A Sensitive and Rapid Method to Determine the Adhesion Capacity of Probiotics and Pathogenic Microorganisms to Human Gastrointestinal Mucins
Abstract
:1. Introduction
2. Methods
2.1. Bacteria and Yeast Culture
2.2. Isolation and Purification of Mucins from Human Tissues and Cell Lines
2.3. Release of Oligosaccharides from Mucin by Alkaline Borohydride Treatment
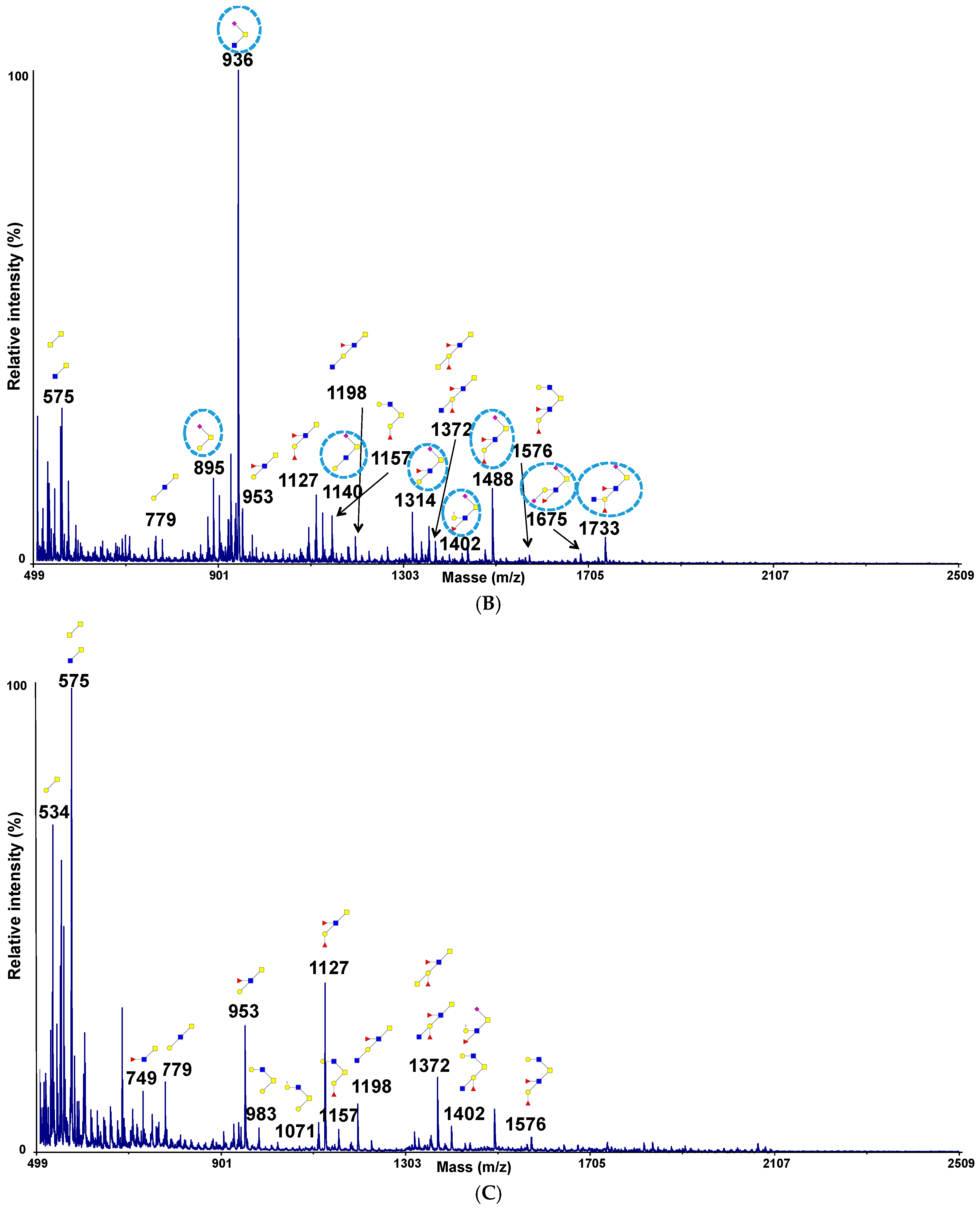
2.4. Permethylation and Mucin Glycosylation Analysis by MALDI TOF MS
2.5. 1-D Bacterial Overlay
2.6. Statistical Analysis
3. Results
3.1. Development of a New Assay to Evaluate the Adhesion of Microorganisms on Mucins
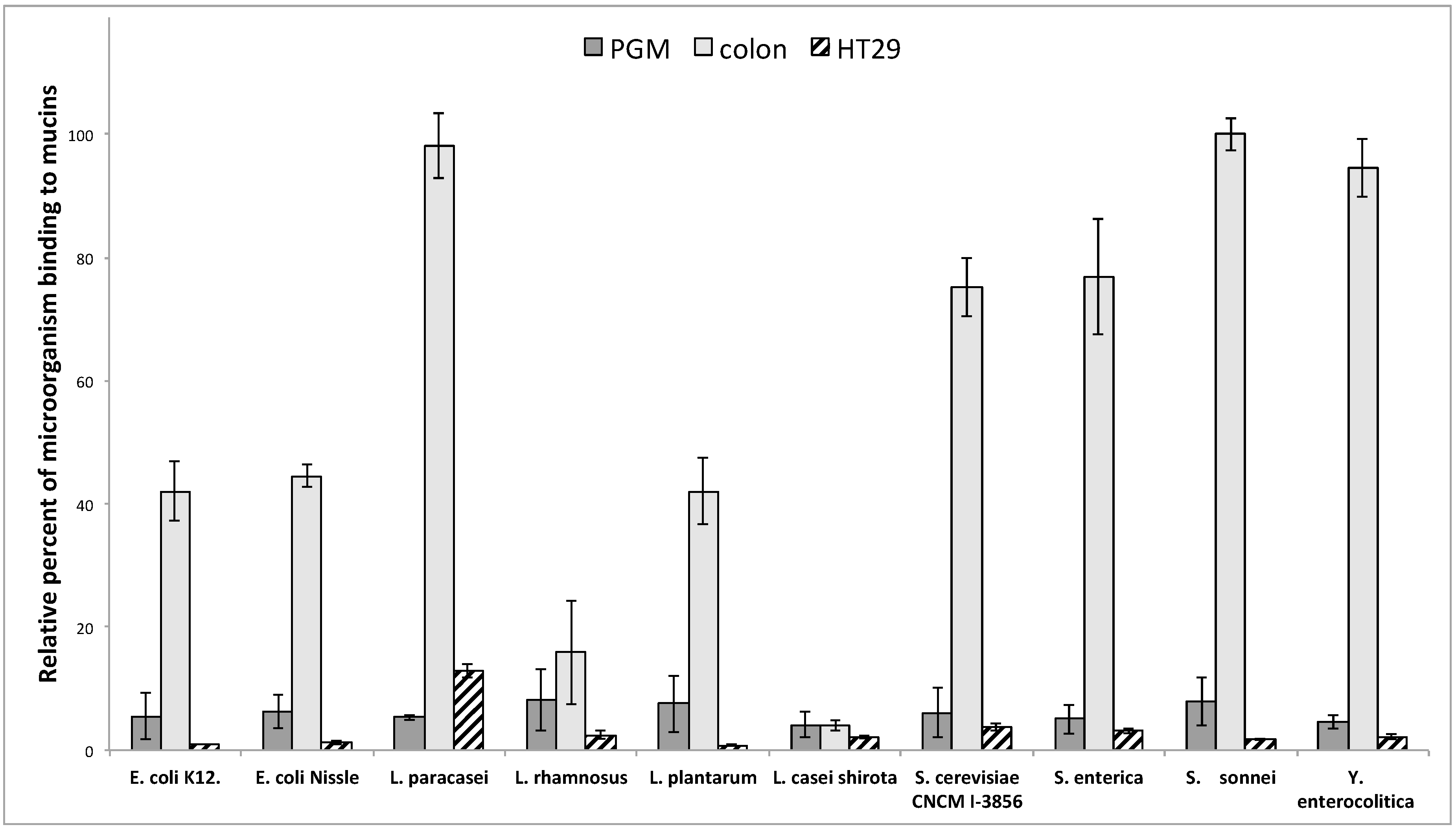
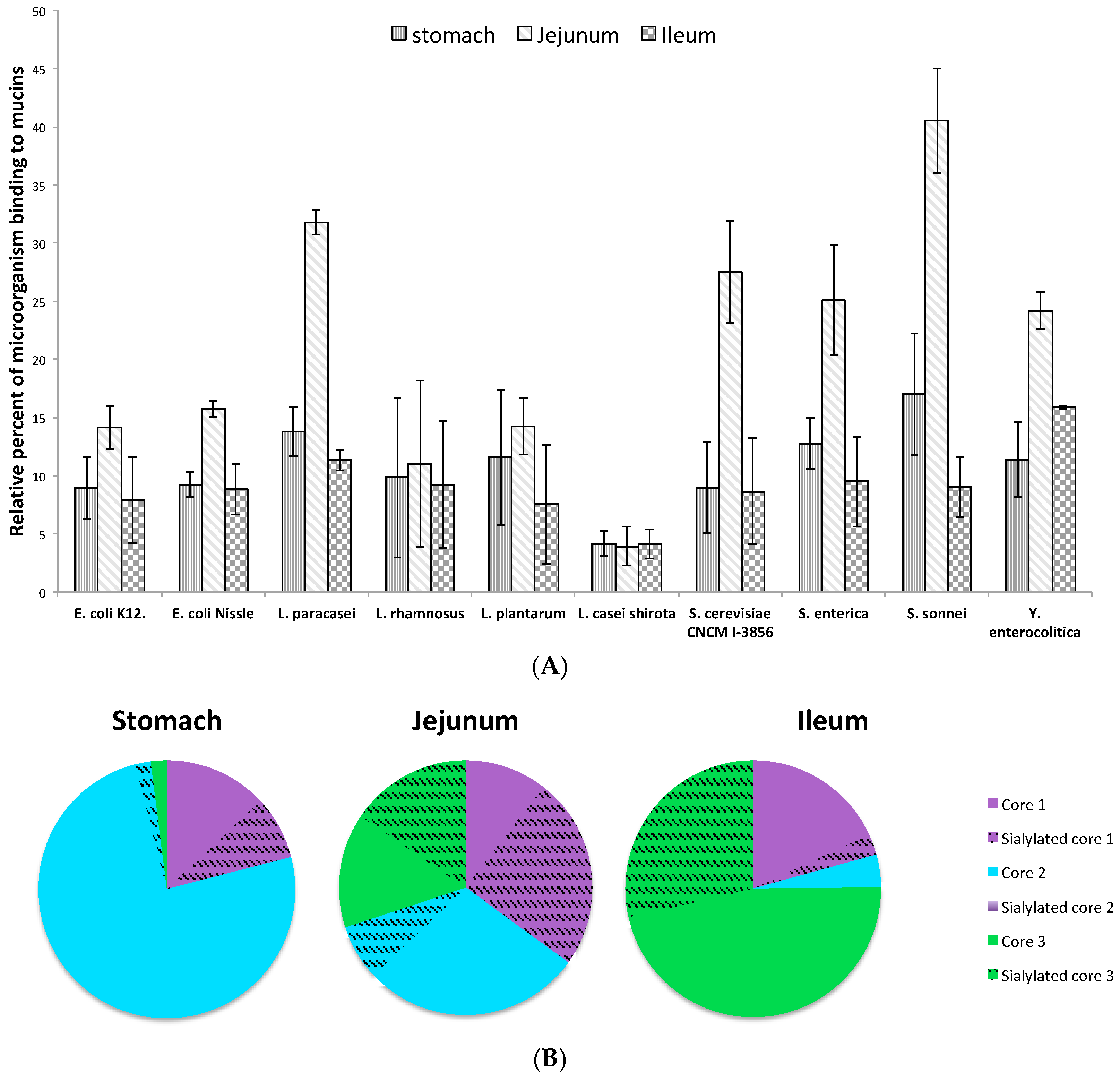
3.2. Adhesion of Microorganisms on Commercially Available Pig Gastric Mucins (PGM) or Mucins from HT29-MTX Cell Lines Does Not Reflect Adhesion on Human Intestinal Mucins
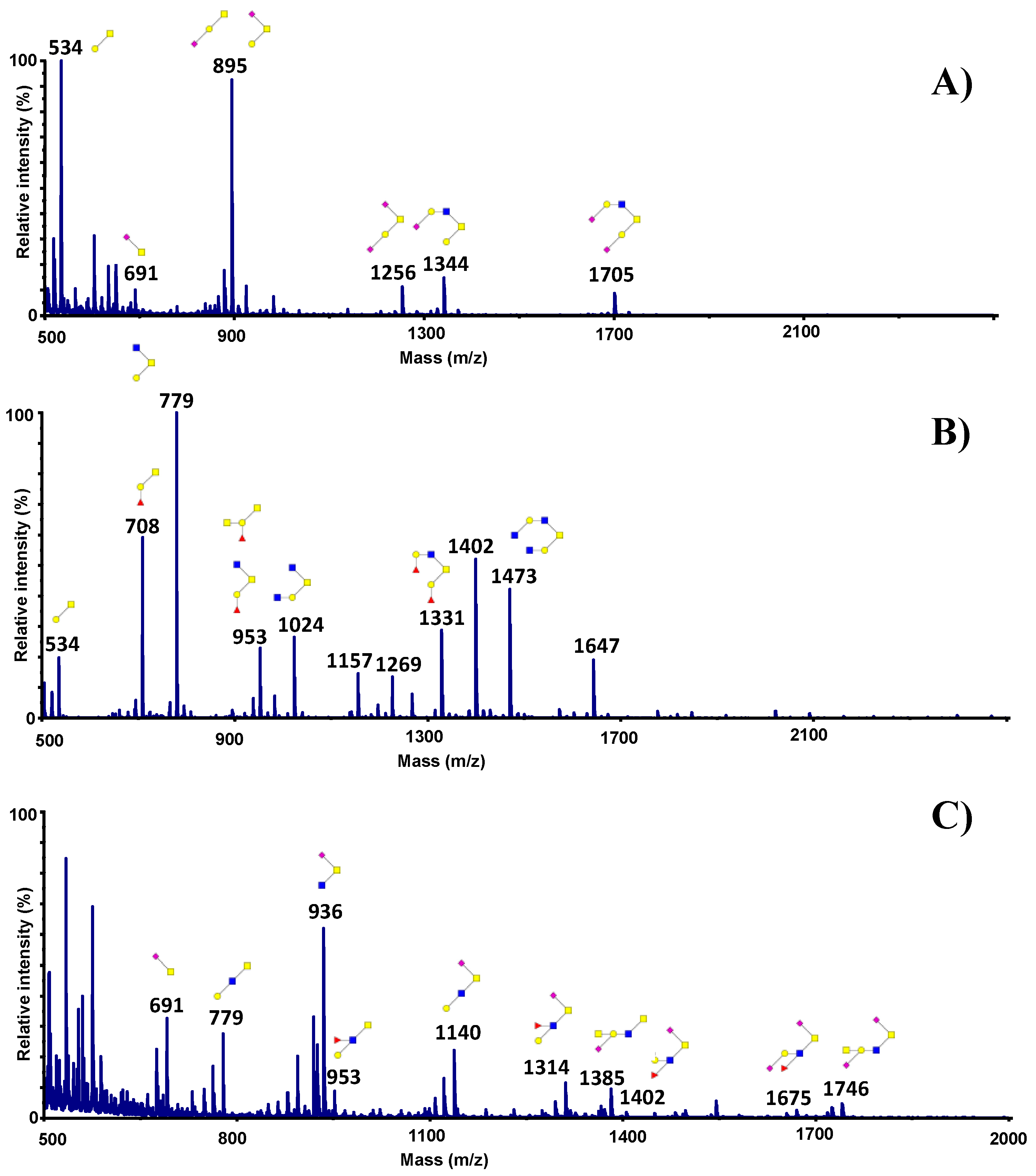
3.3. Influence of Sialic Acid Residues and/or Core Structure on Bacterial and Yeast Adhesion to Human Mucins
3.4. Adhesion of Microorganisms to Mucins Purified along the Gastrointestinal Tract
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Gill, H.S.; Chandra, R.K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur. J. Clin. Nutr. 2000, 54, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J. Clin. Immunol. 2001, 21, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.; Cornish, A.; Soper, P.; McKaigney, C.; Jijon, H.; Yachimec, C.; Doyle, J.; Jewell, L.; De Simone, C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001, 121, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Ben Braïek, O.; Cremonesi, P.; Morandi, S.; Smaoui, S.; Hani, K.; Ghrairi, T. Safety characterisation and inhibition of fungi and bacteria by a novel multiple enterocin-producing Enterococcus lactis 4CP3 strain. Microb. Pathog. 2018, 118, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Vasilchenko, A.V.; Valyshev, A.V.; Rogozhin, E.A. A Novel High-Molecular-Mass Bacteriocin Produced by Enterococcus faecium: Biochemical Features and Mode of Action. Probiotics Antimicrob. Proteins 2018. [Google Scholar] [CrossRef] [PubMed]
- Hecht, A.L.; Casterline, B.W.; Earley, Z.M.; Goo, Y.A.; Goodlett, D.R.; Bubeck Wardenburg, J. Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep. 2016, 17, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Adams, M.; La Ragione, R.M.; Woodward, M.J. Colonisation of poultry by Salmonella Enteritidis S1400 is reduced by combined administration of Lactobacillus salivarius 59 and Enterococcus faecium PXN-33. Vet. Microbiol. 2017, 199, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S. Bacteria, colonic fermentation, and gastrointestinal health. J. AOAC Int. 2012, 95, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ramesh, A. Bacteriocin-producing strains of Lactobacillus plantarum inhibit adhesion of Staphylococcus aureus to extracellular matrix: Quantitative insight and implications in antibacterial therapy. J. Med. Microbiol. 2015, 64, 1514–1526. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Ambort, D.; Pelaseyed, T.; Schütte, A.; Gustafsson, J.K.; Ermund, A.; Subramani, D.B.; Holmén-Larsson, J.M.; Thomsson, K.A.; Bergström, J.H.; et al. Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 2011, 68, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Atuma, C.; Strugala, V.; Allen, A.; Holm, L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G922–G929. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Desseyn, J.L.; Aubert, J.P.; Porchet, N.; Laine, A. Evolution of the large secreted gel-forming mucins. Mol. Biol. Evol. 2000, 17, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.B.; Roberton, A.M.; Shekels, L.L.; Lyftogt, C.T.; Niehans, G.A.; Toribara, N.W. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology 1995, 109, 735–747. [Google Scholar] [CrossRef]
- Reis, C.A.; David, L.; Nielsen, P.A.; Clausen, H.; Mirgorodskaya, K.; Roepstorff, P.; Sobrinho-Simões, M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int. J. Cancer 1997, 74, 112–121. [Google Scholar] [CrossRef]
- De Bolós, C.; Garrido, M.; Real, F.X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology 1995, 109, 723–734. [Google Scholar] [CrossRef]
- Bartman, A.E.; Buisine, M.P.; Aubert, J.P.; Niehans, G.A.; Toribara, N.W.; Kim, Y.S.; Kelly, E.J.; Crabtree, J.E.; Ho, S.B. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J. Pathol. 1998, 186, 398–405. [Google Scholar] [CrossRef]
- Van Klinken, B.J.; Dekker, J.; van Gool, S.A.; van Marle, J.; Büller, H.A.; Einerhand, A.W. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am. J. Physiol. 1998, 274, G871–G878. [Google Scholar] [CrossRef] [PubMed]
- Robbe, C.; Capon, C.; Maes, E.; Rousset, M.; Zweibaum, A.; Zanetta, J.-P.; Michalski, J.-C. Evidence of regio-specific glycosylation in human intestinal mucins: Presence of an acidic gradient along the intestinal tract. J. Biol. Chem. 2003, 278, 46337–46348. [Google Scholar] [CrossRef] [PubMed]
- Robbe, C.; Capon, C.; Coddeville, B.; Michalski, J.-C. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem. J. 2004, 384, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.M.H.; Karlsson, H.; Sjövall, H.; Hansson, G.C. A complex, but uniform O-glycosylation of the human MUC2 mucin from colonic biopsies analyzed by nanoLC/MSn. Glycobiology 2009, 19, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Hounsell, E.F.; Davies, M.J.; Renouf, D.V. O-linked protein glycosylation structure and function. Glycoconj. J. 1996, 13, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Carlsson, S.R.; Klock, J.C.; Dell, A. Structures of O-linked oligosaccharides isolated from normal granulocytes, chronic myelogenous leukemia cells, and acute myelogenous leukemia cells. J. Biol. Chem. 1986, 261, 12796–12806. [Google Scholar] [PubMed]
- Rossez, Y.; Maes, E.; Lefebvre Darroman, T.; Gosset, P.; Ecobichon, C.; Joncquel Chevalier Curt, M.; Boneca, I.G.; Michalski, J.-C.; Robbe-Masselot, C. Almost all human gastric mucin O-glycans harbor blood group A, B or H antigens and are potential binding sites for Helicobacter pylori. Glycobiology 2012, 22, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Capon, C.; Maes, E.; Michalski, J.C.; Leffler, H.; Kim, Y.S. Sd(a)-antigen-like structures carried on core 3 are prominent features of glycans from the mucin of normal human descending colon. Biochem. J. 2001, 358, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, D.K. Oligosaccharide structures of human colonic mucin. J. Biol. Chem. 1985, 260, 8262–8271. [Google Scholar] [PubMed]
- Kruis, W.; Schütz, E.; Fric, P.; Fixa, B.; Judmaier, G.; Stolte, M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 1997, 11, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Henker, J.; Laass, M.; Blokhin, B.M.; Bolbot, Y.K.; Maydannik, V.G.; Elze, M.; Wolff, C.; Schulze, J. The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur. J. Pediatr. 2007, 166, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, E.; O’Toole, P.W. The Genomic Basis of Lactobacilli as Health-Promoting Organisms. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Wang, R.; Gong, F.-M.; Liu, X.-F.; Zheng, H.-J.; Luo, Y.-Y.; Li, X.-R. Complete genome sequences and comparative genome analysis of Lactobacillus plantarum strain 5-2 isolated from fermented soybean. Genomics 2015, 106, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Douillard, F.P.; Ribbera, A.; Xiao, K.; Ritari, J.; Rasinkangas, P.; Paulin, L.; Palva, A.; Hao, Y.; de Vos, W.M. Polymorphisms, Chromosomal Rearrangements, and Mutator Phenotype Development during Experimental Evolution of Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2016, 82, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Campieri, M.; Gionchetti, P. Probiotics in inflammatory bowel disease: New insight to pathogenesis or a possible therapeutic alternative? Gastroenterology 1999, 116, 1246–1249. [Google Scholar] [CrossRef]
- Naito, E.; Yoshida, Y.; Kunihiro, S.; Makino, K.; Kasahara, K.; Kounoshi, Y.; Aida, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Effect of Lactobacillus caseistrain Shirota-fermented milk on metabolic abnormalities in obese prediabetic Japanese men: A randomised, double-blind, placebo-controlled trial. Biosci. Microbiota Food Health 2018, 37, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.; Pélerin, F.; Cayzeele Decherf, A.; Maudet, C.; Housez, B.; Cazaubiel, M.; Jüsten, P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United Eur. Gastroenterol. J. 2016, 4, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Aber, R.C. Yersinia enterocolitica. N. Engl. J. Med. 1989, 321, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Saebø, A.; Lassen, J. Acute and chronic gastrointestinal manifestations associated with Yersinia enterocolitica infection. A Norwegian 10-year follow-up study on 458 hospitalized patients. Ann. Surg. 1992, 215, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Gossman, W.G. Shigella (Shigellosis). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Zhang, J.S.; Guri, A.; Corredig, M.; Morales-Rayas, R.; Hassan, A.; Griffiths, M.; LaPointe, G. Lactococcus lactis subsp. cremoris strain JFR1 attenuates Salmonella adhesion to human intestinal cells in vitro. Food Res. Int. 2016, 90, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Odenbreit, S.; Swoboda, K.; Barwig, I.; Ruhl, S.; Borén, T.; Koletzko, S.; Haas, R. Outer Membrane Protein Expression Profile in Helicobacter pylori Clinical Isolates. Infect. Immun. 2009, 77, 3782–3790. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Derevitskaya, V.A.; Arbatsky, N.P.; Kochetkov, N.K. The structure of carbohydrate chains of blood-group substance. Isolation and elucidation of the structure of higher oligosaccharides from blood group substance H. Eur. J. Biochem. 1978, 86, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, N.G.; Nordman, H.; Karlsson, H.; Carlstedt, I.; Hansson, G.C. Glycosylation differences between pig gastric mucin populations: A comparative study of the neutral oligosaccharides using mass spectrometry. Biochem. J. 1997, 326, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Thomsson, K.A.; Karlsson, N.G.; Hansson, G.C. Liquid chromatography-electrospray mass spectrometry as a tool for the analysis of sulfated oligosaccharides from mucin glycoproteins. J. Chromatogr. A 1999, 854, 131–139. [Google Scholar] [CrossRef]
- Lee, Y.K.; Salminien, S. The coming of age of probiotics. Trends Food Sci. Technol. 1995, 6, 241–245. [Google Scholar] [CrossRef]
- Andreĭchin, M.A. [Bacterial lesions of the biliary tract in viral hepatitis]. Zhurnal Mikrobiol. Epidemiol. Immunobiol. 1980, 246, 72–76. [Google Scholar]
- Begley, M.; Gahan, C.G.M.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Coeuret, V.; Gueguen, M.; Vernoux, J.P. Numbers and strains of lactobacilli in some probiotic products. Int. J. Food Microbiol. 2004, 97, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Azcarate-Peril, M.A.; Altermann, E.; Hoover-Fitzula, R.L.; Cano, R.J.; Klaenhammer, T.R. Identification and inactivation of genetic loci involved with Lactobacillus acidophilus acid tolerance. Appl. Environ. Microbiol. 2004, 70, 5315–5322. [Google Scholar] [CrossRef] [PubMed]
- Bruno-Bárcena, J.M.; Andrus, J.M.; Libby, S.L.; Klaenhammer, T.R.; Hassan, H.M. Expression of a heterologous manganese superoxide dismutase gene in intestinal lactobacilli provides protection against hydrogen peroxide toxicity. Appl. Environ. Microbiol. 2004, 70, 4702–4710. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Zavala, L.; Carasi, P.; Trejo, S.A.; Bronsoms, S.; Serradell, M.L.Á.; Garrote, G.L.; Abraham, A.G. Simulated gastrointestinal conditions increase adhesion ability of Lactobacillus paracasei strains isolated from kefir to Caco-2 cells and mucin. Food Res. Int. 2018, 103, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.D.; Tailford, L.E.; Monaco, S.; Šuligoj, T.; Vaux, L.; Lallement, R.; Khedri, Z.; Yu, H.; Lecointe, K.; Walshaw, J.; et al. Unravelling the specificity and mechanism of sialic acid recognition by the gut symbiont Ruminococcus gnavus. Nat. Commun. 2017, 8, 2196. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.-N.; Okawara, T.; Watanabe, M.; Kawai, Y.; Kitazawa, H.; Ohnuma, S.; Shibata, C.; Horii, A.; Kimura, K.; Taketomo, N.; et al. New screening methods for probiotics with adhesion properties to sialic acid and sulphate residues in human colonic mucin using the Biacore assay. J. Appl. Microbiol. 2013, 114, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Roos, S.; Jonsson, H. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology 2002, 148, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Etzold, S.; Kober, O.I.; Mackenzie, D.A.; Tailford, L.E.; Gunning, A.P.; Walshaw, J.; Hemmings, A.M.; Juge, N. Structural basis for adaptation of lactobacilli to gastrointestinal mucus. Environ. Microbiol. 2014, 16, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Phansopa, C.; Roy, S.; Rafferty, J.B.; Douglas, C.W.I.; Pandhal, J.; Wright, P.C.; Kelly, D.J.; Stafford, G.P. Structural and functional characterization of NanU, a novel high-affinity sialic acid-inducible binding protein of oral and gut-dwelling Bacteroidetes species. Biochem. J. 2014, 458, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Joncquel Chevalier Curt, M.; Lecointe, K.; Mihalache, A.; Rossez, Y.; Gosset, P.; Léonard, R.; Robbe-Masselot, C. Alteration or adaptation, the two roads for human gastric mucin glycosylation infected by Helicobacter pylori. Glycobiology 2015, 25, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Marcos, N.T.; Magalhães, A.; Ferreira, B.; Oliveira, M.J.; Carvalho, A.S.; Mendes, N.; Gilmartin, T.; Head, S.R.; Figueiredo, C.; David, L.; et al. Helicobacter pylori induces beta3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J. Clin. Investig. 2008, 118, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; Marcos-Pinto, R.; Nairn, A.V.; dela Rosa, M.; Ferreira, R.M.; Junqueira-Neto, S.; Freitas, D.; Gomes, J.; Oliveira, P.; Santos, M.R.; et al. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, J.; Sondén, B.; Hurtig, M.; Olfat, F.O.; Forsberg, L.; Roche, N.; Angstrom, J.; Larsson, T.; Teneberg, S.; Karlsson, K.-A.; et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 2002, 297, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Bensing, B.A.; Khedri, Z.; Deng, L.; Yu, H.; Prakobphol, A.; Fisher, S.J.; Chen, X.; Iverson, T.M.; Varki, A.; Sullam, P.M. Novel aspects of sialoglycan recognition by the Siglec-like domains of streptococcal SRR glycoproteins. Glycobiology 2016, 26, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Urano-Tashiro, Y.; Takahashi, Y.; Oguchi, R.; Konishi, K. Correction: Two Arginine Residues of Streptococcus gordonii Sialic Acid-Binding Adhesin Hsa Are Essential for Interaction to Host Cell Receptors. PLoS ONE 2016, 11, e0161900. [Google Scholar] [CrossRef] [PubMed]
- Moonens, K.; Bouckaert, J.; Coddens, A.; Tran, T.; Panjikar, S.; De Kerpel, M.; Cox, E.; Remaut, H.; De Greve, H. Structural insight in histo-blood group binding by the F18 fimbrial adhesin FedF. Mol. Microbiol. 2012, 86, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Garrido, D.; Kim, J.H.; German, J.B.; Raybould, H.E.; Mills, D.A. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS ONE 2011, 6, e17315. [Google Scholar] [CrossRef]
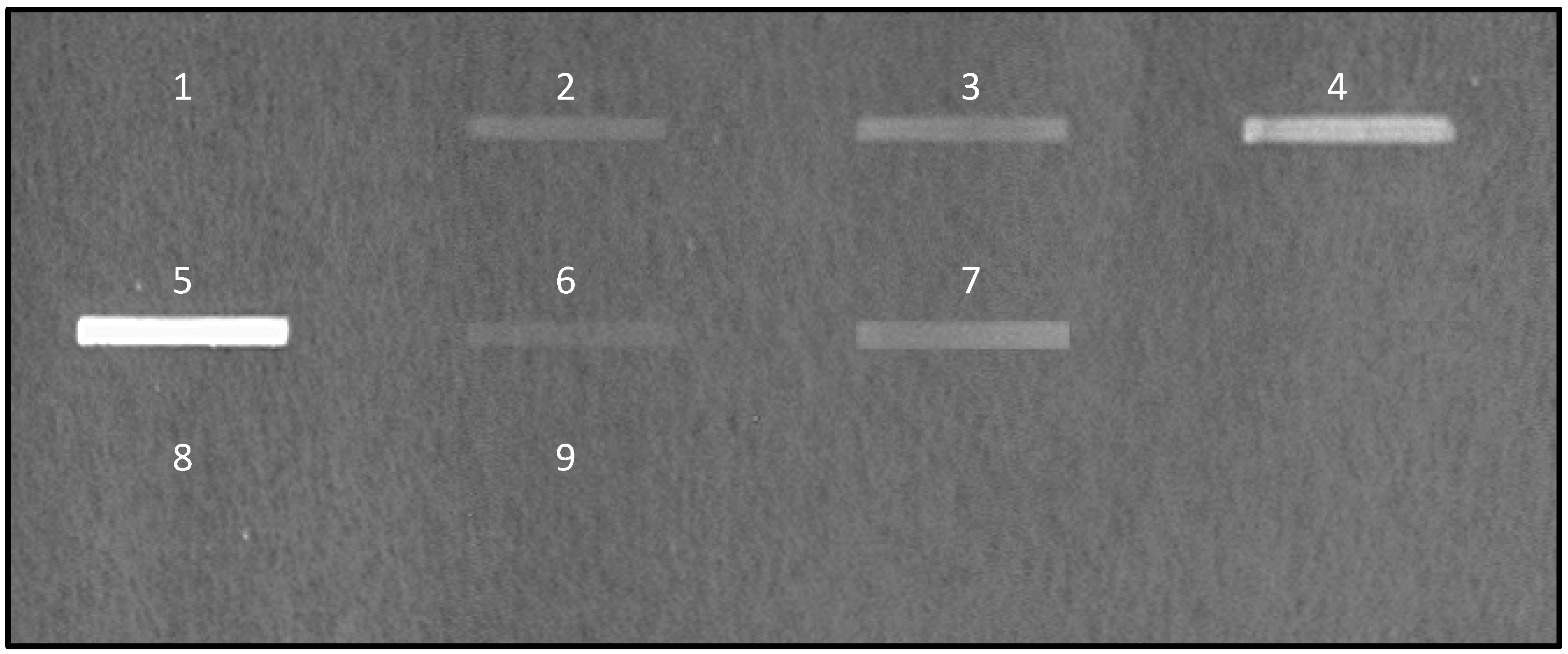
 ) were spotted at different quantities on nitrocellulose membrane and the signal of bound fluorescent-labeled E. coli K12 was quantified. Above 20 μg of mucins per spot of 9 mm2, the signal reaches a plateau.
) were spotted at different quantities on nitrocellulose membrane and the signal of bound fluorescent-labeled E. coli K12 was quantified. Above 20 μg of mucins per spot of 9 mm2, the signal reaches a plateau.
 ) were spotted at different quantities on nitrocellulose membrane and the signal of bound fluorescent-labeled E. coli K12 was quantified. Above 20 μg of mucins per spot of 9 mm2, the signal reaches a plateau.
) were spotted at different quantities on nitrocellulose membrane and the signal of bound fluorescent-labeled E. coli K12 was quantified. Above 20 μg of mucins per spot of 9 mm2, the signal reaches a plateau.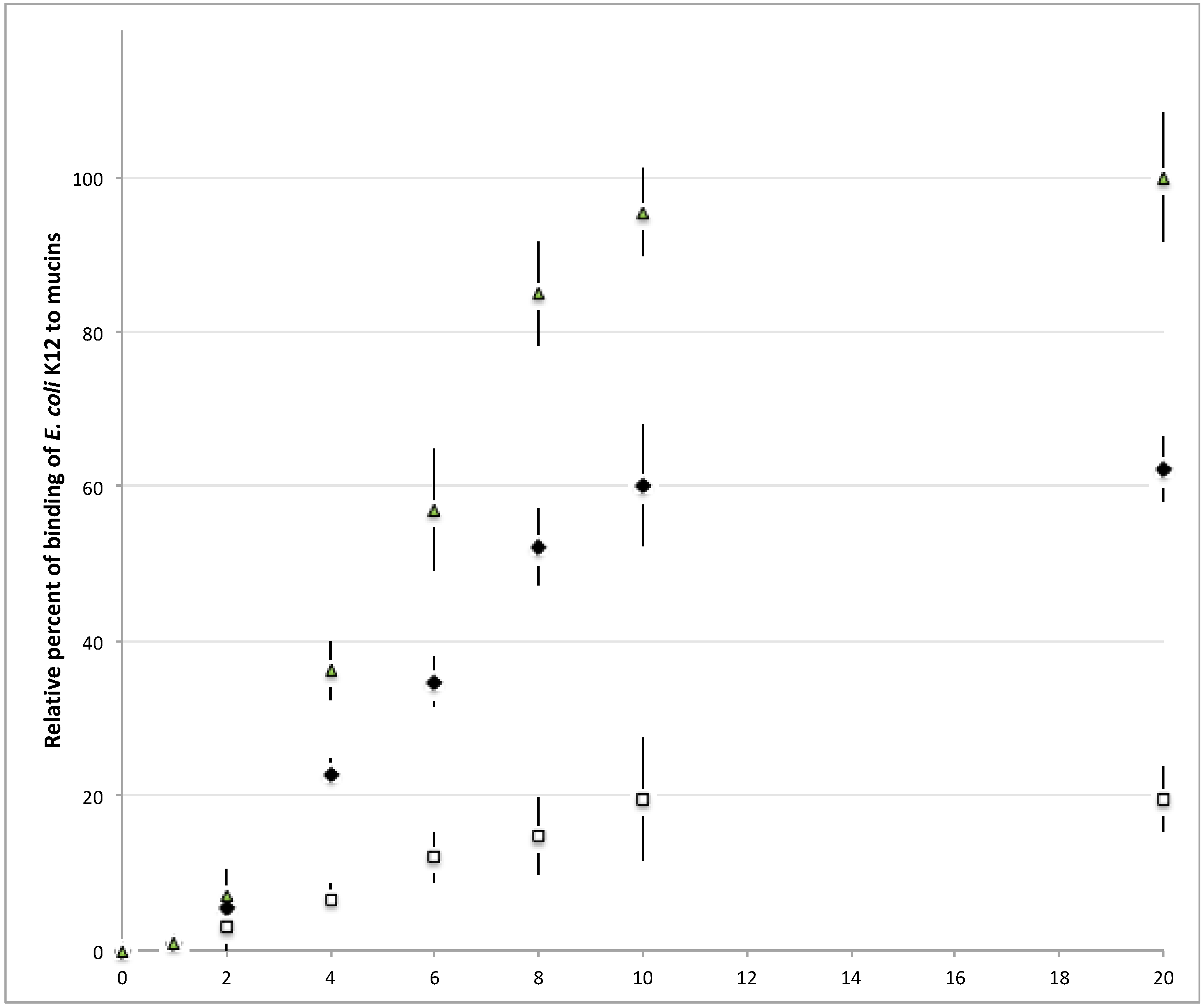


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ringot-Destrez, B.; D’Alessandro, Z.; Lacroix, J.-M.; Mercier-Bonin, M.; Léonard, R.; Robbe-Masselot, C. A Sensitive and Rapid Method to Determine the Adhesion Capacity of Probiotics and Pathogenic Microorganisms to Human Gastrointestinal Mucins. Microorganisms 2018, 6, 49. https://doi.org/10.3390/microorganisms6020049
Ringot-Destrez B, D’Alessandro Z, Lacroix J-M, Mercier-Bonin M, Léonard R, Robbe-Masselot C. A Sensitive and Rapid Method to Determine the Adhesion Capacity of Probiotics and Pathogenic Microorganisms to Human Gastrointestinal Mucins. Microorganisms. 2018; 6(2):49. https://doi.org/10.3390/microorganisms6020049
Chicago/Turabian StyleRingot-Destrez, Bélinda, Zéa D’Alessandro, Jean-Marie Lacroix, Muriel Mercier-Bonin, Renaud Léonard, and Catherine Robbe-Masselot. 2018. "A Sensitive and Rapid Method to Determine the Adhesion Capacity of Probiotics and Pathogenic Microorganisms to Human Gastrointestinal Mucins" Microorganisms 6, no. 2: 49. https://doi.org/10.3390/microorganisms6020049




