Symbiosis in Sustainable Agriculture: Can Olive Fruit Fly Bacterial Microbiome Be Useful in Pest Management?
Abstract
:1. Introduction
2. Symbiotic Microorganisms in Pest Control
3. The Iconic Mediterranean Crop Olives and Its Main Insect Pest
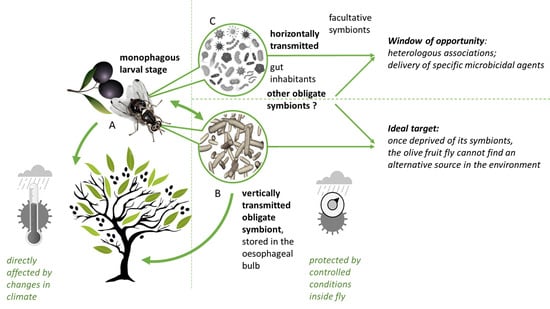
4. Symbiotic Bacteria and the Olive Fruit Fly
5. Symbiosis-Based Olive Fruit Fly Control
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilbert, S.F.; Sapp, J.; Tauber, A.I. A symbiotic view of life: We have never been individuals. Q. Rev. Biol. 2012, 87, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Booth, A. Symbiosis, selection, and individuality. Biol. Philos. 2014, 29, 657–673. [Google Scholar] [CrossRef]
- Gilbert, S.F.; McDonald, E.; Boyle, N.; Buttino, N.; Gyi, L.; Mai, M.; Prakash, N.; Robinson, J. Symbiosis as a source of selectable epigenetic variation: Taking the heat for the big guy. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 671–678. [Google Scholar] [CrossRef] [PubMed]
- De Bary, A. Die Erscheinung der Symbiose. In Vortrag auf der Versammlung der Naturforscher und Ärtze zu Cassel; Trubner, K., Ed.; Verlag: Strasburg, Germany, 1879; pp. 1–30. [Google Scholar]
- Martin, B.D.; Schwab, E. Current usage of symbiosis and associated terminology. Int. J. Biol. 2012, 5, 32–45. [Google Scholar] [CrossRef]
- Wilkinson, D.M. At cross purposes. Nature 2001, 412, 485. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Conflict, cheats and the persistence of symbioses. New Phytol. 2008, 177, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Gano-Cohen, K.A.; Wendlandt, C.E.; Stokes, P.J.; Blanton, M.A.; Quides, K.W.; Zomorrodian, A.; Adinata, E.S.; Sachs, J.L. Interspecific conflict and the evolution of ineffective rhizobia. Ecol. Lett. 2019, 22, 914–924. [Google Scholar] [CrossRef]
- Baker, D.M.; Freeman, C.J.; Wong, J.C.Y.; Fogel, M.L.; Knowlton, N. Climate change promotes parasitism in a coral symbiosis. ISME J. 2018, 12, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, N.; Barber, P.H.; Weisz, J.B. Episymbiotic microbes as food and defence for marine isopods: Unique symbioses in a hostile environment. Proc. R. Soc. B Biol. Sci. 2005, 272, 1209–1216. [Google Scholar] [CrossRef]
- West, P.C.; Gerber, J.S.; Engstrom, P.M.; Mueller, N.D.; Brauman, K.A.; Carlson, K.M.; Cassidy, E.S.; Johnston, M.; MacDonald, G.K.; Ray, D.K.; et al. Leverage points for improving global food security and the environment. Science 2014, 345, 325–328. [Google Scholar] [CrossRef] [Green Version]
- Lusk, J.L.; McCluskey, J. Understanding the impacts of food consumer choice and food policy outcomes. Appl. Econ. Perspect. Policy 2018, 40, 5–21. [Google Scholar] [CrossRef]
- Knorr, D.; Khoo, C.S.H.; Augustin, M.A. Food for an urban planet: Challenges and research opportunities. Front. Nutr. 2018, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luise, K.; Fritz, O.; van der Heijden, M.G.A. Agricultural practices indirectly influence plant productivity and ecosystem services through effects on soil biota. Ecol. Appl. 2014, 24, 1842–1853. [Google Scholar]
- Cooper, J.; Baranski, M.; Stewart, G.; Nobel-de Lange, M.; Bàrberi, P.; Fließbach, A.; Peigné, J.; Berner, A.; Brock, C.; Casagrande, M.; et al. Shallow non-inversion tillage in organic farming maintains crop yields and increases soil C stocks: A meta-analysis. Agron. Sustain. Dev. 2016, 36, 22. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; Heijden, M.G.A.; et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Friesen, M.L. Microbially mediated plant functional traits. In Molecular Microbial Ecology of the Rhizosphere; John Wiley and Sons: Hoboken, NJ, USA, 2013; Volume 1, pp. 87–102. [Google Scholar]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Soman, C.; Dangl, J.L.; Bennett, A.; Morsy, M.; Friesen, M.L.; Busby, P.E.; Leach, J.E.; Eisen, J.A.; Wagner, M.R.; Kremer, J. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar]
- Hohmann, P.; Messmer, M.M. Breeding for mycorrhizal symbiosis: Focus on disease resistance. Euphytica 2017, 213, 113. [Google Scholar] [CrossRef]
- Nogales, A.; Nobre, T.; Valadas, V.; Ragonezi, C.; Döring, M.; Polidoros, A.; Arnholdt-Schmitt, B. Can functional hologenomics aid tackling current challenges in plant breeding? Brief. Funct. Genom. 2015, 15, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Battisti, A.; Larsson, S. Climate change and insect pest distribution range. In Climate Change and Insect Pests; Björkman, C., Niemelä, P., Eds.; CABI: Wallingford, UK, 2015; pp. 1–15. ISBN 978-1-78064-378-6. [Google Scholar]
- Furlong, M.J.; Zalucki, M.P. Climate change and biological control: The consequences of increasing temperatures on host–parasitoid interactions. Curr. Opin. Insect Sci. 2017, 20, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Thomson, L.J.; Macfadyen, S.; Hoffmann, A.A. Predicting the effects of climate change on natural enemies of agricultural pests. Biol. Control 2010, 52, 296–306. [Google Scholar] [CrossRef]
- Ishikawa, H. Insect symbiosis: An introduction. In Insect Symbiosis, Vol1; Bourtzis, K., Miller, T.A., Eds.; CRC press: Boca Raton, FL, USA, 2003; Volume 1, pp. 1–21. ISBN 0-8493-1286-8. [Google Scholar]
- Huang, J.H.; Jing, X.; Douglas, A.E. The multi-tasking gut epithelium of insects. Insect Biochem. Mol. Biol. 2015, 67, 15–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.C.-N.; Luo, Y.; Jing, X.; Franzenburg, S.; Bost, A.; Douglas, A.E. The host as the driver of the microbiota in the gut and external environment of drosophila melanogaster. Appl. Environ. Microbiol. 2015, 81, 6232–6240. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Lemaitre, B. Gut homeostasis in a microbial world: Insights from Drosophila melanogaster. Nat. Rev. Microbiol. 2013, 11, 615–626. [Google Scholar] [CrossRef]
- Tamas, I.; Andersson, S.; Bourtzis, K.; Miller, T.A. Comparative genomics of insect endosymbionts. In Insect Symbiosis, Vol1; Bourtzis, K., Miller, T.A., Eds.; CRC press: Boca Raton, FL, USA, 2003; pp. 39–52. ISBN 0-8493-1286-8. [Google Scholar]
- Appel, H.M.; Martin, M.M. Gut redox conditions in herbivorous lepidopteran larvae. J. Chem. Ecol. 1990, 16, 3277–3290. [Google Scholar] [CrossRef] [Green Version]
- Šustr, V.; Stingl, U.; Brune, A. Microprofiles of oxygen, redox potential, and pH, and microbial fermentation products in the highly alkaline gut of the saprophagous larva of Penthetria holosericea (Diptera: Bibionidae). J. Insect Physiol. 2014, 67, 64–69. [Google Scholar] [CrossRef]
- Lemke, T.; Stingl, U.; Egert, M.; Friedrich, M.W.; Brune, A. Physicochemical conditions and microbial activities in the highly alkaline gut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2003, 69, 6650–6658. [Google Scholar] [CrossRef]
- Johnson, K.S.; Barbehenn, R.V. Oxygen levels in the gut lumens of herbivorous insects. J. Insect Physiol. 2000, 46, 897–903. [Google Scholar] [CrossRef]
- Brune, A.; Emerson, D.; Breznak, J.A. The termite gut microflora as an oxygen sink: Microelectrode determination of oxygen and pH gradients in guts of lower and higher termites. Appl. Environ. Microbiol. 1995, 61, 2681–2687. [Google Scholar] [PubMed]
- Brune, A.; Friedrich, M. Microecology of the termite gut: Structure and function on a microscale. Curr. Opin. Microbiol. 2000, 3, 263–269. [Google Scholar] [CrossRef]
- Engel, M.S. Insect evolution. Curr. Biol. 2015, 25, R868–R872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brune, A. Microbial symbioses in the digestive tract of lower termites. In Beneficial Microorganisms in Multicellular Life Forms; Rosenberg, E., Gophna, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–25. ISBN 978-3-642-21679-4. [Google Scholar]
- Duarte, S.; Nunes, L.; Borges, P.A.V.; Foss-Dal, C.G.; Nobre, T. Living inside termites: An overview of symbiotic interactions, with emphasis on flagellate protists. Arquipel. Life Mar. Sci. 2017, 34, 21–43. [Google Scholar]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Andongma, A.A.; Wan, L.; Xue-ping, D.; Akami, M.; He, J.; Clarke, A.R.; Chang-Ying, N. The impact of nutritional quality and gut bacteria on the fitness of Bactrocera minax (Diptera: Tephritidae). R. Soc. Open Sci. 2018, 5, 180237. [Google Scholar] [CrossRef]
- Richard, F.-J.; Mora, P.; Errard, C.; Rouland, C. Digestive capacities of leaf-cutting ants and the contribution of their fungal cultivar to the degradation of plant material. J. Comp. Physiol. B 2005, 175, 297–303. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Cui, H.L.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [Green Version]
- Visser, A.A.; Nobre, T.; Currie, C.R.; Aanen, D.K.; Poulsen, M. Exploring the potential for actinobacteria as defensive symbionts in fungus-growing termites. Microb. Ecol. 2012, 63, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the oriental fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-D.; Guo, H.-F. Importance of endosymboints Wolbachia and Rickettsia in insect resistance development. Curr. Opin. Insect Sci. 2019, 33, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, M.; Jurkevitch, E.; Yuval, B. Effect of bacteria on nutritional status and reproductive success of the Mediterranean fruit fly Ceratitis capitata. Physiol. Entomol. 2008, 33, 145–154. [Google Scholar] [CrossRef]
- Markov, A.V.; Lazebny, O.E.; Goryacheva, I.I.; Antipin, M.I.; Kulikov, A.M. Symbiotic bacteria affect mating choice in Drosophila melanogaster. Anim. Behav. 2009, 77, 1011–1017. [Google Scholar] [CrossRef]
- Bordenstein, S.R.; O’Hara, F.P.; Werren, J.H. Wolbachia-induced incompatibility precedes other hybrid incompatibilities in Nasonia. Nature 2001, 409, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Andongma, A.A.; Wan, L.; Dong, Y.-C.; Li, P.; Desneux, N.; White, J.A.; Niu, C.-Y. Pyrosequencing reveals a shift in symbiotic bacteria populations across life stages of Bactrocera dorsalis. Sci. Rep. 2015, 5, 9470. [Google Scholar] [CrossRef]
- Dunbar, H.E.; Wilson, A.C.C.; Ferguson, N.R.; Moran, N.A. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 2007, 5, 1006–1015. [Google Scholar] [CrossRef]
- Arora, A.K.; Douglas, A.E. Hype or opportunity? Using microbial symbionts in novel strategies for insect pest control. J. Insect Physiol. 2017, 103, 10–17. [Google Scholar] [CrossRef]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef]
- Nobre, T.; Rouland-Lefèvre, C.; Aanen, D.K. Comparative biology of fungus cultivation in termites and ants. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 193–210. ISBN 978-90-481-3977-4. [Google Scholar]
- Ferrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1389–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldhaar, H.; Gross, R. Insects as hosts for mutualistic bacteria. Int. J. Med. Microbiol. 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zientz, E.; Dandekar, T.; Gross, R. Metabolic interdependence of obligate intracellular bacteria and their insect hosts. Microbiol. Mol. Biol. Rev. 2004, 68, 745–770. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Symbiotic microorganisms: Untapped resources for insect pest control. Trends Biotechnol. 2007, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, R.; Krebs, J.R. Arms races between and within species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1979, 205, 489–511. [Google Scholar]
- IOC. World Olive Oil Figures—Production; International Olive Council: Madrid, Spain, 2017. [Google Scholar]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Biotechnol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Uylaşer, V.; Yildiz, G. The historical development and nutritional importance of olive and olive oil constituted an important part of the Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef]
- Delrio, G. Biological control of olive pests in the Mediterranean region. In Proceedings of the Integrated Protection of Olive Crops, Braganca, Portugal, 10–12 October 2007; pp. 85–92. [Google Scholar]
- Haniotakis, G.E. Olive pest control: Present status and prospects. IOBC WPRS Bull. 2005, 28, 1–9. [Google Scholar]
- Haber, G.; Mifsud, D. Pests and diseases associated with olive trees in the Maltese Islands (Central Mediterranean). Cent. Mediterr. Nat. 2007, 4, 143–161. [Google Scholar]
- Mansour, A.A.; Ouanaimi, F.; Chemseddine, M.; Boumezzough, A. Study of the flight dynamics of Prays oleae (Lepidoptera: Yponomeutidae) using sexual trapping in olive orchards of Essaouira region, Morocco. J. Entomol. Zool. Stud. 2017, 5, 943–952. [Google Scholar]
- Serrano, A.M.V. Ecological Infrastructures in Sustainable Olive Growing: Studies about Prays Oleae (Bernard) and its Natural Enemies; ISA-UL: Lisboa, Portugal, 2016. [Google Scholar]
- Ramos, P.; Campos, M.; Ramos, J.M. Long-term study on the evaluation of yield and economic losses caused by Prays oleae Bern. In the olive crop of Granada (Southern Spain). Crop Prot. 1998, 17, 645–647. [Google Scholar] [CrossRef]
- Bueno, A.M.; Jones, O. Alternative methods for controlling the olive fly. IOBC WPRS Bull. 2002, 25, 147–156. [Google Scholar]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A review of Bactrocera oleae (Rossi) impact in olive products: From the tree to the table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Kakani, E.G.; Ioannides, I.M.; Margaritopoulos, J.T.; Seraphides, N.A.; Skouras, P.J.; Tsitsipis, J.A.; Mathiopoulos, K.D. A small deletion in the olive fly acetylcholinesterase gene associated with high levels of organophosphate resistance. Insect Biochem. Mol. Biol. 2008, 38, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Nardi, F.; Carapelli, A.; Vontas, J.G.; Dallai, R.; Roderick, G.K.; Frati, F. Geographical distribution and evolutionary history of organophosphate-resistant Ace alleles in the olive fly (Bactrocera oleae). Insect Biochem. Mol. Biol. 2006, 36, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.G.; Hejazi, M.J.; Hawkes, N.J.; Cosmidis, N.; Loukas, M.; Janes, R.W.; Hemingway, J. Resistance-associated point mutations of organophosphate insensitive acetylcholinesterase, in the olive fruit fly Bactrocera oleae. Insect Mol. Biol. 2002, 11, 329–336. [Google Scholar] [CrossRef]
- Vontas, J.G.; Cosmidis, N.; Loukas, M.; Tsakas, S.; Hejazi, J.; Ayoutanti, A.; Hemingway, J. Altered acetylcholinesterase confers organophosphate resistance in the olive fruit fly Bactrocera oleae. Pestic. Biochem. Physiol. 2001, 71, 124–132. [Google Scholar] [CrossRef]
- Margaritopoulos, J.T.; Skavdis, G.; Kalogiannis, N.; Nikou, D.; Morou, E.; Skouras, P.J.; Tsitsipis, J.A.; Vontas, J. Efficacy of the pyrethroid alpha-cypermethrin against Bactrocera oleae populations from Greece, and improved diagnostic for an iAChE mutation. Pest Manag. Sci. 2008, 64, 900–908. [Google Scholar] [CrossRef]
- Kakani, E.G.; Zygouridis, N.E.; Tsoumani, K.T.; Seraphides, N.; Zalom, F.G.; Mathiopoulos, K.D. Spinosad resistance development in wild olive fruit fly Bactrocera oleae (Diptera: Tephritidae) populations in California. Pest Manag. Sci. 2010, 66, 447–453. [Google Scholar]
- Tanasijevic, L.; Todorovic, M.; Pereira, L.S.; Pizzigalli, C.; Lionello, P. Impacts of climate change on olive crop evapotranspiration and irrigation requirements in the Mediterranean region. Agric. Water Manag. 2014, 144, 54–68. [Google Scholar] [CrossRef]
- Gabaldón-Leal, C.; Ruiz-Ramos, M.; de la Rosa, R.; León, L.; Belaj, A.; Rodríguez, A.; Santos, C.; Lorite, I.J. Impact of changes in mean and extreme temperatures caused by climate change on olive flowering in southern Spain. Int. J. Climatol. 2017, 37, 940–957. [Google Scholar] [CrossRef]
- Ponti, L.; Gutierrez, A.P.; Ruti, P.M.; Dell’Aquila, A. Fine-scale ecological and economic assessment of climate change on olive in the Mediterranean Basin reveals winners and losers. Proc. Natl. Acad. Sci. USA 2014, 111, 5598–5603. [Google Scholar] [CrossRef] [Green Version]
- Broumas, T.; Haniotakis, G.; Liaropoulos, C.; Tomazou, T.; Ragoussis, N. The efficacy of an improved form of the mass-trapping method, forthe control of the olive fruit fly, Bactrocera oleae (Gmelin) (Dipt., Tephritidae): Pilot-scale feasibility studies. J. Appl. Entomol. 2002, 126, 217–223. [Google Scholar] [CrossRef]
- Yasin, S.; Rempoulakis, P.; Nemny-Lavy, E.; Levi-Zada, A.; Tsukada, M.; Papadopoulos, N.T.; Nestel, D. Assessment of lure and kill and mass-trapping methods against the olive fly, Bactrocera oleae (Rossi), in desert-like environments in the Eastern Mediterranean. Crop Prot. 2014, 57, 63–70. [Google Scholar] [CrossRef]
- Petacchi, R.; Rizzi, I.; Guidotti, D. The “lure and kill” technique in Bactrocera oleae (Gmel.) control: Effectiveness indices and suitability of the technique in area-wide experimental trials. Int. J. Pest Manag. 2003, 49, 305–311. [Google Scholar] [CrossRef]
- Rempoulakis, P.; Nestel, D. Dispersal ability of marked, irradiated olive fruit flies [Bactrocera oleae (Rossi) (Diptera: Tephritidae)] in arid regions. J. Appl. Entomol. 2012, 136, 171–180. [Google Scholar] [CrossRef]
- Ant, T.; Koukidou, M.; Rempoulakis, P.; Gong, H.F.; Economopoulos, A.; Vontas, J.; Alphey, L. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol. 2012, 10, 4–11. [Google Scholar] [CrossRef]
- Ahmad, S.; Haq, I.U.; Cáceres, C.; Tomas, U.S.; Dammalage, T.; Gembinsky, K.; Paulus, H.; Vreysen, M.J.B.; Rempoulakis, P. One for all: Mating compatibility among various populations of olive fruit fly (Diptera: Tephritidae) for application of the sterile insect technique. PLoS ONE 2018, 13, e0206739. [Google Scholar] [CrossRef]
- Saour, G.; Makee, H. A kaolin-based particle film for suppression of the olive fruit fly Bactrocera oleae Gmelin (Dip., Tephritidae) in olive groves. J. Appl. Entomol. 2004, 128, 28–31. [Google Scholar] [CrossRef]
- Pascual, S.; Cobos, G.; Seris, E.; González-Núñez, M. Effects of processed kaolin on pests and non-target arthropods in a Spanish olive grove. J. Pest Sci. (2004) 2010, 83, 121–133. [Google Scholar] [CrossRef]
- Caleca, V.; Lo Verde, G.; Lo Verde, V.; Piccionello, M.P.; Rizzo, R. Control of Bactrocera oleae and Ceratitis capitata in organic orchards: Use of clays and copper products. Acta Hortic. 2010, 873, 227–234. [Google Scholar] [CrossRef]
- Bigiotti, G.; Pastorelli, R.; Belcari, A.; Sacchetti, P. Symbiosis interruption in the olive fly: Effect of copper and propolis on Candidatus Erwinia dacicola. J. Appl. Entomol. 2019, 143, 357–364. [Google Scholar] [CrossRef]
- Belcari, A.; Sacchetti, P.; Rosi, M.C.; Del Pianta, R. The use of copper products to control the olive fly (Bactrocera oleae) in central Italy. IOBC WPRS Bull. 2005, 28, 45. [Google Scholar]
- Groves, O.; Vitanovic, E. Use of Cu fungicides in vineyards and olive groves. In Fungicides for Plant and Animal Diseases; IntechOpen: London, UK, 2012; p. 298. ISBN 978-953-307-804-5. [Google Scholar]
- Roca, L.F.; Moral, J.; Viruega, J.R.; Ávila, A.; Oliveira, R.; Trapero, A. Copper fungicides in the control of olive diseases. FAO Olive Netw. 2007, 26, 48–50. [Google Scholar]
- Ballabio, C.; Panagos, P.; Lugato, E.; Huang, J.H.; Orgiazzi, A.; Jones, A.; Fernández-Ugalde, O.; Borrelli, P.; Montanarella, L. Copper distribution in European topsoils: An assessment based on LUCAS soil survey. Sci. Total Environ. 2018, 636, 282–298. [Google Scholar] [CrossRef]
- Capuzzo, C.; Firrao, G.; Mazzon, L.; Squartini, A.; Girolami, V. “Candidatus Erwinia dacicola”, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). Int. J. Syst. Evol. Microbiol. 2005, 55, 1641–1647. [Google Scholar] [CrossRef]
- Estes, A.M.; Hearn, D.J.; Burrack, H.J.; Rempoulakis, P.; Pierson, E.A. Prevalence of Candidatus Erwinia dacicola in wild and laboratory olive fruit fly populations and across developmental stages. Environ. Entomol. 2012, 41, 265–274. [Google Scholar] [CrossRef]
- Savio, C.; Mazzon, L.; Martinez-Sañudo, I.; Simonato, M.; Squartini, A.; Girolami, V. Evidence of two lineages of the symbiont “Candidatus Erwinia dacicola” in Italian populations of Bactrocera oleae (Rossi) based on 16S rRNA gene sequences. Int. J. Syst. Evol. Microbiol. 2011, 62, 179–187. [Google Scholar] [CrossRef]
- Sacchetti, P.; Granchietti, A.; Landini, S.; Viti, C.; Giovannetti, L.; Belcari, A. Relationships between the olive fly and bacteria. J. Appl. Entomol. 2008, 132, 682–689. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open Sci. 2015, 2, 150170. [Google Scholar] [CrossRef] [Green Version]
- Pavlidi, N.; Gioti, A.; Wybouw, N.; Dermauw, W.; Ben-Yosef, M.; Yuval, B.; Jurkevich, E.; Kampouraki, A.; Van Leeuwen, T.; Vontas, J. Transcriptomic responses of the olive fruit fly Bactrocera oleae and its symbiont Candidatus Erwinia dacicola to olive feeding. Sci. Rep. 2017, 7, 42633. [Google Scholar] [CrossRef]
- Estes, A.M.; Segura, D.F.; Jessup, A.; Wornoayporn, V.; Pierson, E.A. Effect of the symbiont Candidatus Erwinia dacicola on mating success of the olive fly Bactrocera oleae (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2014, 34, S123–S131. [Google Scholar] [CrossRef]
- Estes, A.M.; Hearn, D.J.; Bronstein, J.L.; Pierson, E.A. The olive fly endosymbiont, “Candidatus Erwinia dacicola,” switches from an intracellular existence to an extracellular existence during host insect development. Appl. Environ. Microbiol. 2009, 75, 7097–7106. [Google Scholar] [CrossRef]
- Christenson, L.D.; Foote, R.H. Biology of Fruit Flies. Annu. Rev. Entomol. 1960, 5, 171–192. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Courtice, A.C.; Teakle, D.S. Bacteria as a natural source of food for adult fruit flies (Diptera: Tephritidae). Oecologia 1983, 60, 279–284. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Yuval, B. The evolution of fruit fly feeding behavior. In Fruit Flies (Tephritidae) Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 731–749. ISBN 0849312752. [Google Scholar]
- Sacchetti, P.; Ghiardi, B.; Granchietti, A.; Stefanini, F.M.; Belcari, A. Development of probiotic diets for the olive fly: Evaluation of their effects on fly longevity and fecundity. Ann. Appl. Biol. 2014, 164, 138–150. [Google Scholar] [CrossRef]
- Daser, U.; Brandl, R. Microbial gut floras of eight species of tephritids. Biol. J. Linn. Soc. 1992, 45, 155–165. [Google Scholar] [CrossRef]
- Konstantopoulou, M.A.; Raptopoulos, D.G.; Stavrakis, N.G.; Mazomenos, B.E. Microflora species and their volatile compounds affecting development of an alcohol dehydrogenase homozygous strain (Adh-I) of Bactrocera (Dacus) oleae (Diptera: Tephritidae). J. Econ. Entomol. 2009, 98, 1943–1949. [Google Scholar] [CrossRef]
- Luthy, P.; Studer, D.; Jaquet, F.; Yamvrias, C. Morphology and in vitro cultivation of the bacterial symbiote of Dacus oleae. Mitteilungen der Schweizerischen Entomol. Gesellschaft 1983, 56, 67–72. [Google Scholar]
- Rempoulakis, P.; Sela, S.; Nemny-Lavy, E.; Pinto, R.; Birke, A.; Nestel, D. Microbial composition affects the performance of an artificial Tephritid larval diet. Bull. Entomol. Res. 2018, 108, 434–441. [Google Scholar] [CrossRef]
- Yamvrias, C.; Panagopiulos, C.G.; Psallidas, P.G. Preliminary study of the internal bacterial flora of the olive fruit fly (Dacus oleae Gmelin). Ann. l’Institut Phytopathol. Benaki 1970, 9, 201–206. [Google Scholar]
- Mazzon, L.; Martinez-Sanudo, I.; Simonato, M.; Squartini, A.; Savio, C.; Girolami, V. Phylogenetic relationships between flies of the Tephritinae subfamily (Diptera, Tephritidae) and their symbiotic bacteria. Mol. Phylogenet. Evol. 2010, 56, 312–326. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Aharon, Y.; Jurkevitch, E.; Yuval, B. Give us the tools and we will do the job: Symbiotic bacteria affect olive fly fitness in a diet-dependent fashion. Proc. R. Soc. B Biol. Sci. 2010, 277, 1545–1552. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J. Evol. Biol. 2014, 27, 2695–2705. [Google Scholar] [CrossRef]
- Kounatidis, I.; Crotti, E.; Sapountzis, P.; Sacchi, L.; Rizzi, A.; Chouaia, B.; Bandi, C.; Alma, A.; Daffonchio, D.; Mavragani-Tsipidou, P.; et al. Acetobacter tropicalis is a major symbiont of the olive fruit fly (Bactrocera oleae). Appl. Environ. Microbiol. 2009, 75, 3281–3288. [Google Scholar] [CrossRef]
- Blow, F.; Gioti, A.; Goodhead, I.B.; Kalyva, M. Functional genomics of a symbiotic community: Shared traits in the olive fruit fly gut microbiota. bioRxiv 2019. [Google Scholar] [CrossRef]
- Douglas, A.E. Buchnera bacteria and other symbionts of aphids. Insect Symbiosis 2003, 1, 23–38. [Google Scholar]
- Kost, C.; Lakatos, T.; Böttcher, I.; Arendholz, W.-R.; Redenbach, M.; Wirth, R. Non-specific association between filamentous bacteria and fungus-growing ants. Naturwissenschaften 2007, 94, 821–828. [Google Scholar] [CrossRef]
- Darby, A.C.; Douglas, A.E. Elucidation of the transmission patterns of an insect-borne bacterium. Appl. Environ. Microbiol. 2003, 69, 4403–4407. [Google Scholar] [CrossRef]
- Sacchetti, P.; Pastorelli, R.; Bigiotti, G.; Guidi, R.; Ruschioni, S.; Viti, C.; Belcari, A. Olive fruit fly rearing procedures affect the vertical transmission of the bacterial symbiont Candidatus Erwinia dacicola. bioRxiv 2018, 367417. [Google Scholar] [CrossRef]
- Bigiotti, G.; Pastorelli, R.; Guidi, R.; Belcari, A.; Sacchetti, P. Horizontal transfer and finalization of a reliable detection method for the olive fruit fly endosymbiont, Candidatus Erwinia dacicolax. bioRxiv 2018, 326090. [Google Scholar] [CrossRef]
- Petri, L. Ricerche Sopra i Batteri Intestinali Della Mosca Olearia; Tipografia nazionale di G. Bertero: Roma, Italy, 1909. [Google Scholar]
- Konstantopoulos, D.; Cosmidis, N. Letter to the Editor: An integrated pipeline for the pest management of Bactrocera oleae. J. Mol. Biochem. 2019, 8, 13–14. [Google Scholar]
- Estes, A.M.; Hearn, D.J.; Agrawal, S.; Pierson, E.A.; Dunning Hotopp, J.C. Comparative genomics of the Erwinia and Enterobacter olive fly endosymbionts. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Pavlidi, N.; Dermauw, W.; Rombauts, S.; Chrisargiris, A.; Van Leeuwen, T.; Vontas, J. Analysis of the olive fruit fly Bactrocera oleae transcriptome and phylogenetic classification of the major detoxification gene families. PLoS ONE 2013, 8, e66533. [Google Scholar] [CrossRef]
- Blow, F.; Gioti, A.; Starns, D.; Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Vontas, J.; Darby, A.C. Draft genome sequence of the Bactrocera oleae symbiont “Candidatus Erwinia dacicola.”. Genome Announc. 2016, 4, e00896-16. [Google Scholar] [CrossRef]
- Apostolaki, A.; Livadaras, I.; Saridaki, A.; Chrysargyris, A.; Savakis, C.; Bourtzis, K. Transinfection of the olive fruit fly Bactrocera oleae with Wolbachia: Towards a symbiont-based population control strategy. J. Appl. Entomol. 2011, 135, 546–553. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Tsitsipis, J.A. An improved method for the mass rearing of the olive fruit fly, Dacus oleae (Gmel.) (Diptera, Tephritidae). Zeitschrift für Angew. Entomol. 1977, 83, 419–426. [Google Scholar] [CrossRef]
- Tsitsipis, J.A. Mass rearing of the olive fruit fly, Dacus oleae (Gmelin), at “Democritos.”. In Controlling Fruit Flies by the Sterile-Insect Technique; IAEA: Vienna, Austria, 1975; pp. 93–100. [Google Scholar]
- Tzanakakis, M.E.; Economopoulos, A.P.; Tsitsipis, J.A. Rearing and nutrition of the olive fruit fly. 1. Improved larval diet and simple containers. J. Econ. Entomol. 1970, 63, 317–318. [Google Scholar] [CrossRef]
- Dimou, I.; Rempoulakis, P.; Economopoulos, A.P. Olive fruit fly [Bactrocera (Dacus) oleae (Rossi) (Diptera: Tephritidae)] adult rearing diet without antibiotic. J. Appl. Entomol. 2010, 134, 72–79. [Google Scholar] [CrossRef]
- Ahmad, S.; ul Haq, I.; Rempoulakis, P.; Orozco, D.; Jessup, A.; Caceres, C.; Paulus, H.; Vreysen, M.J.B. Artificial rearing of the olive fruit fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) for use in the Sterile Insect Technique: Improvements of the egg collection system. Int. J. Ind. Entomol. 2016, 33, 15–23. [Google Scholar] [CrossRef]
- Baratella, V.; Pucci, C.; Paparatti, B.; Speranza, S. Response of Bactrocera oleae to different photoperiods and temperatures using a novel method for continuous laboratory rearing. Biol. Control 2017, 110, 79–88. [Google Scholar] [CrossRef]
- Estes, A.M.; Nestel, D.; Belcari, A.; Jessup, A.; Rempoulakis, P.; Economopoulos, A.P. A basis for the renewal of sterile insect technique for the olive fly, Bactrocera oleae (Rossi). J. Appl. Entomol. 2012, 136, 1–16. [Google Scholar] [CrossRef]
- Ras, E.; Beukeboom, L.W.; Carlos, C. Review of the role of gut microbiota in mass rearing of the olive fruit fly, Bactrocera oleae, and its parasitoids. Entomol. Exp. Appl. 2017, 164, 237–256. [Google Scholar] [CrossRef]
- Beard, C.B.; Durvasula, R.V.; Richards, F.F. Bacterial symbiosis in arthropods and the control of disease transmission. Emerg. Infect. Dis. 1998, 4, 581–591. [Google Scholar] [CrossRef]
- Whitten, M.; Dyson, P. Gene silencing in non-model insects: Overcoming hurdles using symbiotic bacteria for trauma-free sustainable delivery of RNA interference: Sustained RNA interference in insects mediated by symbiotic bacteria: Applications as a genetic tool and as a biocid. BioEssays 2017, 39, 1600247. [Google Scholar] [CrossRef]
- Jurkevitch, E. Riding the Trojan horse: Combating pest insects with their own symbionts. Microb. Biotechnol. 2011, 4, 620–627. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobre, T. Symbiosis in Sustainable Agriculture: Can Olive Fruit Fly Bacterial Microbiome Be Useful in Pest Management? Microorganisms 2019, 7, 238. https://doi.org/10.3390/microorganisms7080238
Nobre T. Symbiosis in Sustainable Agriculture: Can Olive Fruit Fly Bacterial Microbiome Be Useful in Pest Management? Microorganisms. 2019; 7(8):238. https://doi.org/10.3390/microorganisms7080238
Chicago/Turabian StyleNobre, Tânia. 2019. "Symbiosis in Sustainable Agriculture: Can Olive Fruit Fly Bacterial Microbiome Be Useful in Pest Management?" Microorganisms 7, no. 8: 238. https://doi.org/10.3390/microorganisms7080238