Carbon Dioxide and Nitrogen Infused Compressed Air Foam for Depopulation of Caged Laying Hens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Subjects
2.2. Experimental Design
2.3. Foam Production and Application
2.4. CAF Infused with Gases
2.5. Gas Inhalation Treatments
2.6. Measurement of Expansion Ratio and CO2 Concentration
2.7. Assessment of Stress Hormones
2.8. Determination of Cessation of Movement
2.9. Statistical Analysis
3. Results and Discussion
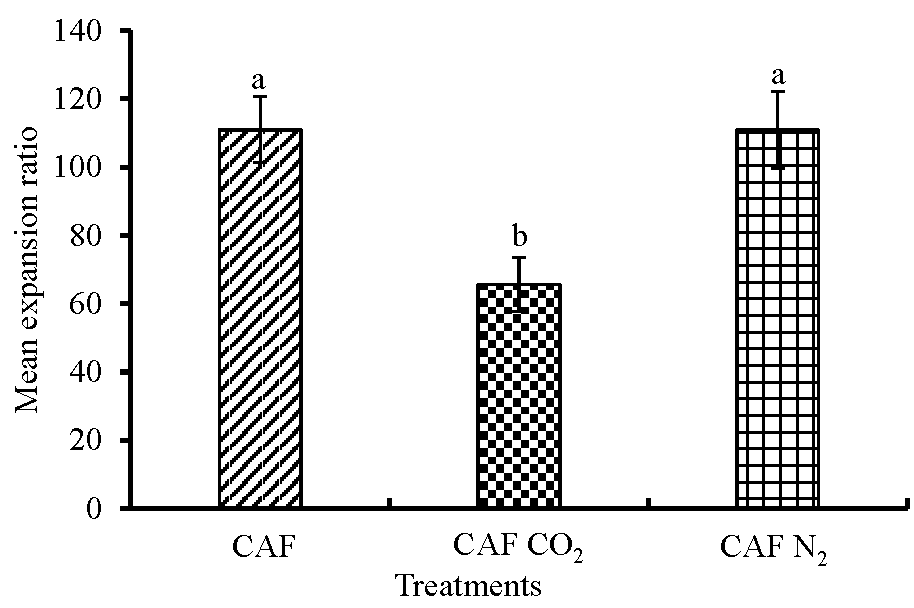
3.1. Foam Quality Parameters
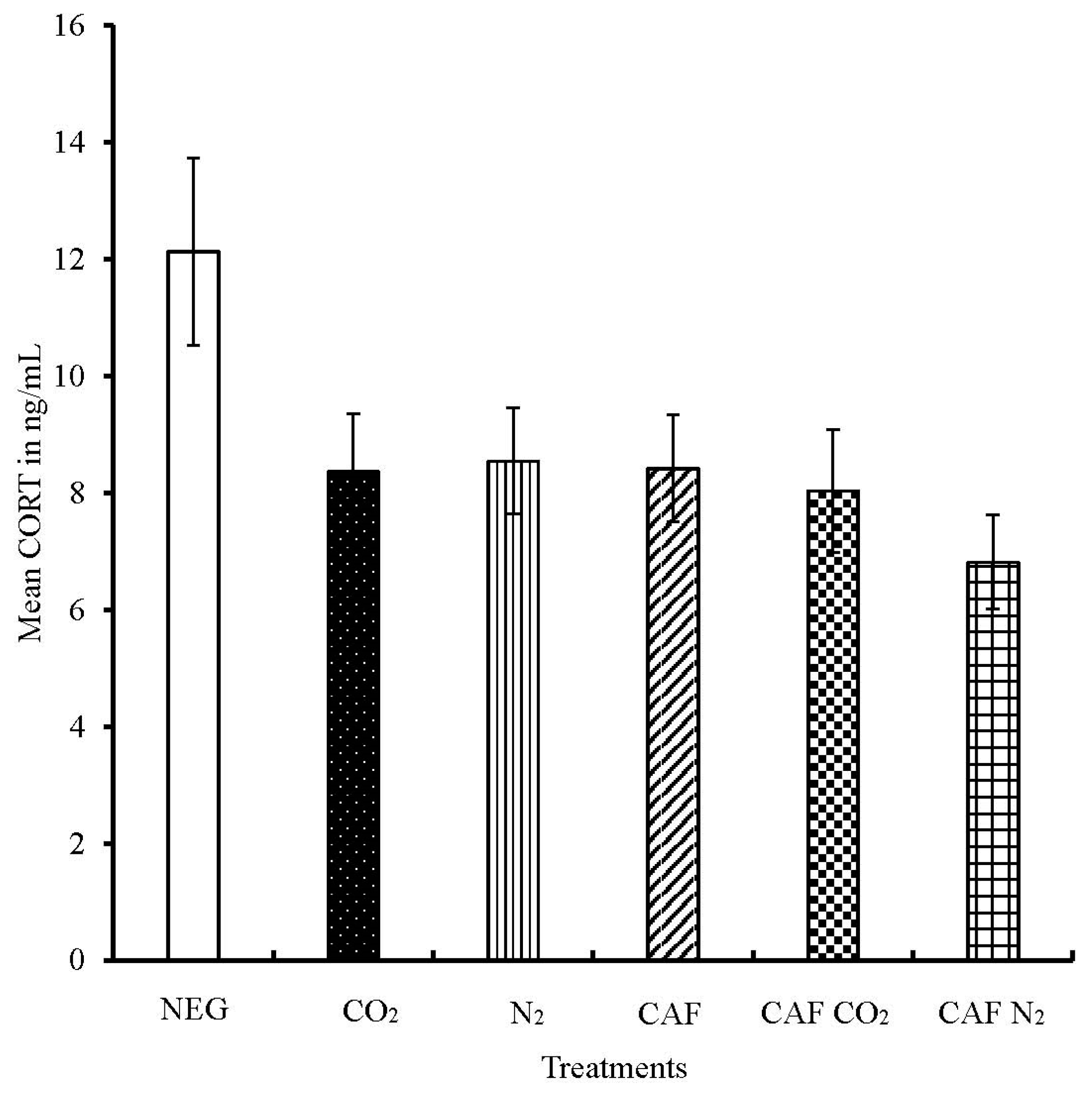
3.2. Serum Corticosterone
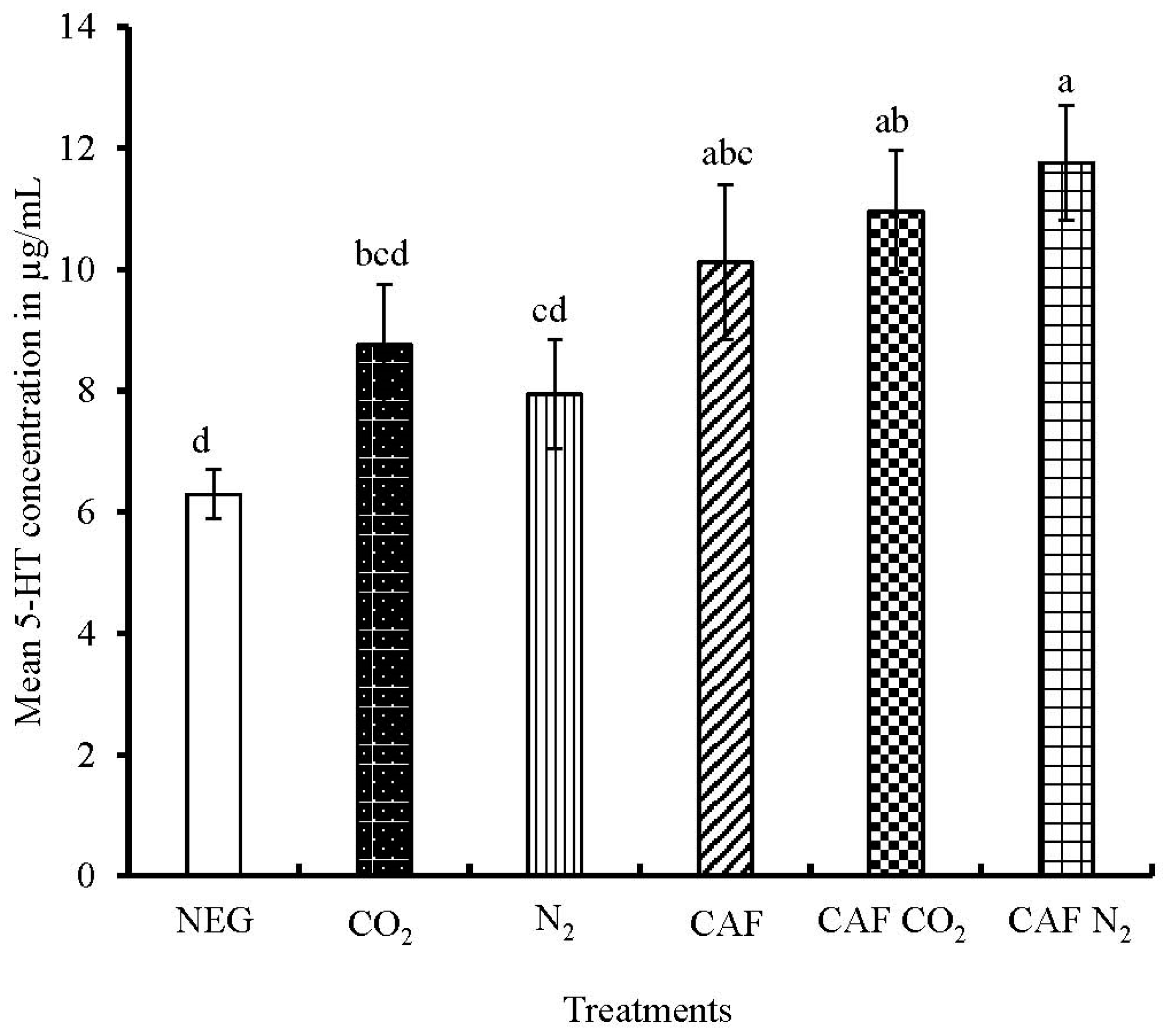
3.3. Serum Serotonin
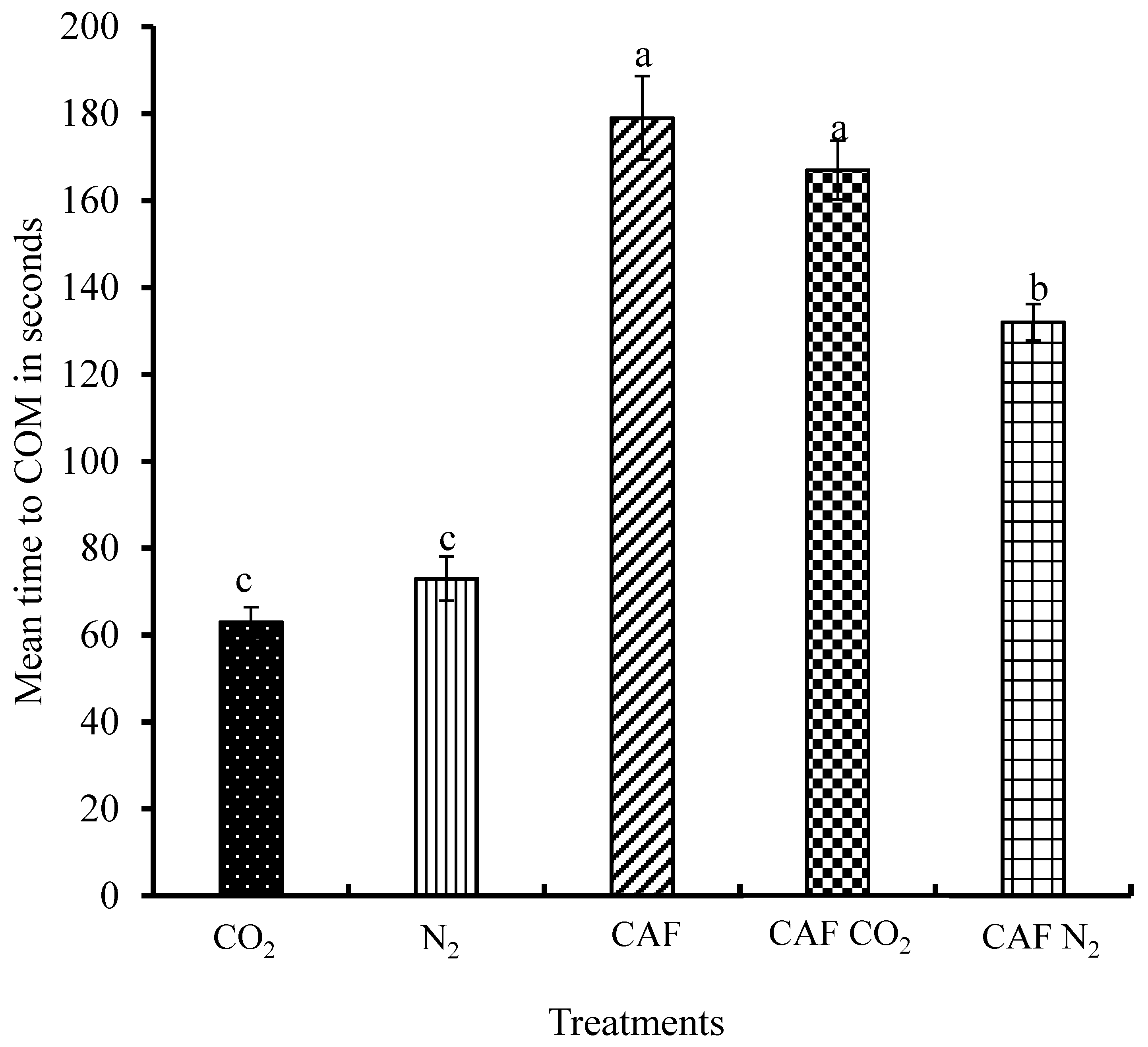
3.4. Time to Cessation of Movement
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USDA APHIS. Final Report for the 2014–2015 Outbreak of Highly Pathogenic Avian Influenza (HPAI) in the United States. 2016. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai/2015-hpai-final-report.pdf (accessed on 12 September 2017).
- Greene, J.L. Update on the Highly-Pathogenic Avian Influenza Outbreak of 2014–2015; Congressional Research Service: Washington, DC, USA, 2015. Available online: http://nationalaglawcenter.org/wp-content/uploads/assets/crs/R44114.pdf (accessed on 10 August 2017).
- U.S. National List of Reportable Animal Diseases (NLRAD)-National Animal Health Reporting System (NAHRS) Operational Manual. Available online: https://www.aphis.usda.gov/animal_health/nahrs/downloads/nahrsoperationalmanual.pdf (accessed on 12 September 2017).
- Kinde, H.; Utterback, W.; Takeshita, K.; McFarland, M. Survival of exotic Newcastle disease virus in commercial poultry environment following removal of infected chickens. Avian Dis. 2004, 48, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Thornton, G. 9 Challenges Facing US Poultry Producers in 2017. 2016. Available online: http://www.wattagnet.com/blogs/6-all-things-poultry/post/29141-challenges-facing-us-poultry-producers-in-2017 (accessed on 20 October 2017).
- AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (accessed on 10 August 2017).
- USDA. HPAI Outbreak 2014–2015 Stamping-Out & Depopulation Policy. 2015. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai/depopulationpolicy.pdf (accessed on 15 August 2017).
- USDA. Highly Pathogenic Avian Influenza Response Plan: The RedBook. 2017. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai_response_plan.pdf (accessed on 15 August 2017).
- Hackbarth, H.; Küppers, N.; Bohnet, W. Euthanasia of rats with carbon dioxide—Animal welfare aspects. Lab. Anim. 2000, 34, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.E.; Whitley, J.T.; Morrow, W.E.M. Effect of physical and inhaled euthanasia methods on hormonal measures of stress in pigs. J. Swine Health Prod. 2013, 21, 261–269. [Google Scholar]
- Shields, S.J.; Raj, A.B.M. A critical review of electrical water-bath stun systems for poultry slaughter and recent developments in alternative technologies. J. Appl. Anim. Welf. Sci. 2010, 13, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Otsuguro, K.; Yasutake, S.; Yamaji, Y.; Ban, M.; Ohta, T.; Ito, S. Why does carbon dioxide produce analgesia? In Proceedings of the 6th World Congress on Alternatives & Animal Use in the Life Sciences, Tokyo, Japan, 21–25 August 2007. [Google Scholar]
- Martoft, L.; Lomholt, L.; Kolthoff, C.; Rodriguez, B.E.; Jensen, E.W.; Jørgensen, P.F.; Pedersen, H.D.; Forslid, A. Effects of CO2 anaesthesia on central nervous system activity in swine. Lab. Anim. 2002, 36, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kingston, S.K.; Dussault, C.A.; Zaidlicz, R.S.; Faltas, N.H.; Geib, M.E.; Taylor, S.; Holt, T.; Porter-Spalding, B.A. Evaluation of two methods for mass euthanasia of poultry in disease outbreaks. J. Am. Vet. Med. Assoc. 2005, 227, 730–738. [Google Scholar] [CrossRef] [PubMed]
- AVMA. Poultry Depopulation. 2015. Available online: https://www.avma.org/KB/Policies/Pages/Poultry-Depopulation.aspx (accessed on 12 August 2017).
- Benson, E.; Malone, G.W.; Alphin, R.L.; Dawson, M.D.; Pope, C.R.; Van Wicklen, G.L. Foam-based mass emergency depopulation of floor-reared meat-type poultry operations. Poult. Sci. 2007, 86, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Thornber, P.M.; Rubira, R.J.; Styles, D.K. Humane killing of animals for disease control purposes. Rev. Off. Int. Epizoot. 2014, 33, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, E. Using Ventilation Shutdown for Emergency Mass Depopulation of Poultry. Available online: http://www.usaha.org/upload/Committee/TransDisPoultry/06-VSD%20for%20Mass%20Depopulation_Gingerich_share.pdf (accessed on 21 October 2017).
- McKeegan, D.E.F.; Reimert, H.G.M.; Hindle, V.A.; Boulcott, P.; Sparrey, J.M.; Wathes, C.M.; Demmers, T.G.M.; Gerritzen, M.A. Physiological and behavioral responses of poultry exposed to gas-filled high expansion foam. Poult. Sci. 2013, 92, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.B. Aversive reactions of turkeys to argon, carbon dioxide and a mixture of carbon dioxide and argon. Vet. Rec. 1996, 138, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, V.; Raj, A.B.M.; Baker, L.; Sparks, N.H.C. Aversion of chickens to various lethal gas mixtures. Anim. Welf. 2011, 20, 253–262. [Google Scholar]
- Raj, A.B.M. Recent developments in stunning and slaughter of poultry. Worlds Poult. Sci. J. 2006, 62, 467–484. [Google Scholar] [CrossRef]
- Alphin, R.L.; Benson, E.R.; Hougentogler, D.P.; Herrman, E.R. Is foam an option for addressing the challenges associated with the depopulation of caged layers? In Proceedings of the 5th International Symposium Managing Animal Mortalities, Products, By-Products, & Associated Heath Risks: Connecting Research, Regulations, & Responses, Lancaster, PA, USA, 28 September–1 October 2015. [Google Scholar]
- USDA. HPAI Response Guidance: Using Ventilation Shutdown to Control HPAI. 2016. Available online: http://minnesotaturkey.com/wp-content/uploads/2015/03/USDA-NEW-Using-VSD-1.15.2016_V2.pdf (accessed on 9 November 2017).
- USDA. HPAI Outbreak 2014–2015: Ventilation Shutdown Evidence & Policy. 2015. Available online: https://www.aphis.usda.gov/animal_health/emergency_management/downloads/hpai/ventilationshutdownpolicy.pdf (accessed on 12 October 2017).
- Zhang, J.P.; Delichatsios, M.; Neill, A.O. Assessment of gas cooling capabilities of compressed air foam systems in fuel-and ventilation-controlled compartment fires. J. Fire Sci. 2011, 29, 543–554. [Google Scholar] [CrossRef]
- Rie, D.-H.; Lee, J.-W.; Kim, S. Class B fire-extinguishing performance evaluation of a compressed air foam system at different air-to-aqueous foam solution mixing ratios. Appl. Sci. 2016, 6, 191. [Google Scholar] [CrossRef]
- Kim, A.K.; Dlugogorski, B.Z. Multipurpose overhead compressed-air foam system and its fire suppression performance. J. Fire Prot. Eng. 1996, 8, 133–150. [Google Scholar] [CrossRef]
- White, D.; Gurung, S.; Zhao, D.; Tabler, T.; McDaniel, C.; Styles, D.; McKenzie, S.; Farnell, Y.; Farnell, M. Foam or spray application of agricultural chemicals to clean and disinfect layer cages. J. Appl. Poult. Res. 2017, in press. [Google Scholar]
- Magrabi, S.A.; Dlugogorski, B.Z.; Jameson, G.J. A comparative study of drainage characteristics in AFFF and FFFP compressed-air fire-fighting foams. Fire Saf. J. 2002, 37, 21–52. [Google Scholar] [CrossRef]
- Farnell, M.; Caldwell, D.; Bryd, A.; Berghman, L.; Kiess, A.; Stayer, P.; Tabler, T.; Farnell, Y. Use of a compressed air foam system in response to reportable poultry diseases. In Proceedings of the 5th International Symposium Managing Animal Mortalities, Products, By-Products, & Associated Heath Risks: Connecting Research, Regulations, & Responses, Lancaster, PA, USA, 28 September–1 October 2015. [Google Scholar]
- Laundess, A.J.; Rayson, M.S.; Dlugogorski, B.Z.; Kennedy, E.M. Suppression performance comparison for aspirated, compressed-air and in situ chemically generated class B foams. Fire Technol. 2012, 48, 625–640. [Google Scholar] [CrossRef]
- Dawson, M.D.; Lombardi, M.E.; Benson, E.R.; Alphin, R.L.; Malone, G.W. Using accelerometers to determine the cessation of activity of broilers. J. Appl. Poult. Res. 2007, 16, 583–591. [Google Scholar] [CrossRef]
- Menon, D.G.; Bennett, D.C.; Schaefer, A.M.; Cheng, K.M. Hematological and serum biochemical profile of farm emus (Dromaius novaehollandiae) at the onset of their breeding season. Poult. Sci. 2013, 92, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, B.S.; Dlugogorski, B.Z.; Jameson, G.J. Rheology of fire-fighting foams. Fire Saf. J. 1998, 31, 61–75. [Google Scholar] [CrossRef]
- Dlugogorski, B.Z.; Kennedy, E.M.; Schaefer, T.H.; Vitali, J.A. What Properties Matter in Fire-Fighting Foams? National Research Institute of Fire Disaster Symposium: Tokyo, Japan, 2002; pp. 57–78.
- Squires, E.J. Applied Animal Endocrinology; CABI Publishing: Cambridge, MA, USA, 2003. [Google Scholar]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [PubMed]
- Metzger, M.; Toledo, C.; Braun, K. Serotonergic innervation of the telencephalon in the domestic chick. Brain Res. Bull. 2002, 57, 547–551. [Google Scholar] [CrossRef]
- Bolhuis, J.E.; Ellen, E.D.; Van Reenen, C.G.; De Groot, J.; Ten Napel, J.; Koopmanschap, R.E.; De Vries Reilingh, G.; Uitdehaag, K.A.; Kemp, B.; Rodenburg, T.B. Effects of genetic group selection against mortality on behavior and peripheral serotonin in domestic laying hens with trimmed and intact beaks. Physiol. Behav. 2009, 97, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Gruss, M.; Braun, K. Distinct activation of monoaminergic pathways in chick brain in relation to auditory imprinting and stressful situations: A microdialysis study. Neuroscience 1996, 76, 891–899. [Google Scholar] [CrossRef]
- Williams, E.; Stewart-Knox, B.; Helander, A.; McConville, C.; Bradbury, I.; Rowland, I. Associations between whole-blood serotonin and subjective mood in healthy male volunteers. Biol. Psychol. 2006, 71, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Scanes, C.G. Biology of stress in poultry with emphasis on glucocorticoids and the heterophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Uitdehaag, K.A.; Rodenburg, T.B.; Van Reenen, C.G.; Koopmanschap, R.E.; De Vries Reilingh, G.; Engel, B.; Buist, W.G.; Komen, H.; Bolhuis, J.E. Effects of genetic origin and social environment on behavioral response to manual restraint and monoamine functioning in laying hens. Poult. Sci. 2011, 90, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Koyama, T. Effects of acute and chronic administration of high-dose corticosterone and dexamethasone on regional brain dopamine and serotonin metabolism in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1996, 20, 147–156. [Google Scholar] [CrossRef]
- Karten, Y.J.; Nair, S.M.; van Essen, L.; Sibug, R.; Joëls, M. Long-term exposure to high corticosterone levels attenuates serotonin responses in rat hippocampal CA1 neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 13456–13461. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen, M.; Lambooij, B.; Reimert, H.; Stegeman, A.; Spruijt, B. A note on behaviour of poultry exposed to increasing carbon dioxide concentrations. Appl. Anim. Behav. Sci. 2007, 108, 179–185. [Google Scholar] [CrossRef]
- Erasmus, M.A.; Turner, P.V.; Widowski, T.M. Measures of insensibility used to determine effective stunning and killing of poultry. J. Appl. Poult. Res. 2010, 19, 288–298. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurung, S.; White, D.; Archer, G.; Styles, D.; Zhao, D.; Farnell, Y.; Byrd, J.; Farnell, M. Carbon Dioxide and Nitrogen Infused Compressed Air Foam for Depopulation of Caged Laying Hens. Animals 2018, 8, 6. https://doi.org/10.3390/ani8010006
Gurung S, White D, Archer G, Styles D, Zhao D, Farnell Y, Byrd J, Farnell M. Carbon Dioxide and Nitrogen Infused Compressed Air Foam for Depopulation of Caged Laying Hens. Animals. 2018; 8(1):6. https://doi.org/10.3390/ani8010006
Chicago/Turabian StyleGurung, Shailesh, Dima White, Gregory Archer, Darrel Styles, Dan Zhao, Yuhua Farnell, James Byrd, and Morgan Farnell. 2018. "Carbon Dioxide and Nitrogen Infused Compressed Air Foam for Depopulation of Caged Laying Hens" Animals 8, no. 1: 6. https://doi.org/10.3390/ani8010006




