Effect of Mn Doping on the Microstructure and Electrical Properties of Potassium Niobate Ceramics Using Plasma Spraying
Abstract
:1. Introduction
2. Experimental Procedure
2.1. KNN Powder and Coating Preparation
2.2. Powder and Coating Performance Testing
3. Results and Discussion
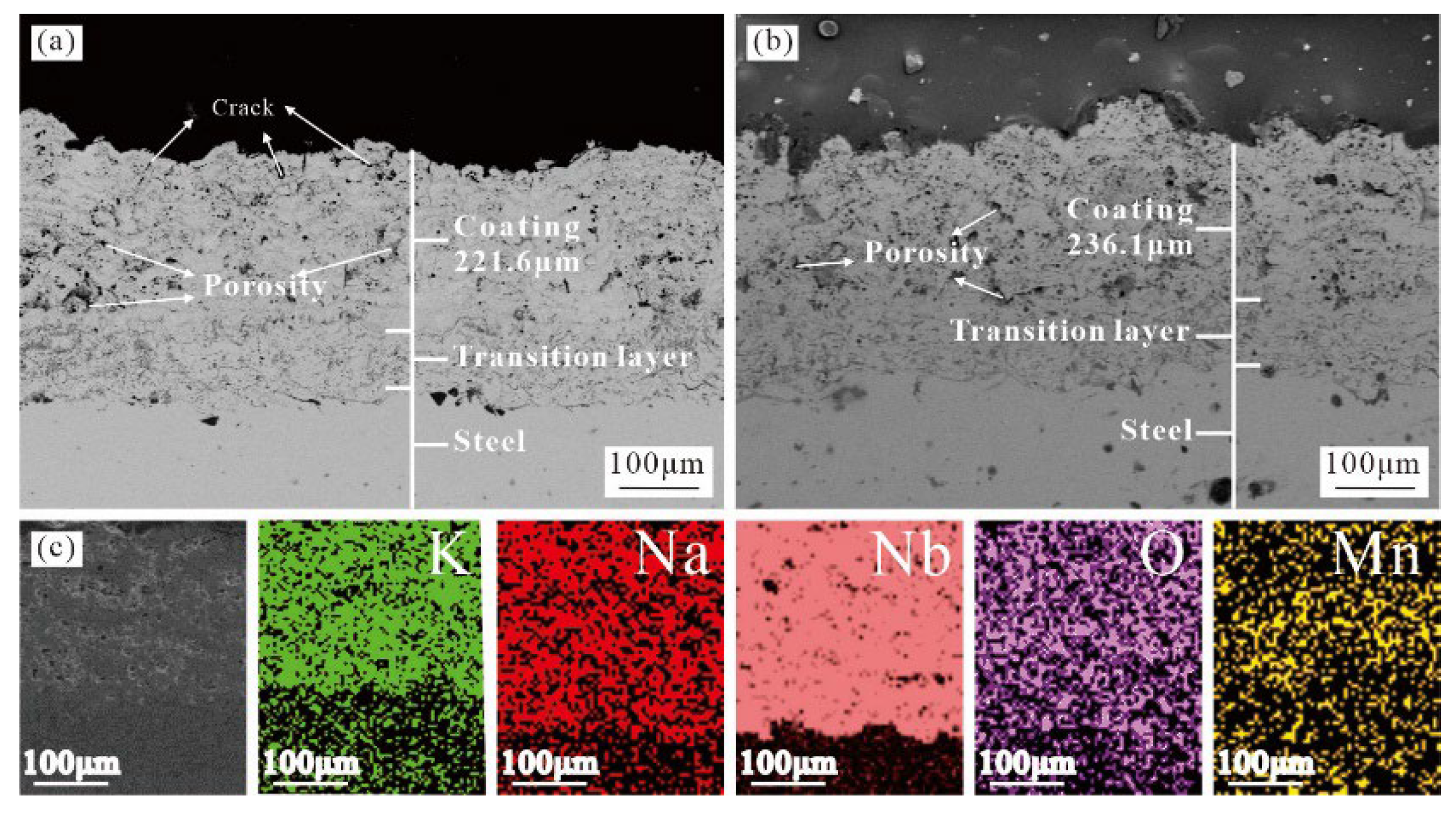
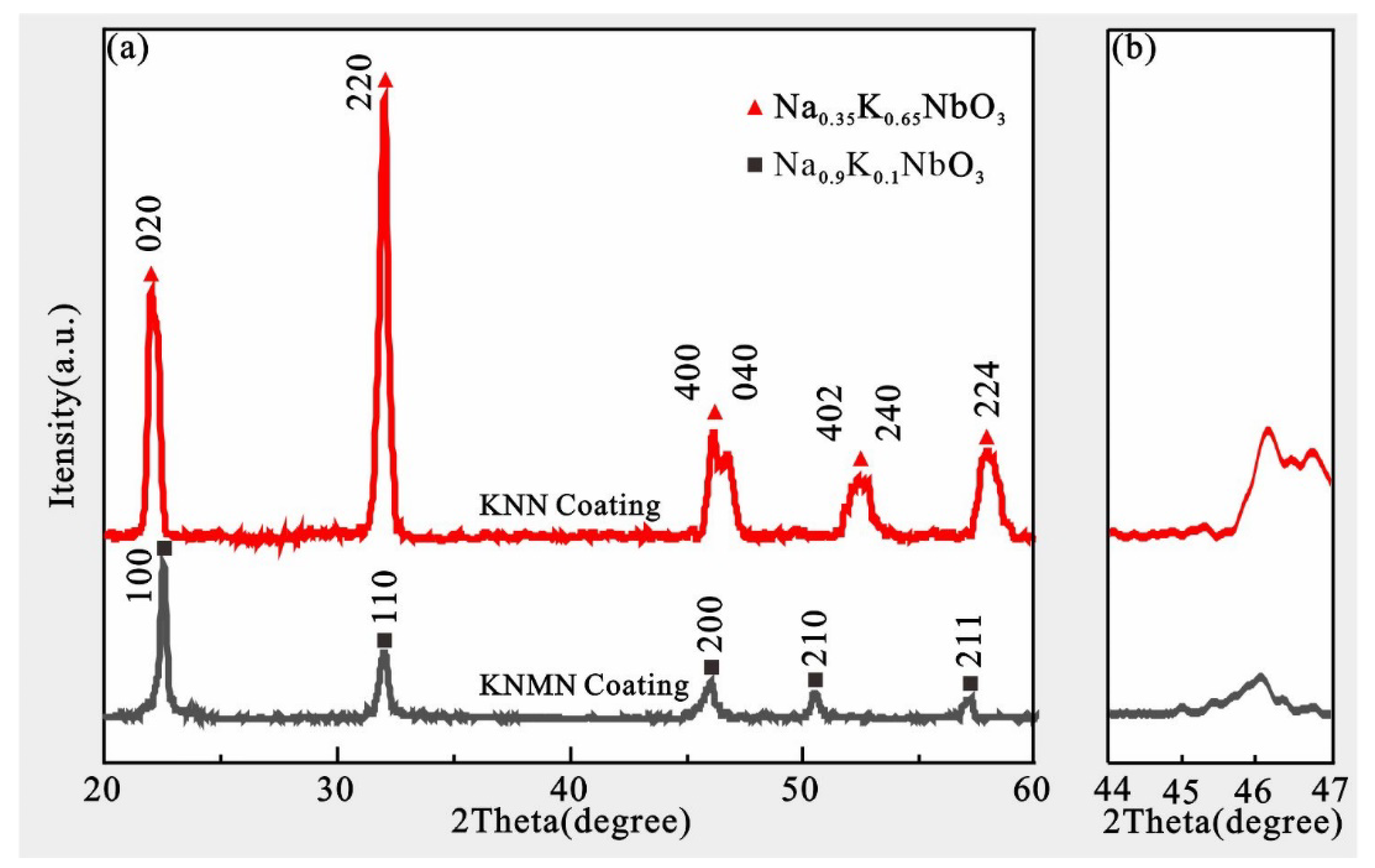
3.1. Organizational Structure and Phase Structure
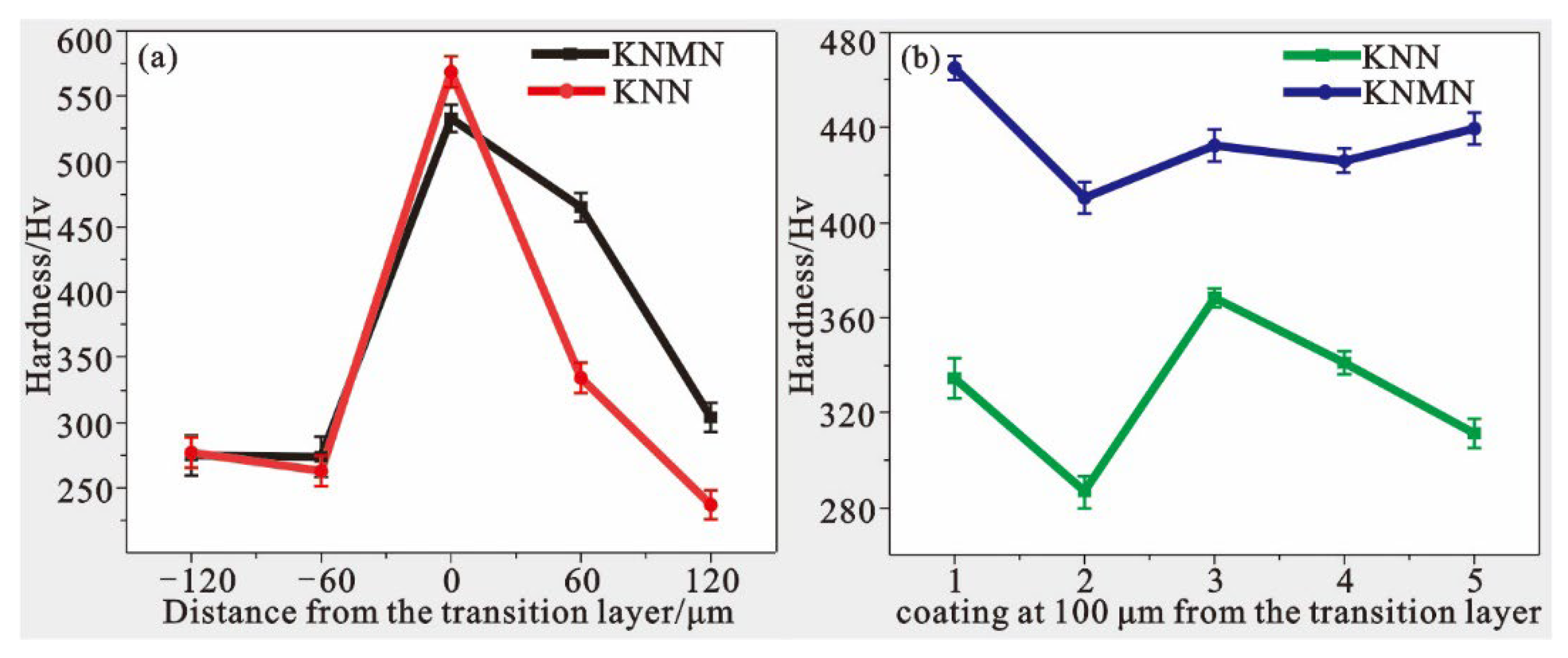
3.2. Mechanical Properties
3.3. Electrical Properties
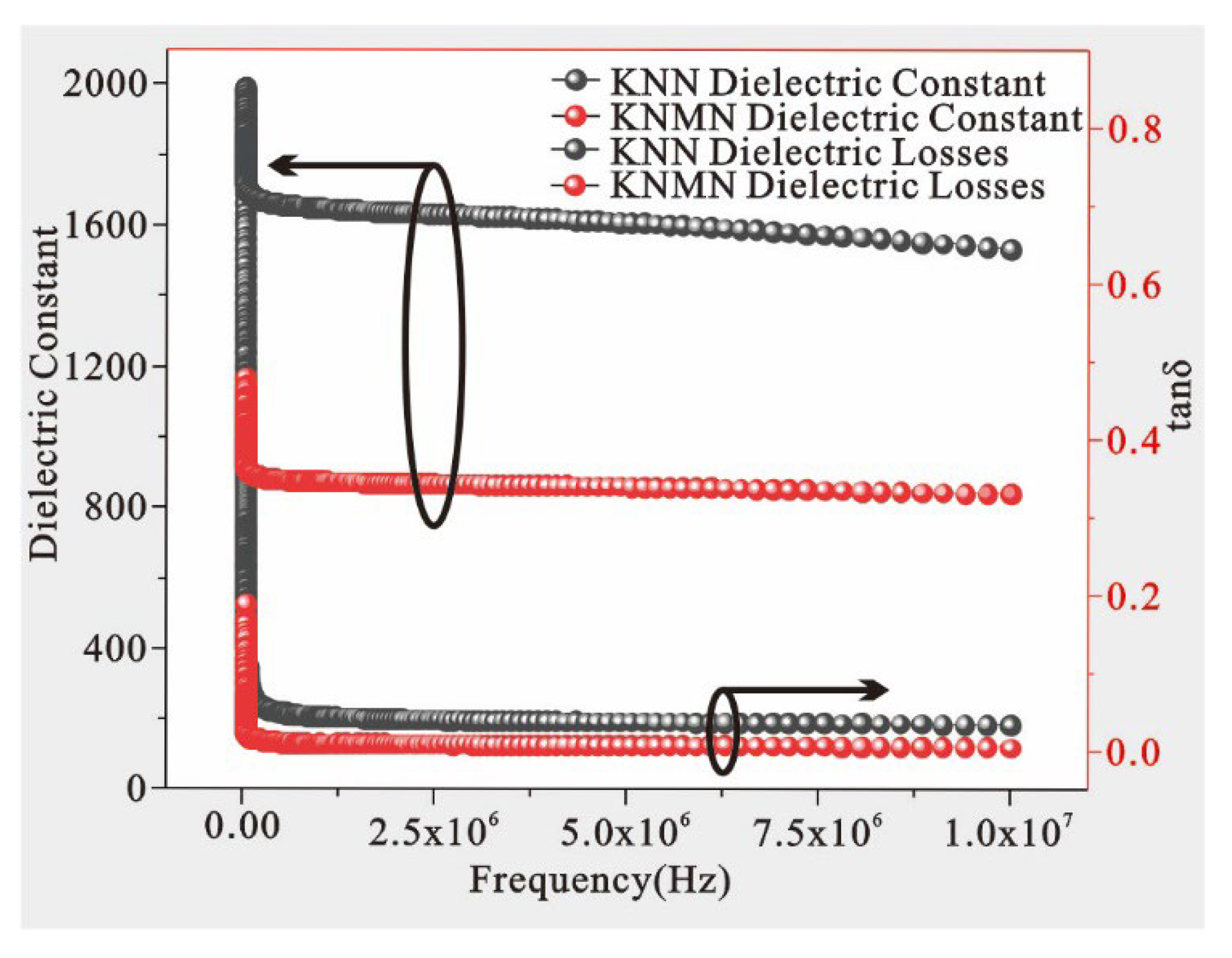
3.3.1. Dielectric Properties
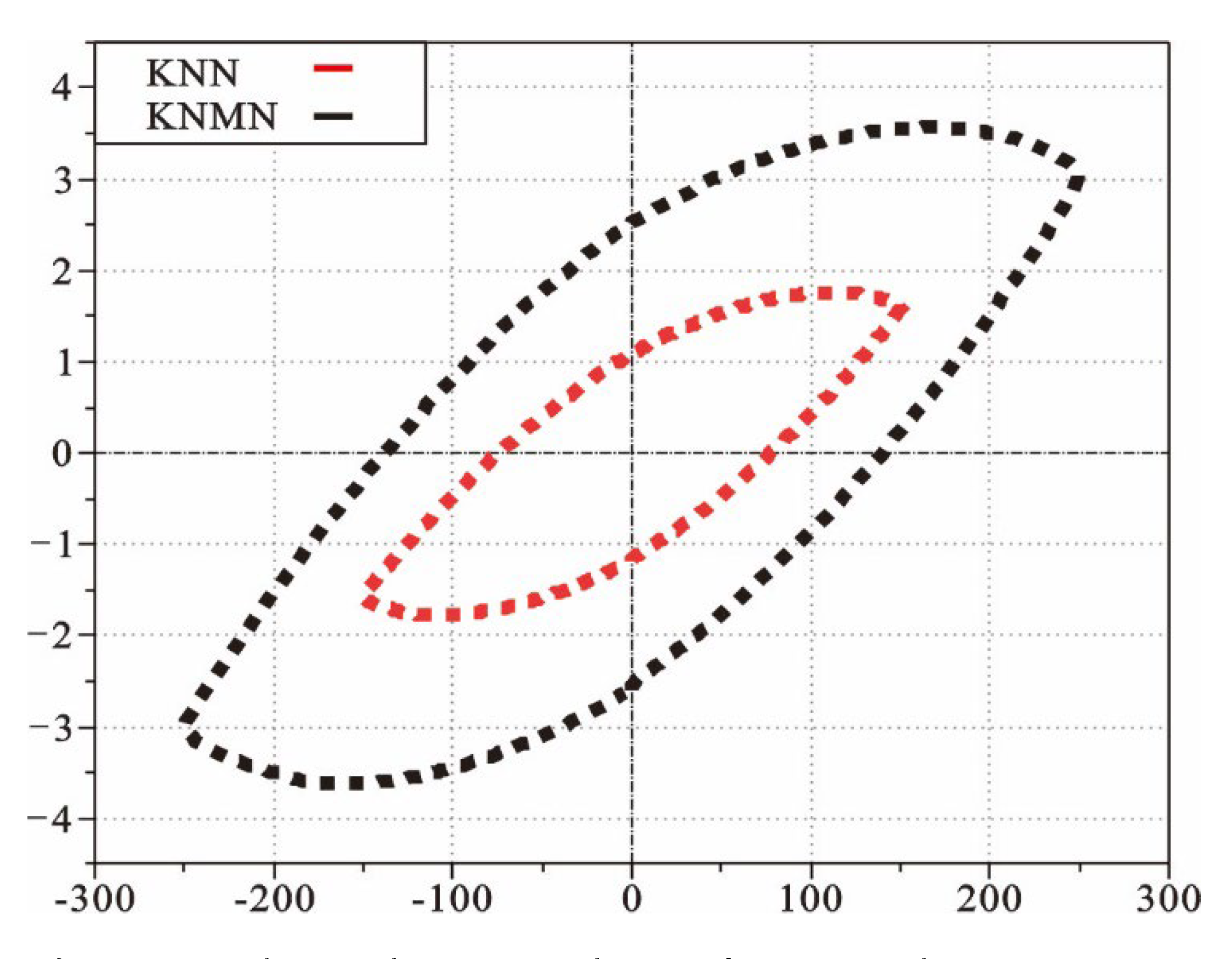
3.3.2. Ferroelectric Properties
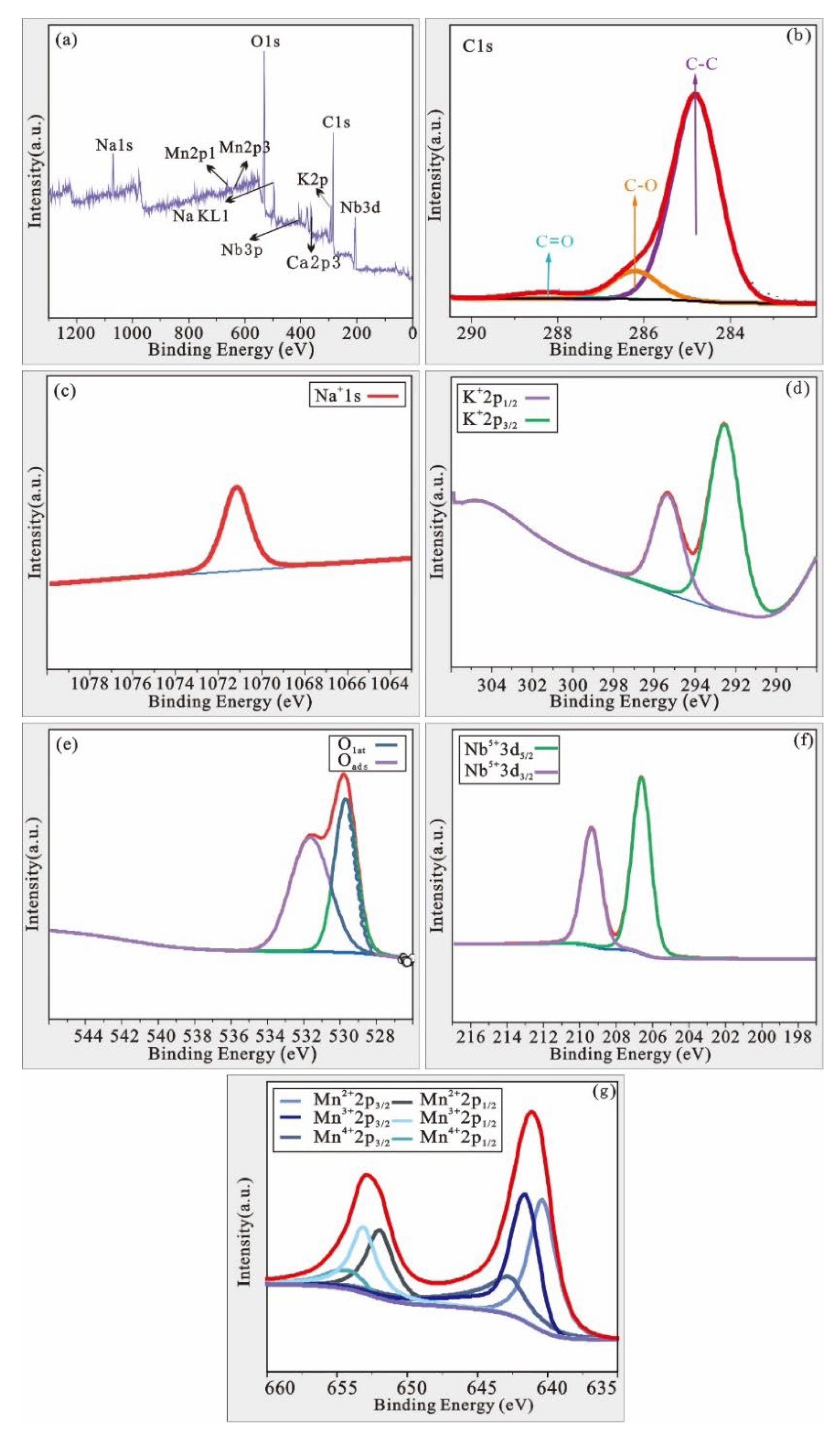
3.3.3. XPS Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shibnath, S.; Sankaranarayanan, V.; Sethupathi, K. Band gap, piezoelectricity and temperature dependence of differential permittivity and energy storage density of PZT with different Zr/Ti ratios. Vacuum 2018, 156, 456–462. [Google Scholar]
- Sun, S.D.; Liu, H.; Fan, L.L.; Ren, Y.; Xing, X.R.; Chen, J. Structural origin of size effect on piezoelectric performance of Pb(Zr,Ti)O3. Ceram. Int. 2020, 47, 5256–5264. [Google Scholar] [CrossRef]
- Samanta, S.; Sankaranarayanan, V.; Sethupathi, K.; Rao, M.S.R. Enhanced ferroelectricity in PLZT ceramic by precise La-doping, minimizing pyrochlore phase and lead loss. Vacuum 2018, 157, 514–523. [Google Scholar] [CrossRef]
- Pereira, M.; Boerasu, I.; Gomes, M. Characterization of Nb-doped PZT (65/35/1) ferroelectric thin films deposited by pulsed laser ablation. Vacuum 2008, 82, 1379–1382. [Google Scholar] [CrossRef]
- Li, X.W.; Wang, H.X.; Shi, T.; Zhang, C.W.; Jiang, X.N.; Zhou, X.G.; Li, C. Efficient Preparation and Anticorrosion Mechanism of Superhydrophobic 7075 Aviation Aluminum Alloy. Rare Met. Mater. Eng. 2022, 51, 6–10. [Google Scholar]
- Coondoo, I.; Panwar, N.; Maiwa, H.; Kholkin, A.L. Improved piezoelectric and energy harvesting characteristics in lead-free Fe2O3 modified KNN ceramics. J. Electroceram. 2015, 34, 255–261. [Google Scholar] [CrossRef]
- Zhao, J.B.; Shao-Bo, Q.U.; Hong-Liang, D.U.; Zheng, Y.J.; Mo, W.D.; Yang, M. Actuality and Prospect of Potassium Sodium Niobium Based Lead-free Piezoelectric Ceramics. J. Air Force Eng. Univ. Nat. Sci. Ed. 2010, 11, 89–94. [Google Scholar]
- Li, X.W.; Yan, J.Y.; Yu, T.; Zhang, B.B. Versatile nonfluorinated superhydrophobic coating with self-cleaning, anti-fouling, anti-corrosion and mechanical stability. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128701. [Google Scholar] [CrossRef]
- Qiao, L.; Li, G.; Tao, H.; Wu, J.G.; Xu, Z.; Li, F. Full characterization for material constants of a promising KNN-based lead-free piezoelectric ceramic. Ceram. Int. 2019, 46, 5641–5644. [Google Scholar] [CrossRef]
- Venet, M.; Santa-Rosa, W.; Silva, P.; Peko, J.C.; Ramos, P.; Amorin, H.; Alguero, M. Selection and Optimization of a K0.5Na0.5NbO3-Based Material for Environmentally-Friendly Magnetoelectric Composites. Materials 2020, 13, 731. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.F.; Wu, W.J.; Xiao, D.Q.; Zhu, J.G. Effect of nonstoichiometry on the phase transitions and properties of(K0.465Na0.465Li0.07)(Nb0.95Sb0.05)O3 lead-free piezoelectric ceramics. J. Funct. Mater. 2012, 43, 126–129. [Google Scholar]
- Bassi, M.; Tripathi, S.L.; Verma, S. Analysis and Design of high-K Material Nanowire Transistor for Improved Performance. In Proceedings of the IEEE 10th Annual Information Technology, Electronics and Mobile Communication Conference (IEMCON), Vancouver, BC, Canada, 17–19 October 2019; pp. 613–618. [Google Scholar]
- Hong, X.; Wei, R.; Wu, X.; Shi, P. Effect of Mn doping on structures and properties of chemical solution deposited lead zirconate titanate thick films with (100) preferential orientation. J. Appl. Phys. 2013, 114, 317. [Google Scholar]
- Qi, Z.; Whatmore, R.R. Hysteretic properties of Mn-doped Pb(Zr,Ti)O3 thin films. J. Eur. Ceram. Soc. 2004, 24, 277–282. [Google Scholar]
- Huang, X.; Peng, J.; Zeng, J.; Zheng, L.; Li, G.; Karaki, T. The high piezoelectric properties and high temperature stability in Mn doped Pb(Mg0.5W0.5)O3-Pb(Zr,Ti)O3 ceramics. Ceram. Int. 2019, 45, 6523–6527. [Google Scholar] [CrossRef]
- Geng, W.; Chen, X.; Pan, L.; Qiao, X.J.; He, J.; Mu, J.L.; Hou, X.J.; Chou, X.J. Improved crystallization, domain, and ferroelectricity by controlling lead/oxygen vacancies in Mn-doped PZT thin films. Mater. Charact. 2021, 176, 111131. [Google Scholar] [CrossRef]
- Eml, A.; Ya, B.; Jys, A. Effects of Mn doping on BaTiO3 thin films grown on highly oriented pyrolytic graphite substrates-ScienceDirect. Curr. Appl. Phys. 2020, 20, 755–759. [Google Scholar]
- Peng, W.; Li, L.; Yu, S.; Yang, P.; Xu, K.L. Dielectric properties, microstructure and charge compensation of MnO2-doped BaTiO3-based ceramics in a reducing atmosphere. Ceram. Int. 2021, 47, 29191–29196. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.; Huang, F.; Hu, C.P.; Tan, P.; Wang, Y.; Huang, X.L.; Zhou, Z.X.; Tian, H. Effects of Mn-doping on anti-fatigue and anti-leakage current characteristics in KNN single crystals. Appl. Phys. Lett. 2021, 118, 042903. [Google Scholar] [CrossRef]
- Wang, L.; Wei, R.; Peng, S.; Wu, X.Q. Structures, electrical properties, and leakage current behaviors of un-doped and Mn-doped lead-free ferroelectric K0.5Na0.5NbO3 films. J. Appl. Phys. 2014, 115, 1400. [Google Scholar] [CrossRef]
- Abazari, M.; Safari, A. Effects of doping on ferroelectric properties and leakage current behavior of KNN-LT-LS thin films on SrTiO3 substrate. J. Appl. Phys. 2009, 105, 241. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Gupta, V.; Tomar, M. Dielectric and ferroelectric studies of KNN thin film grown by pulsed laser deposition technique. Vacuum 2019, 160, 233–237. [Google Scholar] [CrossRef]
- Jia, Z.; Huang, C.; Sekino, T.; Kim, S.H.; Lee, S.W. Residual stress determination in plasma sprayed Al2O3 coatings. J. Ceram. Process. Res. 2008, 9, 317–320. [Google Scholar]
- Fang, S.; Zhao, H.Y.; Liu, C.G.; Zhong, X.H.; Zhuang, Y.; Ni, J.X.; Tao, S.Y. Dense yttria-stabilized zirconia coatings fabricated by plasma spray-physical vapor deposition. Ceram. Int. 2017, 43, 2305–2313. [Google Scholar]
- Dong, X.Y.; Luo, X.T.; Zhang, S.L.; Li, C.J. A Novel Strategy for Depositing Dense Self-fluxing Alloy Coatings with Sufficiently Bonded Splats by One-Step Atmospheric Plasma Spraying. J. Therm. Spray Technol. 2020, 29, 173–184. [Google Scholar] [CrossRef]
- Waldbillig, D.; Kesler, O. Effect of suspension plasma spraying process parameters on YSZ coating microstructure and permeability. Surf. Coat. Technol. 2011, 205, 5483–5492. [Google Scholar] [CrossRef]
- Chen, S.; Tan, C.K.I.; Yao, K. Potassium-Sodium Niobate-Based Lead-Free Piezoelectric Ceramic Coatings by Thermal Spray Process. J. Am. Ceram. Soc. 2016, 99, 3293–3299. [Google Scholar] [CrossRef]
- Chen, S.; Tan, C.; Tan, S.Y.; Guo, S.F.; Zhang, L.; Yao, K. Potassium sodium niobate (KNN)-based lead-free piezoelectric ceramic coatings on steel structure by thermal spray method. J. Am. Ceram. Soc. 2018, 101, 5524–5533. [Google Scholar] [CrossRef]
- Zhou, L.L.; Li, X.W.; He, D.Y.; Guo, W.L.; Huang, Y.F.; He, G.C.; Xing, Z.G.; Wang, H.D. Study on Properties of Potassium Sodium Niobate Coating Prepared by High Efficiency Supersonic Plasma Spraying. Actuators 2022, 11, 28. [Google Scholar] [CrossRef]
- Song, Y.Y.; Huang, Y.F.; Guo, W.L.; Zhou, X.Y.; Xing, Z.G.; He, D.Y.; Lv, Z.L. Electrical Properties of Li+-Doped Potassium Sodium Niobate Coating Prepared by Supersonic Plasma Spraying. Actuators 2022, 11, 39. [Google Scholar] [CrossRef]
- Tnase, L.C.; Abramiuc, L.E.; Popescu, D.G.; Trandafir, A.M.; Apostol, N.G.; Bucur, I.C.; Hrib, L.; Pintilie, L.; Pasuk, I.; Trupina, L.; et al. Polarization Orientation in Lead Zirconate Titanate (001) Thin Films Driven by the Interface with the Substrate. Phys. Rev. Appl. 2018, 10, 034020. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, F.; Lu, Q.; Zhao, S.F. Improved Ferroelectric Photovoltaic Effect in Mn-doped Lead-free K0.5Na0.5NbO3 Films. Ceram. Int. 2018, 44, 13994–13998. [Google Scholar] [CrossRef]
- Wang, L.; Wei, R.; Goh, P.C.; Goh, P.C.; Yao, K.; Shi, P.; Wu, X.Q.; Yao, X. Structures and electrical properties of Mn- and Co-doped lead-free ferroelectric K0.5Na0.5NbO3 films prepared by a chemical solution deposition method. Thin Solid Film. 2013, 537, 65–69. [Google Scholar] [CrossRef]
- Won, S.S.; Lee, J.; Venugopal, V.; Venugopal, V.; Kim, D.J.; Lee, J.; Kim, I.W.; Kinggon, A.L.; Kim, S.H. Lead-free Mn-doped (K0.5,Na0.5)NbO3 piezoelectric thin films for MEMS-based vibrational energy harvester applications. Appl. Phys. Lett. 2016, 108, 1131. [Google Scholar] [CrossRef]
- Wang, L.Y.; Ren, R.; Shi, P.; Chen, X.F.; Wu, X.Q.; Yao, X. Enhanced ferroelectric properties in Mn-doped K0.5Na0.5NbO3 thin films derived from chemical solution deposition. Appl. Phys. Lett. 2010, 97, 209. [Google Scholar] [CrossRef]







| Process Parameters | |
|---|---|
| Ar gas flow (m3/h) | 50 |
| Spraying voltage (V) | 96 |
| H2 gas flow (m3/h) | 8 |
| Spraying current (A) | 400 |
| Spraying distance (mm) | 100 |
| Powder feeding speed (g/min) | 20 |
| Element | K | Na | Nb | O | Mn | C |
|---|---|---|---|---|---|---|
| wt% | 11.27 | 2.48 | 60.26 | 24.93 | 0.58 | 0.48 |
| at% | 10.9 | 4 | 24.5 | 58.69 | 0.4 | 1.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Guo, W.; Wang, H.; Li, X.; Xing, Z. Effect of Mn Doping on the Microstructure and Electrical Properties of Potassium Niobate Ceramics Using Plasma Spraying. Actuators 2022, 11, 343. https://doi.org/10.3390/act11120343
Gao R, Guo W, Wang H, Li X, Xing Z. Effect of Mn Doping on the Microstructure and Electrical Properties of Potassium Niobate Ceramics Using Plasma Spraying. Actuators. 2022; 11(12):343. https://doi.org/10.3390/act11120343
Chicago/Turabian StyleGao, Rui, Weiling Guo, Hongxing Wang, Xuewu Li, and Zhiguo Xing. 2022. "Effect of Mn Doping on the Microstructure and Electrical Properties of Potassium Niobate Ceramics Using Plasma Spraying" Actuators 11, no. 12: 343. https://doi.org/10.3390/act11120343




