | 1.90 | 1.33 | 1.13 | 1.08 | 1.07 |
| Vac (A) | 1.19 | 0.20 | 0.04 | 0.10 | 0.67 | 0.87 | 0.92 | 0.93 |
| Si | 0.22 | 0.00 | 0.01 | 0.02 | 0.04 | 0.01 | 0.02 | 0.05 |
| Ti | 0.42 | 0.25 | 0.22 | 0.23 | 0.19 | 0.22 | 0.20 | 0.13 |
| Fe | 0.17 | 0.02 | 0.01 | 0.01 | 0.05 | 0.04 | 0.05 | 0.04 |
| Zr | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Nb | 1.19 | 1.73 | 1.76 | 1.73 | 1.72 | 1.73 | 1.73 | 1.78 |
| Ta | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Σ cat (B) | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| F | 0.00 | 0.80 | 0.54 | 0.53 | 0.00 | 0.00 | 0.00 | 0.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, E.; Fomina, E.; Sidorov, M.; Shilovskikh, V. Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia). Geosciences 2018, 8, 281. https://doi.org/10.3390/geosciences8080281
Kozlov E, Fomina E, Sidorov M, Shilovskikh V. Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia). Geosciences. 2018; 8(8):281. https://doi.org/10.3390/geosciences8080281
Chicago/Turabian StyleKozlov, Evgeniy, Ekaterina Fomina, Mikhail Sidorov, and Vladimir Shilovskikh. 2018. "Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia)" Geosciences 8, no. 8: 281. https://doi.org/10.3390/geosciences8080281
APA StyleKozlov, E., Fomina, E., Sidorov, M., & Shilovskikh, V. (2018). Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia). Geosciences, 8(8), 281. https://doi.org/10.3390/geosciences8080281






