Effect of Provision of Feed and Water during Transport on the Welfare of Weaned Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Design
- (1)
- Not weaned: pigs remained with the sow (CON)
- (2)
- Weaned, not transported, but had access to feed and water: pigs removed from the sow and moved into nursery pens that had feed and water (WEAN+)
- (3)
- Weaned but had no access to feed and water: pigs were removed from the sow and moved into nursery pens that did not have feed and water (WEAN−)
- (4)
- Weaned and transported with access to feed and water: pigs were removed from the sow and transported for 32 h with access to feed and water (TRANS+)
- (5)
- Weaned and transported without access to feed and water (as normally done): pigs were removed from the sow and transported 32 h without access to feed and water (TRANS−)
| Behavior | Definition |
|---|---|
| Lying | Animal is recumbent, flat on its side, or ventrally |
| Standing | Animal is upright on all fours, legs are extended |
| Sitting | Resting on the caudal part of the body with the forelimbs extended |
| Drinking | Animal placing its mouth on the water nipple and consuming water |
| Eating/Nursing | Animal placing its mouth in the feed container and consuming feed or latching on to the sows’ nipple. |
3. Results and Discussion
3.1. Weight
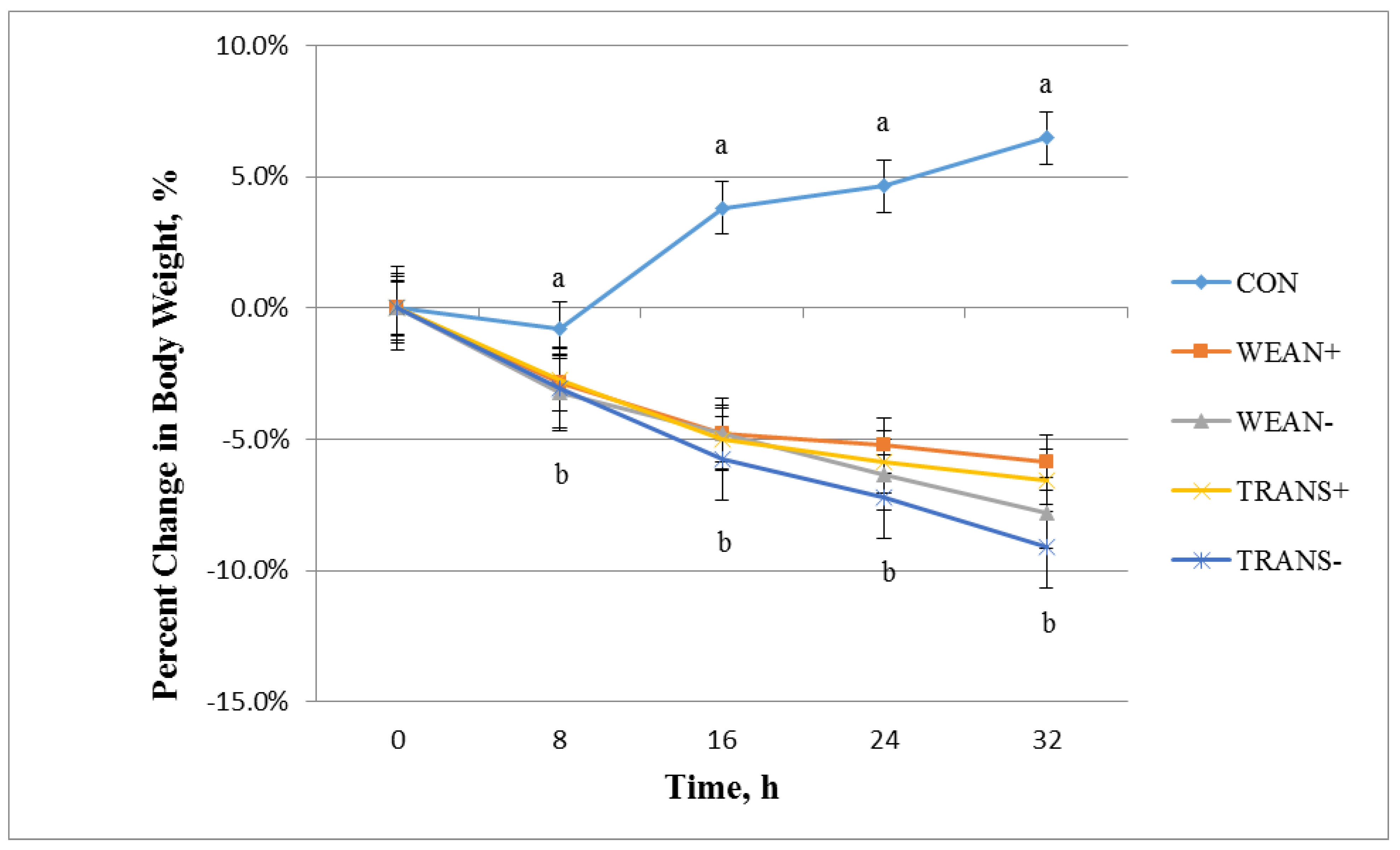
3.2. Physiology
3.2.1. Neutrophil to Lymphocyte Ratio Interaction
| Treatment | 0 h | 8 h | 16 h | 24 h | 32 h |
|---|---|---|---|---|---|
| CON | 0.59 | 0.64 a | 0.70 a | 0.69 | 0.86 |
| WEAN+ | 0.77 | 1.10 b | 0.84 a | 0.61 | 0.76 |
| WEAN− | 0.67 | 1.16 b | 1.10 b | 0.87 | 0.82 |
| TRANS+ | 0.62 | 1.0b | 1.00 b | 0.84 | 1.06 |
| TRANS− | 0.73 | 1.0b | 1.10 b | 0.69 | 0.64 |
3.2.2. Blood Glucose Interaction
| Treatment | 0 h | 8 h | 16 h | 24 h | 32 h |
|---|---|---|---|---|---|
| CON | 122.45 | 135.01 a | 121.89 a | 130.45 a | 124.16 a |
| WEAN+ | 123.15 | 116.12 b | 104.96 b | 82.24 b | 87.12 b |
| WEAN− | 124.05 | 114.38 b | 104.66 b | 86.82 b | 83.85 b |
| TRANS+ | 117.03 | 121.42 b | 114.53 a | 103.08 b | 103.36 b |
| TRANS− | 116.24 | 122.19 b | 119.30 a | 107.13 b | 102.96 b |
3.2.3. Creatine Kinase Interaction
| Treatment | Male | Female | SE |
|---|---|---|---|
| CON | 1153.60 | 2002.70 | 453.30 |
| WEAN+ | 1749.09 | 1042.71 | 332.45 |
| WEAN− | 647.05 | 1392.65 | 350.05 |
| TRANS+ | 543.84 a | 1687.34 b | 311.80 |
| TRANS− | 547.02 | 1083.38 | 325.50 |
3.2.4. Total Plasma Protein Interaction
| Treatment | 0 h | 8 h | 16 h | 24 h | 32 h |
|---|---|---|---|---|---|
| CON | 5.33 a | 5.17 a | 5.18 a | 5.09 a | 4.97 a |
| WEAN+ | 5.14 b | 5.22 a | 5.28 a | 5.17 a | 5.22 b |
| WEAN− | 5.06 b | 5.17 a | 5.35 a | 5.16 a | 5.21 b |
| TRANS+ | 5.12 b | 5.16 a | 5.27 a | 5.17 a | 5.18 b |
| TRANS− | 5.15 b | 5.29 b | 5.50 b | 5.40 b | 5.44 b |
3.2.5. Cortisol
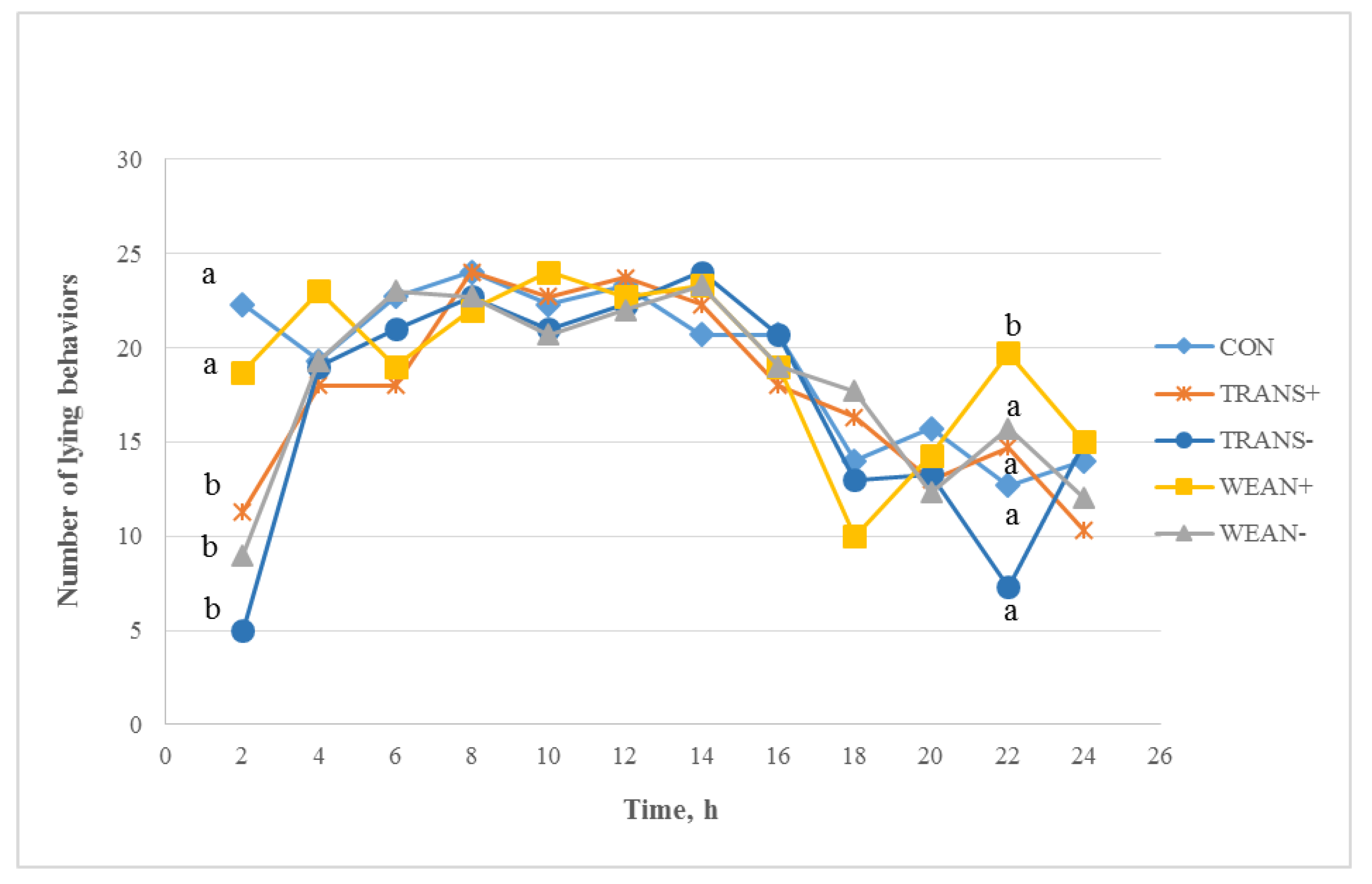
3.3. Behavior
3.3.1. During Transport
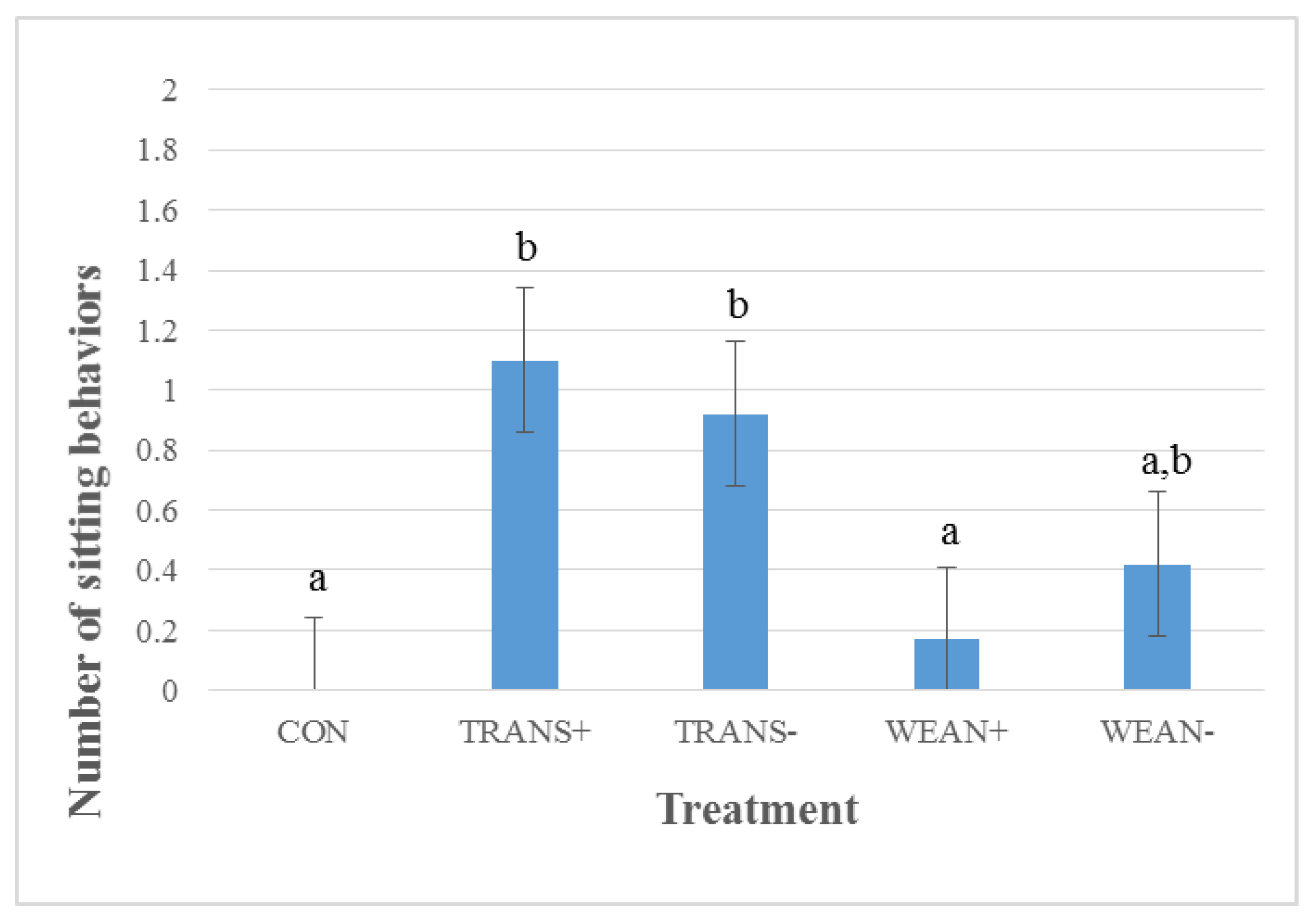
3.3.2. Post-Treatment Behavior
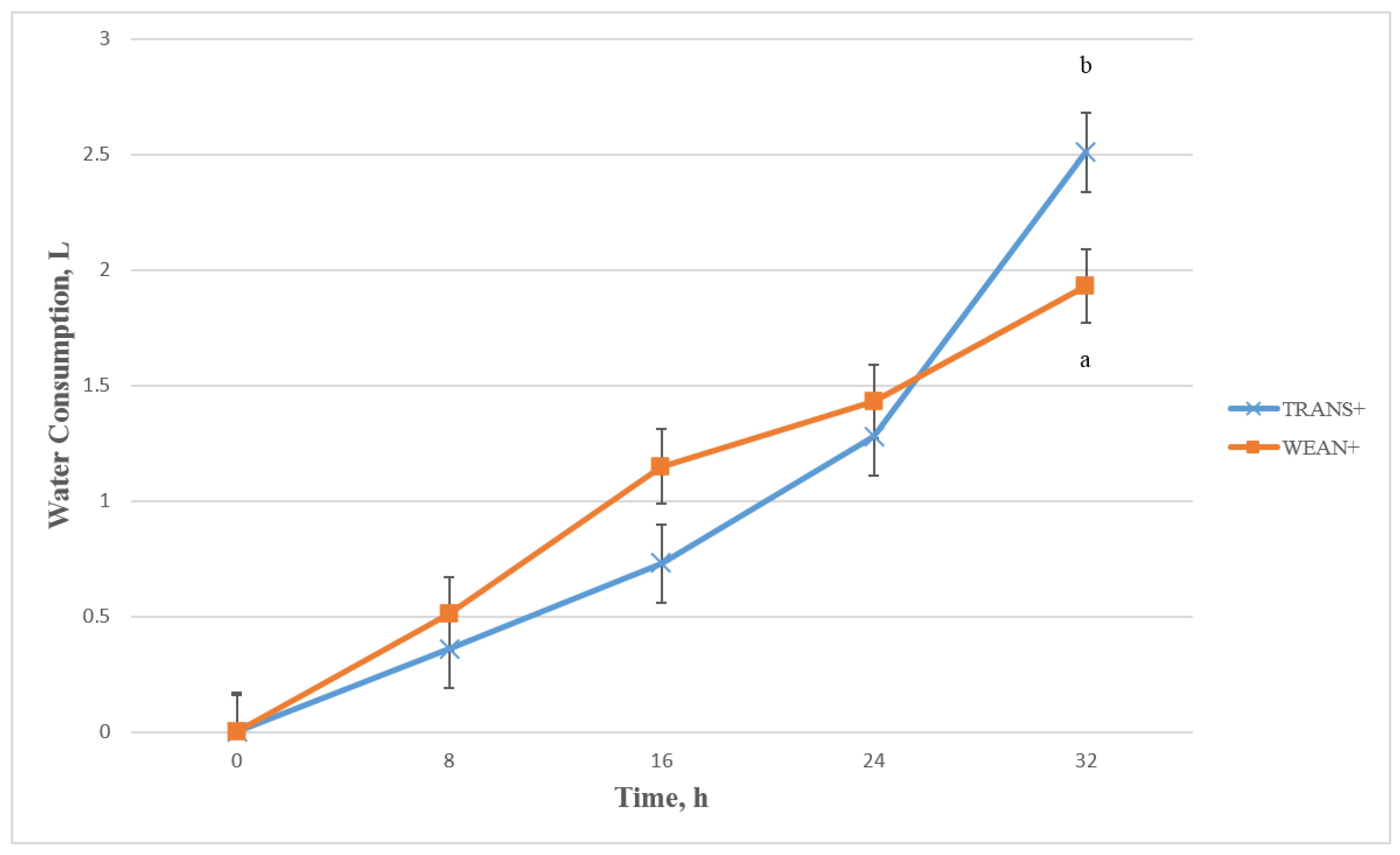
3.3.3. Feed and Water Consumption during and after Transportation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lambooiji, W. Transport of pigs. In Livestock Handling and Transport; Granding, T., Ed.; CAB International: Wallingford, UK, 1993; pp. 213–231. [Google Scholar]
- Sutherland, M.A.; Backus, B.L.; McGlone, J.J. Effects of transport at weaning on the behavior, physiology and performance of pigs. Animals 2014, 4, 657–669. [Google Scholar] [CrossRef]
- Barton-Gade, P.; Christensen, L. Effect of different stocking densities during transport on welfare and meat quality in Danish slaughter pigs. Meat Sci. 1998, 48, 237–247. [Google Scholar] [CrossRef]
- Warriss, P.D.; Brown, S.D.; Knowles, T.G.; Edwards, J.E.; Kettlewell, P.J.; Guise, H.J. The effect of stocking density in transit on the carcass quality and welfare of slaughter pigs: Results from the analysis of blood and meat samples. Meat Sci. 1998, 50, 447–456. [Google Scholar] [CrossRef]
- Kim, D.H.; Woo, J.H.; Lee, C.Y. Effects of stocking density and transportation time of market pigs on their behaviour, plasma concentrations of glucose and stress-associated enzymes and carcass quality. Asian-Australas. J. Anim. Sci. 2004, 17, 116–121. [Google Scholar] [CrossRef]
- National Pork Producers Council. 28 Hour Law. Available online: http://www.nppc.org/issues/animal-health-safety/28-hour-law/ (accessed on 7 October 2014).
- Sutherland, M.A.; McDonald, A.; McGlone, J.J. Environmental, trailer, and gender effects on the percentage of dead and non-ambulatory pigs during transport. Vet. Rec. 2009, 165, 13–18. [Google Scholar] [CrossRef]
- Rademacher, C.; Davies, P. Factors associated with the incidence of mortality during transport of market hogs. In Proceedings of the Allen D. Leman Swine Conference, 17–22 September 2005; pp. 186–191.
- Sutherland, M.A.; Bryer, P.J.; Davis, B.L.; McGlone, J.J. Space requirements of weaned pigs during a 60 minute transport in summer. J. Anim. Sci. 2009, 87, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.R.G.; McGlone, J.J. Moving finishing pigs in different group sizes: Cardiovascular responses, time, and ease of handling. Livest. Sci. 2007, 107, 86–90. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Barnett, J.L.; Hofmeyr, C.; Coleman, G.J.; Dowling, S.; Boyce, J. The effects of fear of humans and pre-slaughter handling on the meat quality of pigs. Aust. J. Agric. Res. 2002, 53, 493–501. [Google Scholar] [CrossRef]
- Hamilton, D.N.; Ellis, M.; Bertol, T.M.; Miller, K.D. Effects of handling intensity and live weight on blood acid-base status in finishing pigs. J. Anim. Sci. 2004, 82, 2405–2409. [Google Scholar] [PubMed]
- Fàbrega, E.; Manteca, X.; Font, J.; Gispert, M.; Carrión, D.; Velarde, A.; Ruiz-dela-Torre, J.L.; Diestre, A. Effects of halothane gene and preslaughter treatment on meat quality and welfare from two pig crosses. Meat Sci. 2002, 62, 463–472. [Google Scholar] [CrossRef]
- Fazio, E.; Medica, P.; Alberghina, D.; Cavaleri, S.; Ferlazzo, A. Effect of long distance road transport on thyroid and adrenal function and haematocrit values in Limousin cattle: Influence of body weight decrease. Vet. Res. Commun. 2005, 29, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Helmreich, D.L.; Crouch, M.; Dorr, N.P.; Parfitt, D.B. Peripheral triiodothyronine (T3) levels during escapable and inescapable footshock. Physiol. Behav. 2006, 87, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Stiegmann, G.V.; Yamamoto, M.; Berguer, R. Neuroendocrine stress response after minimally invasive surgery in pigs. Surg. Endosc. 1992, 6, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Qingquing, H.; Endong, B.; Miao, Z.; Zhenhua, Y.; Hartung, J. Variation in the expression of Hsp27, Hsp70, Hsp90 and their corresponding mRNA transcripts in the hearts of pigs during different transportation durations. Livest. Sci. 2010, 129, 88–94. [Google Scholar]
- Hay, M.; Orgeur, P.; Levy, F.; Le Dividich, J.; Concordet, D.; Nowak, R.; Schaal, B.; Mormede, P. Neuroendocrine consequences of very early weaning in swine. Physiol. Behav. 2001, 72, 263–269. [Google Scholar] [CrossRef]
- Kanitz, E.; Tuchscherer, M.; Tuchscherer, A.; Stabenow, B.; Manteuffel, G. Neuroendocrine and immune response to acute endotoxemia in suckling pigs. Biol. Neonate 2002, 81, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Le Dividch, J.; Seve, B. Energy requirement of the young piglet. The weaner pig: Nutrition and management. In Proceedings of a British Society of Animal Science Occasional Meeting, University of Nottingham, UK, September 2000.
- Tokach, M.D.; Goodbank, R.D.; Nelssen, J.L.; Kats, L.J. Influence of weaning weight and growth during the first week post-weaning on subsequent pig performance. Available online: http://krex.k-state.edu/dspace/bitstream/handle/2097/2556/Swine92pg15-17.pdf?sequence=1 (accessed on 7 October 2014).
- Azain, M.J. Impact of Starter Diet on Nursery Performance; Swine Report; University of Georgia: Athens, GA, USA, 1993; pp. 49–54. [Google Scholar]
- Sutherland, M.A.; Krebs, N.; Smith, J.S.; Dailey, J.W.; Caroll, J.A.; McGlone, J.J. The effect of three space allowances on the physiology and behavior of weaned pigs during transportation. Livest. Sci. 2009, 126, 183–188. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Bryer, P.J.; Davis, B.L.; McGlone, J.J. A multidisciplinary approach to assess the welfare of weaned pigs during transport at three space allowances. J. Appl. Anim. Welf. Sci. 2010, 13, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Lambooy, E.; Garson, G.J.; Walstra, P.; Mateman, G.; Merkus, G.S. Transport of pigs by car for two ds; some aspects of watering and loading density. Livest. Prod. Sci. 1985, 13, 289–299. [Google Scholar] [CrossRef]
- Jones, S.D.; Rompala, R.E.; Haworth, C.R. Effects of fasting and water restriction on carcass shrink in pork quality. Can. J. Anim. Sci. 1985, 65, 613–618. [Google Scholar] [CrossRef]
- Brumm, M.C.; Jesse, G.W.; Mayes, H.F.; Sinn, G.M.; Clemens, E.T. Effects of feed and water restriction receiving diet crude protein on feeder pig performance. J. Anim. Sci. 1987, 64, 1606–1611. [Google Scholar] [PubMed]
- Lambooy, E. Road transport of pigs over a long distance: Some aspects of behavior, temperature and humidity during transport and some effects of the last two factors. Anim. Prod. 1988, 46, 257–263. [Google Scholar] [CrossRef]
- Lewis, N.J.; Berry, R.J. Effects of season on the behavior of early-weaned piglets during and immediately following transport. Appl. Anim. Behav. Sci. 2006, 100, 182–192. [Google Scholar] [CrossRef]
- Lewis, N.J. Transport of early weaned pigs. Behav. Sci. 2008, 110, 128–135. [Google Scholar]
- Sutherland, M.A.; Bryer, P.J.; Davis, B.L.; Smith, J.F.; McGlone, J. The combined effects of transport and food and water deprivation on the physiology of breeding age gilts. Livest. Sci. 2012, 144, 124–131. [Google Scholar] [CrossRef]
- Becerril-Herrera, M.; Alonso-Spilsberry, M.; Trujillo Ortega, M.E.; Guerrerro-Legarreta, I.; Ramirez-Necoechea, R.; Roldan-Santiago, P.; Perez-Sato, M.; Soni-Guillermo, E.; Mota-Rojas, D. Changes in blood constituents of swine transported for 8 or 16 h to an Abattoir. Meat Sci. 2010, 86, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.E.; Katz, A.; Spencer, M.K.; Yan, Z.; Nyomba, B.L. Fasting inhibits insulin mediated glycolysis and anaplerosis in human skeletal muscle. Am. J. Physiol. 1991, 261, E598–E605. [Google Scholar] [PubMed]
- Fernández, X.; Meunier-Salaun, M.C.; Ecolan, P.; Mormède, P. Interactive effect of food deprivation and agonistic behavior on blood parameters and muscle glycogen in pigs. Physiol. Behav. 1995, 58, 337–345. [Google Scholar] [CrossRef]
- Pégorier, J.P.; Duée, P.H.; Simoes-Nunes, C.; Peret, J.; Girard, J.R. Glucose turnover and recycling in unrestrained and unaesthetized 48-hr-old fasting or post-absorptive newborn pigs. Br. J. Nutr. 1984, 52, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R.; Kramer, J.K.G. Fat metabolism in growing swine: A review. Can. J. Anim. Sci. 1987, 67, 301–318. [Google Scholar] [CrossRef]
- Le Dividich, J.; Seve, B. Effects of underfeeding during the weaning period on growth, metabolism, and hormonal adjustments in the piglet. Domest. Anim. Endocr. 2000, 19, 63–74. [Google Scholar] [CrossRef]
- Van der Meulen, J.H.; Kuipers, H.; Drukker, J. Relationship between exercise-induced muscle damage and enzyme release in rats. J. Appl. Physiol. 1991, 71, 999–1004. [Google Scholar] [PubMed]
- Yu, H.; Bao, E.D.; Zhao, R.Q.; Lu, Q.X. Effect of transportation stress on heat shock protein 70 concentration and mRNA expression in heart and kidney tissues and serum enzyme activities and hormone concentrations of pigs. Am. J. Vet. Res. 2007, 68, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Averos, X.; Herranz, A.; Sanchez, R.; Gozalvez, L.F. Effect of duration of commercial journeys between rearing farms and growing-finishing farms on the physiological stress response of weaned piglets. Livest. Sci. 2009, 122, 339–344. [Google Scholar] [CrossRef]
- Wammes, S.; Lewis, N.J.; Berry, R.J. The behavior of early-weaned piglets following transport; Effect of season and weaning weight. Can. J. Anim. Sci. 2008, 88, 357–367. [Google Scholar] [CrossRef]
- Warriss, P.D. The consequences of fighting between mixed groups and unfamiliar pigs before slaughter. Meat Focus Int. 1996, 5, 89–92. [Google Scholar]
- Guàrdia, M.D.; Estany, J.; Balasch, S.; Oliver, M.A.; Gispert, M.; Diestre, A. Risk assessment of PSE condition due to pre-slaughter conditions in RYR1 gene in pigs. Meat Sci. 2004, 67, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, G.; Addis, P.B.; Rempel, W.E.; Madero, C.; Martin, F.B.; Anderson, D.B.; Marple, D.N. Stress Response and muscle properties in Pietrain (P), Minnestota No. 1 (M) and PxM pigs. J. Anim. Sci. 1976, 43, 1004–1014. [Google Scholar] [PubMed]
- Metz, J.H.M.; Gonyou, H.W. Effect of age and housing conditions on the behavioral and haemolytic reaction of piglets to weaning. Appl. Anim. Sci. 1990, 27, 299–309. [Google Scholar] [CrossRef]
- Dybkjaer, L. The identification of behavioral indicators of “stress” in early weaned piglets. Appl. Anim. Behav. Sci. 1992, 35, 135–147. [Google Scholar] [CrossRef]
- Gonyou, H.W.; Beltranena, E.; Whittington, D.L.; Patience, J.F. The behavior of weaned pigs at 12 and 21 ds of age from weaning to market. Can J. Anim. Sci. 1998, 78, 517–523. [Google Scholar] [CrossRef]
- Worbec, E.K.; Duncan, I.J.; Widowski, T.M. The effects of weaning at 7, 14, 28 ds on piglet behavior. Appl. Anim. Behav. Sci. 1999, 62, 173–182. [Google Scholar] [CrossRef]
- Villé, H.; Bertels, S.; Geers, R.; Janssens, S.; Goedseels, V.; Parduyns, G.; van Bael, J.; Goossens, K.; Bosschaerts, L.; de Ley, J.; et al. Electrocardiogram parameters of piglets during housing, handling and transport. Anim. Prod. 1993, 56, 211–216. [Google Scholar] [CrossRef]
- Geers, R.; Bleus, E.; Van Schie, E.; Villé, H.; Gerard, H.; Janssens, S.; Nackaerts, G.; Decuypere, E.; Jourquin, J. Transport of pigs different with respect to the halothane gene: Stress assessment. J. Anim. Sci. 1994, 72, 2552–2558. [Google Scholar] [PubMed]
- Hicks, T.A.; Mcglone, J.J.; Whisnant, C.S.; Kattesh, H.G.; Norman, R.L. Behavioral, endocrine, immune and performance measures for pigs exposed to acute stress. J. Anim. Sci. 1998, 76, 474–483. [Google Scholar] [PubMed]
- Berry, R.J.; Lewis, N.J. The effect of duration and temperature of simulated transport on the performance of early-weaned piglets. Can. J. Anim. Sci. 2001, 81, 199–204. [Google Scholar] [CrossRef]
- Lewis, N.J.; Berry, R.J.; Crow, G.; Wamnes, S. Assessment of the effects of season of transport on the performance of early-weaned piglets. Can. J. Anim. Sci. 2005, 85, 449–454. [Google Scholar] [CrossRef]
- Bench, C.; Schaefer, A.; Faucitano, L. The welfare of pigs during transport. In Welfare of Pigs from Birth to Slaughter; Wageningen Academic Publishers: Wageningen, The Netherlands, 2008; pp. 161–195. [Google Scholar]
- Pineiro, M.; Pineiro, C.; Carpintero, R.; Morales, J.; Campbell, F.M.; Eckersall, P.D.; Toussaint, M.; Lampreave, F. Characteristics of the pig acute phase protein response to road transport. Vet. J. 2007, 173, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kietzmann, M.; Jablonski, H. Blocking of stress in swine with magnesium aspartate hydrochloride. Prakt. Tierz. 1985, 661, 328–335. [Google Scholar]
- D’Souza, D.N.; Warner, R.D.; Leury, B.J.; Dunshea, F.R. The effect of dietary magnesium aspartate supplementation on pork quality. J. Anim. Sci. 1998, 76, 104–109. [Google Scholar] [PubMed]
- D’Souza, D.N.; Warner, R.D.; Dunshea, F.R.; Leury, B.J. Comparison of different dietary magnesium supplements on pork quality. Meat Sci. 1999, 51, 221–225. [Google Scholar] [CrossRef]
- Kuhn, G.; Nowak, A.; Otto, E.; Albrecht, V.; Gassmann, B.; Sandner, E.; Przybilski, H.; Zahn, L. Studies on the control of meat quality by special treatment of swine. 1. Effects of stress and preventative magnesium feeding on selected parameters of carcass value and blood serum. Arch. Tierz. 1981, 24, 217–225. [Google Scholar]
- Eckersall, P.E. Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Rev. Med. Vet. 2000, 151, 577–584. [Google Scholar]
- Murata, H.; Shimada, N.; Yoshioka, M. Current research on acute phase proteins in veterinary diagnosis: An overview. Vet. J. 2004, 168, 28–40. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [PubMed]
- Van Putten, G. Handling of slaughter pigs prior to loading and during loading on a lorry. In Transport of Animals Intended for Breeding, Production and Slaughter; Springer: Dordrecht, The Netherlands, 1982; Volume 18, pp. 15–27. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.; Pirner, G.; Picinin, G.; May, M.; Guay, K.; Backus, B.; Sutherland, M.; McGlone, J. Effect of Provision of Feed and Water during Transport on the Welfare of Weaned Pigs. Animals 2015, 5, 407-425. https://doi.org/10.3390/ani5020363
Garcia A, Pirner G, Picinin G, May M, Guay K, Backus B, Sutherland M, McGlone J. Effect of Provision of Feed and Water during Transport on the Welfare of Weaned Pigs. Animals. 2015; 5(2):407-425. https://doi.org/10.3390/ani5020363
Chicago/Turabian StyleGarcia, Arlene, Glenna Pirner, Guilherme Picinin, Matthew May, Kimberly Guay, Brittany Backus, Mhairi Sutherland, and John McGlone. 2015. "Effect of Provision of Feed and Water during Transport on the Welfare of Weaned Pigs" Animals 5, no. 2: 407-425. https://doi.org/10.3390/ani5020363






