Prevalence of Aggregatibacter actinomycetemcomitans and Periodontal Findings among 14 to 15-Year Old Danish Adolescents: A Descriptive Cross-Sectional Study
Abstract
1. Introduction
2. Results
2.1. Study Population
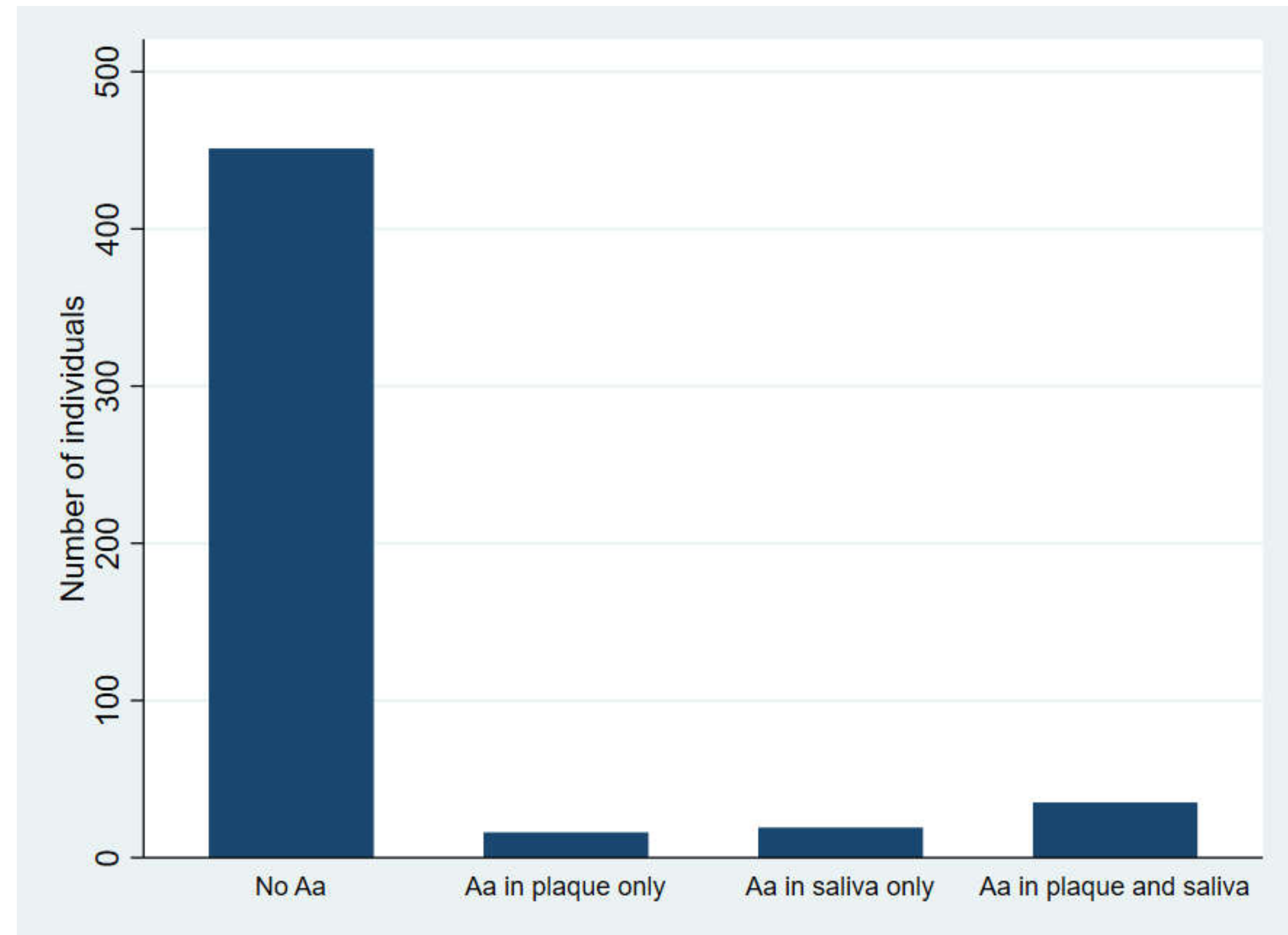
2.2. Carrier Status of A. actinomycetemcomitans
2.3. Periodontal Outcomes of the Participants According to A. actinomycetemcomitans-Carrier Status
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical Examination
4.3. Periodontal Outcomes
4.4. Biological Samples
4.4.1. Stimulated Saliva Samples
4.4.2. Subgingival Plaque Samples
4.4.3. Real-Time Polymerase Chain Reaction
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C.H.; Haubek, D.; Kwamin, F.; Johansson, A.; Claesson, R. Leukotoxic activity of Aggregatibacter actinomycetemcomitans and periodontal attachment loss. PLoS ONE 2014, 9, e104095. [Google Scholar] [CrossRef]
- Haubek, D.; Ennibi, O.-K.; Poulsen, K.; Væth, M.; Poulsen, S.; Kilian, M. Risk of Aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) Aactinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Furgang, D.; Fairlie, K.; Ferrandiz, J.; Nasri, C.; McKiernan, M.; Gunsolley, J. Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: Longitudinal cohort study of initially healthy adolescents. J. Clin. Microbiol. 2007, 45, 3859–3869. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Poulsen, K.; Kilian, M. Microevolution and patterns of dissemination of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infect. Immun. 2007, 75, 3080–3088. [Google Scholar] [CrossRef]
- Rylev, M.; Kilian, M. Prevalence and distribution of principal periodontal pathogens worldwide. J. Clin. Periodontol. 2008, 35, 346–361. [Google Scholar] [CrossRef]
- Haubek, D.; Johansson, A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J. Oral Microbiol. 2014, 6, 23980. [Google Scholar] [CrossRef]
- Elamin, A.; Albandar, J.M.; Poulsen, K.; Ali, R.W.; Bakken, V. Prevalence of Aggregatibacter actinomycetemcomitans in Sudanese patients with aggressive periodontitis: A case-control study. J. Periodontal Res. 2011, 46, 285–291. [Google Scholar] [CrossRef]
- Eick, S.; Pietkiewicz, M.; Sculean, A. Oral microbiota in Swiss adolescents. Clin. Oral Investig. 2013, 17, 79–86. [Google Scholar] [CrossRef][Green Version]
- Gajardo, M.; Silva, N.; Gómez, L.; León, R.; Parra, B.; Contreras, A.; Gamonal, J. Prevalence of periodontopathic bacteria in aggressive periodontitis patients in a Chilean population. J. Periodontol. 2005, 76, 289–294. [Google Scholar] [CrossRef]
- Macheleidt, A.; Müller, H.-P.; Eger, T.; Putzker, M.; Fuhrmann, A.; Zöller, L. Absence of an especially toxic clone among isolates of Actinobacillus actinomycetemcomitans recovered from army recruits. Clin. Oral Investig. 1999, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Hayashi, F.; Nagasaka, N. Detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in dental plaque samples from children 2 to 12 years of age. J. Clin. Periodontol. 2000, 27, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Mínguez, M.; Pousa, X.; Herrera, D.; Blasi, A.; Sánchez, M.C.; León, R.; Sanz, M. Characterization and serotype distribution of Aggregatibacter actinomycetemcomitans isolated from a population of periodontitis patients in Spain. Arch. Oral Biol. 2014, 59, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; Di Bonaventura, G.; Di Placido, G.; Tumini, V.; Catamo, G.; Di Donato, A.; Piccolomini, R. Prevalence of Actinobacillus actinomycetemcomitans and clinical conditions in children and adolescents from rural and urban areas of central Italy. J. Clin. Periodontol. 2000, 27, 549–557. [Google Scholar] [CrossRef]
- Roman-Torres, C.V.G.; Aquino, D.R.; Cortelli, S.C.; Franco, G.C.N.; dos Santos, J.G.; Corraini, P.; Holzhausen, M.; Diniz, M.G.; Gomez, R.S.; Cortelli, J.R. Prevalence and distribution of serotype-specific genotypes of Aggregatibacter actinomycetemcomitans in chronic periodontitis Brazilian subjects. Arch. Oral Biol. 2010, 55, 242–248. [Google Scholar] [CrossRef]
- Sakellari, D.; Katsikari, A.; Slini, T.; Ioannidis, I.; Konstantinidis, A.; Arsenakis, M. Prevalence and distribution of Aggregatibacter actinomycetemcomitans serotypes and the JP2 clone in a Greek population. J. Clin. Periodontol. 2011, 38, 108–114. [Google Scholar] [CrossRef]
- Torrungruang, K.; Bandhaya, P.; Likittanasombat, K.; Grittayaphong, C. Relationship between the presence of certain bacterial pathogens and periodontal status of urban Thai adults. J. Periodontol. 2009, 80, 122–129. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Yang, M.; Geng, Y.; Chen, H.; Xu, Y.; Sun, Y. Prevalence and distribution of Aggregatibacter actinomycetemcomitans and its CdtB gene in subgingival plaque of Chinese periodontitis patients. BMC Oral Health 2014, 14. [Google Scholar] [CrossRef]
- Ali, R.W.; Velcescu, C.; Jivanescu, M.C.; Lofthus, B.; Skaug, N. Prevalence of 6 putative periodontal pathogens in subgingival plaque samples from Romanian adult periodontitis patients. J. Clin. Periodontol. 1996, 23, 133–139. [Google Scholar] [CrossRef]
- Bandhaya, P.; Saraithong, P.; Likittanasombat, K.; Hengprasith, B.; Torrungruang, K. Aggregatibacter actinomycetemcomitans serotypes, the JP2 clone and cytolethal distending toxin genes in a Thai population. J. Clin. Periodontol. 2012, 39, 519–525. [Google Scholar] [CrossRef]
- Ellwood, R.P.; Worthington, H.V.; Cullinan, M.P.; Hamlet, S.; Clerehugh, V.; Davies, R. Prevalence of suspected periodontal pathogens identified using ELISA in adolescents of differing ethnic origins. J. Clin. Periodontol. 1997, 24, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Fiehn, N.-E.; Nielsen, C.H.; Kirkby, N.; Twetman, S.; Klepac-Ceraj, V.; Paster, B.J.; Holmstrup, P. Differences in bacterial saliva profile between periodontitis patients and a control cohort. J. Clin. Periodontol. 2014, 41, 104–112. [Google Scholar] [CrossRef]
- Claesson, R.; Höglund-Åberg, C.; Haubek, D.; Johansson, A. Age-related prevalence and characteristics of Aggregatibacter actinomycetemcomitans in periodontitis patients living in Sweden. J. Oral Microbiol. 2017, 9, 1334504. [Google Scholar] [CrossRef] [PubMed]
- Hölttä, P.; Alaluusua, S.; Saarela, M.; Asikainen, S. Isolation frequency and serotype distribution of Mutans streptococci and Actinobacillus actinomycetemcomitans, and clinical periodontal status in Finnish and Vietnamese children. Eur. J. Oral Sci. 1994, 102, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Chiang, H.-M.; Lindholm, M.; Åberg, C.H.; Haubek, D.; Johansson, A.; Oscarsson, J. Characterization of Aggregatibacter actinomycetemcomitans serotype b strains with five different, including two novel, leukotoxin promoter structures. Vaccines 2020, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Aarhus i Tal. Aarhus Kommune. Available online: https://ledelsesinformation.aarhuskommune.dk/aarhus-i-tal/default.aspx?doc=vfs://Global/AARHUS-I-TAL/Hjem.xview (accessed on 4 July 2020).
- Nørskov-Lauritsen, N.; Claesson, R.; Jensen, A.B.; Åberg, C.H.; Haubek, D. Aggregatibacter actinomycetemcomitans: Clinical significance of a pathobiont subjected to ample changes in classification and nomenclature. Pathogens 2019, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Åberg, C.H.; Kwamin, F.; Claesson, R.; Dahlén, G.; Johansson, A.; Haubek, D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: A prospective cohort study of a young adolescent population. J. Clin. Periodontol. 2014, 41, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Alaluusua, S.; Asikainen, S. Detection and distribution of Actinobacillus actinomycetemcomitans in the primary dentition. J. Periodontol. 1988, 59, 504–507. [Google Scholar] [CrossRef] [PubMed]
- DiRienzo, J.M.; Slots, J.; Sixou, M.; Sol, M.A.; Harmon, R.; McKay, T.L. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect. Immun. 1994, 62, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Saarela, M.; Asikainen, S.; Alaluusua, S.; Pyhälä, L.; Lai, C.-H.; Jousimies-Somer, H. Frequency and stability of mono-or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol. Immunol. 1992, 7, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Ennibi, O.K.; Væth, M.; Poulsen, S.; Poulsen, K. Stability of the JP2 clone of Aggregatibacter actinomycetemcomitans. J. Dent. Res. 2009, 88, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Kittichotirat, W.; Wang, J.; Jan, M.; Chen, W.; Asikainen, S.; Bumgarner, R.; Chen, C. Genomic stability of Aggregatibacter actinomycetemcomitans during persistent oral infection in human. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S. Microbial etiological agents of destructive periodontal diseases. Periodontol. 2000 1994, 5, 78–111. [Google Scholar] [CrossRef] [PubMed]
- Danmarks Statistik. Indvandrere og efterkommere–Danmarks Statistik. Available online: https://www.dst.dk/da/Statistik/emner/befolkning-og-valg/indvandrere-og-efterkommere/indvandrere-og-efterkommere (accessed on 18 August 2020).
- Collins, J.R.; Chinea, S.; Cuello, R.J.; Florian, A.P.; Palma, P.; Ambrosio, N.; Marín, M.J.; Figuero, E.; Herrera, D. Subgingival microbiological profile of periodontitis patients in Dominican republic. Acta Odontol. Latinoam. 2019, 32, 36. [Google Scholar]
- Psoter, W.J.; Ge, Y.; Russell, S.L.; Chen, Z.; Katz, R.V.; Jean-Charles, G.; Li, Y. PCR detection of Streptococcus mutans and Aggregatibacter actinomycetemcomitans in dental plaque samples from Haitian adolescents. Clin. Oral Investig. 2011, 15, 461. [Google Scholar] [CrossRef]
- Jensen, A.B.; Ennibi, O.K.; Ismaili, Z.; Poulsen, K.; Haubek, D. The JP2 Genotype of Aggregatibacter actinomycetemcomitans and marginal periodontitis in the mixed dentition. J. Clin. Periodontol. 2016, 43, 19. [Google Scholar] [CrossRef]
- Ennibi, O.K.; Claesson, R.; Akkaoui, S.; Reddahi, S.; Kwamin, F.; Haubek, D.; Johansson, A. High salivary levels of JP2 genotype of Aggregatibacter actinomycetemcomitans is associated with clinical attachment loss in Moroccan adolescents. Clin. Exp. Dent. Res. 2019, 5, 44. [Google Scholar] [CrossRef]
- Marin, M.; Ambrosio, N.; Herrera, D.; Sanz, M.; Figuero, E. Validation of a Multiplex qPCR assay for the identification and quantification of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis: In vitro and subgingival plaque samples. Arch. Oral Biol. 2018, 88, 47. [Google Scholar] [CrossRef]
- Orrù, G.; Marini, M.F.; Ciusa, M.L.; Isola, D.; Cotti, M.; Baldoni, M.; Piras, V.; Pisano, E.; Montaldo, C. Usefulness of real time PCR for the differentiation and quantification of 652 and JP2 Actinobacillus actinomycetemcomitans genotypes in dental plaque and saliva. BMC Infect. Dis. 2006, 6. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89, S74. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and Proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45, S162. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.J.; Figuero, E.; Herrera, D.; Sanz, M. Quantitative analysis of periodontal pathogens using real-time polymerase chain reaction (PCR). In Methods in Molecular Biology (Clifton, N.J.); Springer: Berlin/Heidelberg, Germany, 2017; Volume 1537. [Google Scholar] [CrossRef]
- SciPy. SciPy v1.3.0 Reference Guide. Available online: https://docs.scipy.org/doc/scipy-1.3.0/reference/ (accessed on 8 July 2019).
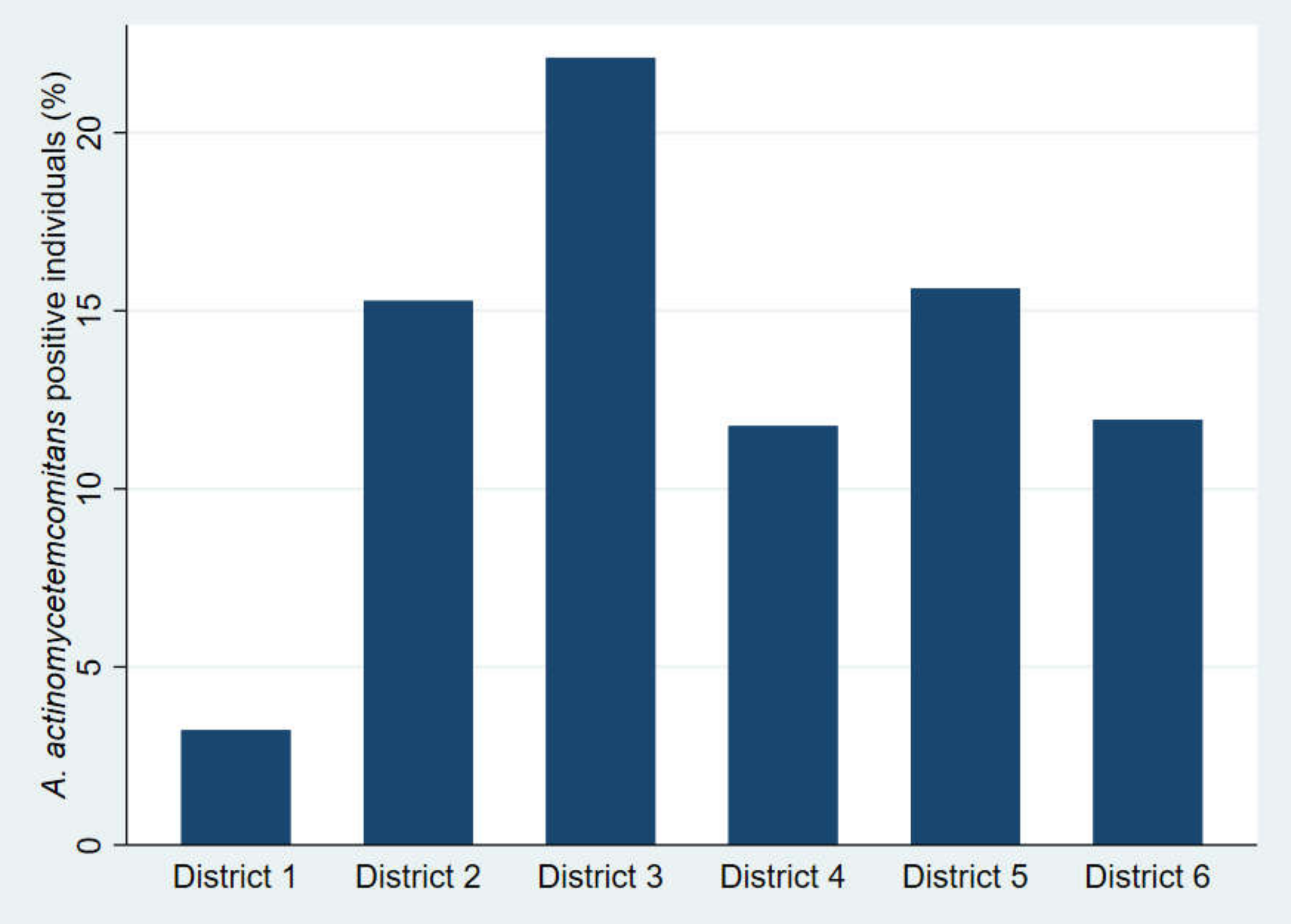
| No. of District of Municipality of Aarhus, Denmark | No. of Participants (%) | District Size According to the Total no. of Individuals Born in Year 2003 in the Municipality (%) | Proportion of Individuals with a Danish Ethnic Background (%) |
|---|---|---|---|
| 1 | 93 (17.7) | 16.9 | 93.9 |
| 2 | 72 (13.7) | 13.9 | 76.9 |
| 3 | 95 (18.1) | 18.0 | 59.4 |
| 4 | 102 (19.4) | 17.7 | 90.4 |
| 5 | 96 (18.3) | 18.5 | 75.0 |
| 6 | 67 (12.7) | 14.9 | 84.0 |
| Periodontal Outcomes | Individuals Positive for Aa (n = 70) | Individuals with no Aa (n = 455) | Total (n = 525) |
|---|---|---|---|
| No. of individuals with PS 1 > 20% (%) | 54 (77.1) | 327 (71.9) | 381 (72.6) |
| No. of individuals with BOP 2 > 10% (%) | 66 (94.3) | 431 (94.6) | 497 (94.7) |
| No. of individuals with PPD 3 ≥ 4 mm (%) | 26 (37.1)* | 83 (18.2) * | 109 (20.0) |
| No. of individuals with interdental CAL 4 ≥ 2 mm (%) | 4 (5.7)* | 5 (1.1) * | 9 (1.7) |
| Periodontal Outcomes | Individuals Positive for Aa in Subgingival Plaque (n = 51) | Individuals Positive for Aa in Stimulated Saliva (n = 54) | Individuals Positive for Aa in Subgingival Plaque only (n = 16) | Individuals Positive for Aa in Saliva only (n = 19) | Individuals Positive for Aa in both Saliva and Subgingival Plaque (n = 35) | Individuals with no Aa (n = 455) |
|---|---|---|---|---|---|---|
| No. of individuals with PS 1 > 20% (%) | 44 (86.3) * | 42 (77.8) | 12 (75.0) | 10 (52.6) | 32 (91.4) * | 327 (71.9) |
| No. of individuals with BOP 2 > 10% (%) | 50 (98.0) | 51 (94.4) | 15 (93.6) | 16 (84.2) | 35 (100) | 431 (94.6) |
| No. of individuals with PPD 3 ≥ 4 mm (%) | 20 (39.2) * | 22 (40.7)* | 4 (25.0) | 6 (31.6) | 16 (45.7) * | 83 (18.2) * |
| No. of individuals with interdental CAL 4 ≥ 2 mm (%) | 4 (7.8) * | 3 (5.6)* | 1 (6.3) | 0 (0.0) | 3 (8.6) * | 5 (1.1) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, A.B.; Isidor, F.; Lund, M.; Væth, M.; Johansson, A.; Lauritsen, N.N.; Haubek, D. Prevalence of Aggregatibacter actinomycetemcomitans and Periodontal Findings among 14 to 15-Year Old Danish Adolescents: A Descriptive Cross-Sectional Study. Pathogens 2020, 9, 1054. https://doi.org/10.3390/pathogens9121054
Jensen AB, Isidor F, Lund M, Væth M, Johansson A, Lauritsen NN, Haubek D. Prevalence of Aggregatibacter actinomycetemcomitans and Periodontal Findings among 14 to 15-Year Old Danish Adolescents: A Descriptive Cross-Sectional Study. Pathogens. 2020; 9(12):1054. https://doi.org/10.3390/pathogens9121054
Chicago/Turabian StyleJensen, Anne Birkeholm, Flemming Isidor, Marianne Lund, Michael Væth, Anders Johansson, Niels Nørskov Lauritsen, and Dorte Haubek. 2020. "Prevalence of Aggregatibacter actinomycetemcomitans and Periodontal Findings among 14 to 15-Year Old Danish Adolescents: A Descriptive Cross-Sectional Study" Pathogens 9, no. 12: 1054. https://doi.org/10.3390/pathogens9121054
APA StyleJensen, A. B., Isidor, F., Lund, M., Væth, M., Johansson, A., Lauritsen, N. N., & Haubek, D. (2020). Prevalence of Aggregatibacter actinomycetemcomitans and Periodontal Findings among 14 to 15-Year Old Danish Adolescents: A Descriptive Cross-Sectional Study. Pathogens, 9(12), 1054. https://doi.org/10.3390/pathogens9121054






