Simultaneous Quantification of Vibrio metoecus and Vibrio cholerae with Its O1 Serogroup and Toxigenic Subpopulations in Environmental Reservoirs
Abstract
:1. Introduction
2. Results and Discussion
2.1. A Specific and Efficient Multiplex qPCR Assay to Detect V. cholerae and V. metoecus
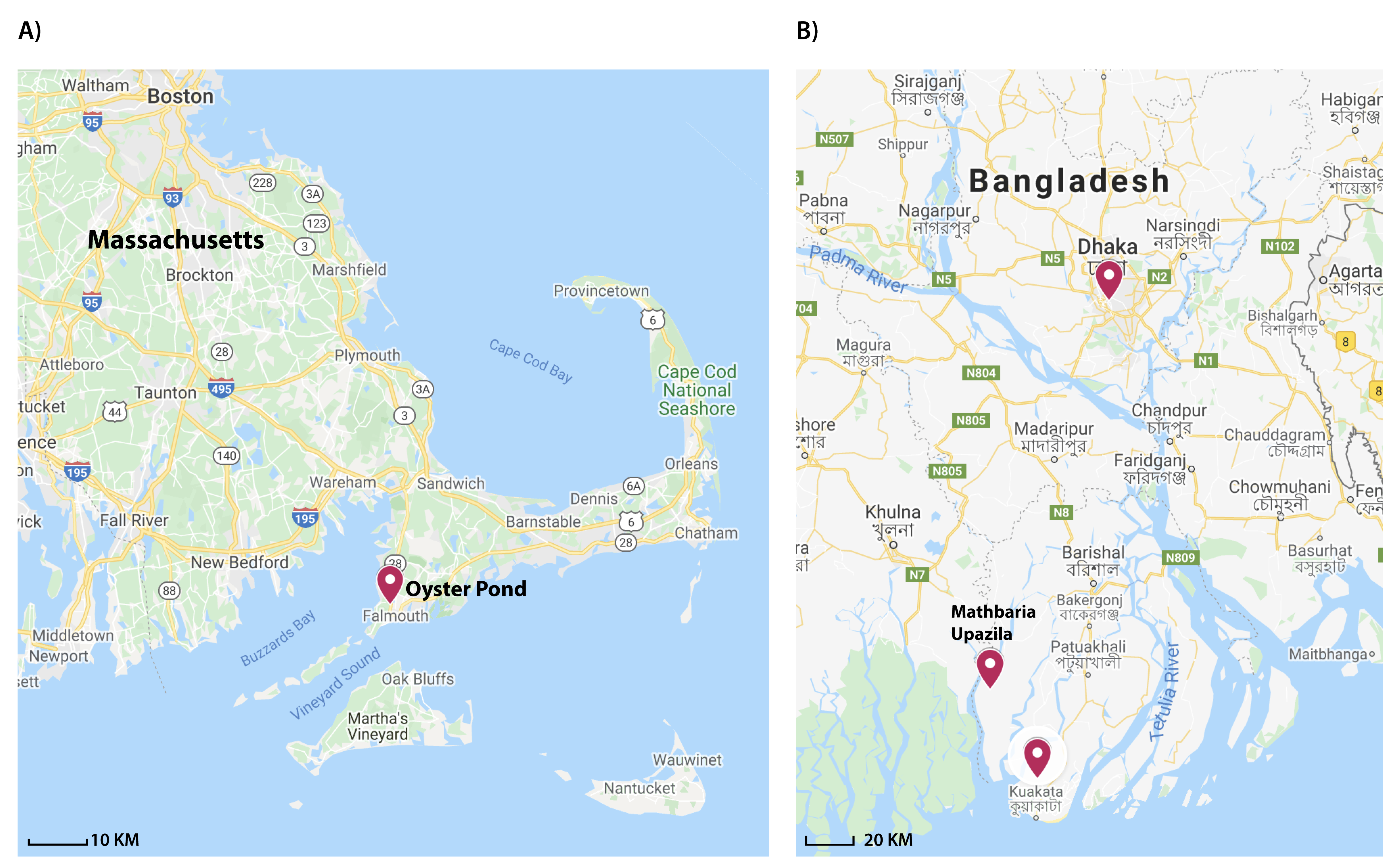
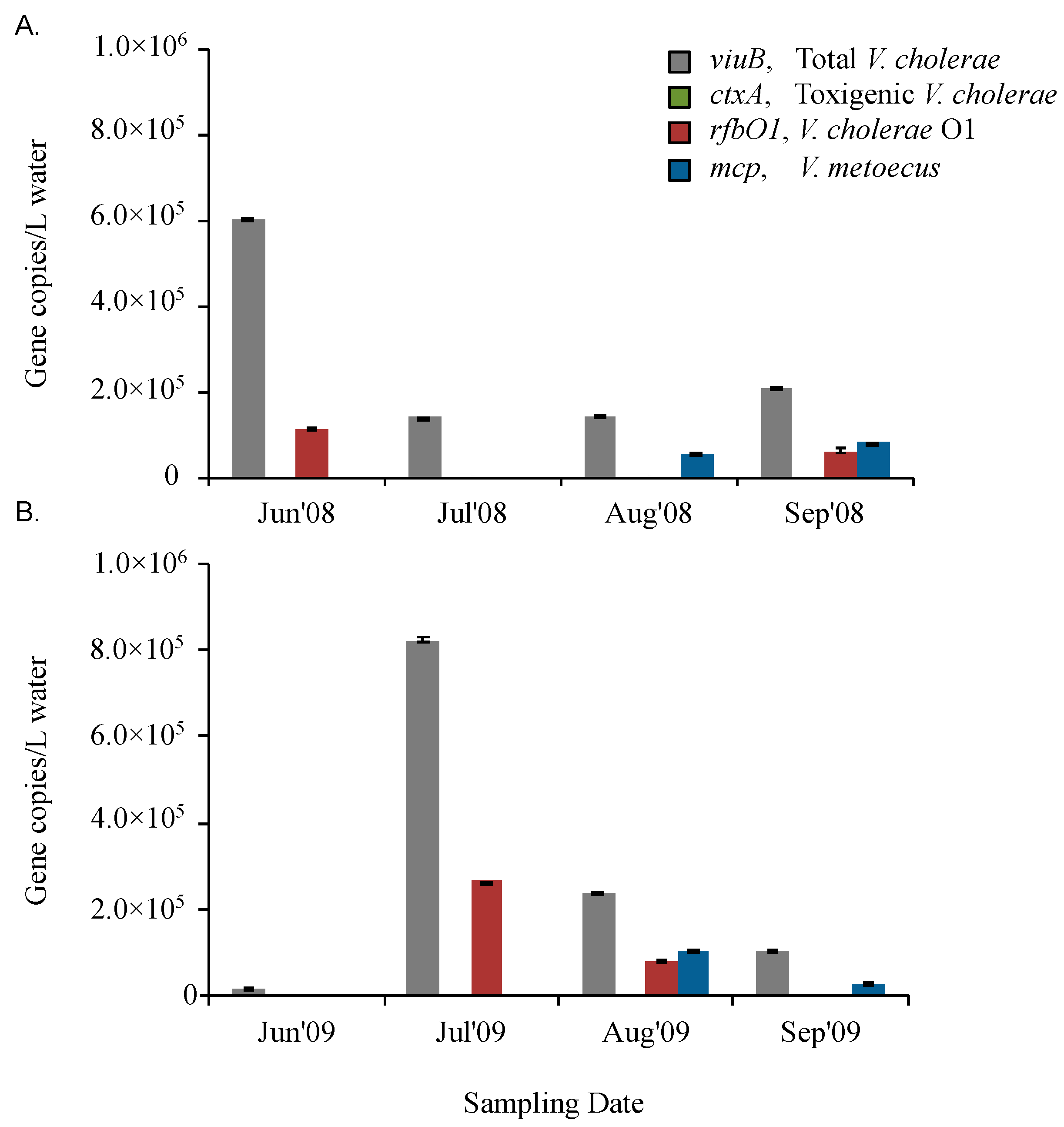
2.2. V. cholerae and V. metoecus Co-Occur Seasonally in a Temperate Coastal Location
2.3. O1 Serogroup Strains Are Important Members of a Temperate Coastal V. cholerae Population
2.4. Toxigenic O1 Serogroup Strains Are Constantly Present at Dangerous Levels in Dhaka Freshwater
3. Materials and Methods
3.1. Bacterial Cultures and DNA Template Preparation
3.2. DNA Extraction from Biomass and Isolation of Organisms from Environmental Water Samples
3.3. Design and Evaluation of Primers and Fluorogenic Probes for Real–Time qPCR
3.4. Real-Time qPCR Amplification
3.5. Generation of Standard Curves and Calculation of qPCR Efficiency
3.6. Limit of Detection (LOD) and Impact of Inhibition Testing
3.7. Specificity Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colwell, R.; Kaper, J.; Joseph, S. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: Occurrence and distribution in Chesapeake Bay. Science 1977, 198, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Hasan, N.A.; Sadique, A.; Bhuiyan, N.A.; Ahmed, K.U.; Nusrin, S.; Nair, G.B.; Siddique, A.K.; Sack, R.B.; Sack, D.A.; et al. Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Appl. Environ. Microbiol. 2006, 72, 4096–4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Number of Reported Cholera Cases. Available online: https://www.who.int/gho/epidemic_diseases/cholera/cases_text/en/ (accessed on 27 August 2020).
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 2004, 68, 403–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaper, J.B.; Morris, J.G., Jr.; Levine, M.M. Cholera. Clin. Microbiol. Rev. 1995, 8, 48–86. [Google Scholar] [CrossRef]
- Ali, M.; Lopez, A.L.; You, Y.A.; Kim, Y.E.; Sah, B.; Maskery, B.; Clemens, J. The global burden of cholera. Bull. World Health Organ. 2012, 90, 209–218A. [Google Scholar] [CrossRef]
- Islam, M.S.; Hasan, M.K.; Miah, M.A.; Yunus, M.; Zaman, K.; Albert, M.J. Isolation of Vibrio cholerae O139 synonym Bengal from the aquatic environment in Bangladesh: Implications for disease transmission. Appl. Environ. Microbiol. 1994, 60, 1684–1686. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.; Grim, C.J.; Hasan, N.A.; Lee, J.H.; Choi, S.Y.; Haley, B.J.; Taviani, E.; Jeon, Y.S.; Kim, D.W.; Lee, J.H.; et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2009, 106, 15442–15447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, D.; Chowdhury, G.; Pazhani, G.P.; Guin, S.; Dutta, S.; Ghosh, S.; Rajendran, K.; Nandy, R.K.; Mukhopadhyay, A.K.; Bhattacharya, M.K.; et al. Vibrio cholerae non-O1, non-O139 serogroups and cholera-like diarrhea, Kolkata, India. Emerg. Infect. Dis. 2013, 19, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Sharma, N.C.; Halder, K.; Bhadra, R.K.; Chowdhury, G.; Pazhani, G.P.; Shinoda, S.; Mukhopadhyay, A.K.; Nair, G.B.; Ramamurthy, T. Phenotypic and Genetic Heterogeneity in Vibrio cholerae O139 Isolated from Cholera Cases in Delhi, India during 2001-2006. Front. Microbiol. 2016, 7, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faruque, S.M.; Sack, D.A.; Sack, R.B.; Colwell, R.R.; Takeda, Y.; Nair, G.B. Emergence and evolution of Vibrio cholerae O139. Proc. Natl. Acad. Sci. USA 2003, 100, 1304–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashed, S.M.; Iqbal, A.; Mannan, S.B.; Islam, T.; Rashid, M.U.; Johura, F.T.; Watanabe, H.; Hasan, N.A.; Huq, A.; Stine, O.C.; et al. Vibrio cholerae O1 El Tor and O139 Bengal strains carrying ctxB (ET), Bangladesh. Emerg. Infect. Dis. 2013, 19, 1713–1715. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, F.; Mather, A.E.; Begum, Y.A.; Asaduzzaman, M.; Baby, N.; Sharmin, S.; Biswas, R.; Uddin, M.I.; LaRocque, R.C.; Harris, J.B.; et al. Vibrio cholerae Serogroup O139: Isolation from Cholera Patients and Asymptomatic Household Family Members in Bangladesh between 2013 and 2014. PLoS Negl. Trop. Dis. 2015, 9, e0004183. [Google Scholar] [CrossRef] [PubMed]
- Mutreja, A.; Kim, D.W.; Thomson, N.R.; Connor, T.R.; Lee, J.H.; Kariuki, S.; Croucher, N.J.; Choi, S.Y.; Harris, S.R.; Lebens, M.; et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature 2011, 477, 462–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, Y.; Orata, F.D.; Alam, M. The out-of-the-delta hypothesis: Dense human populations in low-lying river deltas served as agents for the evolution of a deadly pathogen. Front. Microbiol. 2015, 6, 1120. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, P.C.; Turnsek, M.; Hunt, D.E.; Haley, B.J.; Colwell, R.R.; Polz, M.F.; Tarr, C.L.; Boucher, Y. Vibrio metoecus sp. nov., a close relative of Vibrio cholerae isolated from coastal brackish ponds and clinical specimens. Int. J. Syst. Evol. Microbiol. 2014, 64, 3208–3214. [Google Scholar] [CrossRef]
- Haley, B.J.; Grim, C.J.; Hasan, N.A.; Choi, S.Y.; Chun, J.; Brettin, T.S.; Bruce, D.C.; Challacombe, J.F.; Detter, J.C.; Han, C.S.; et al. Comparative genomic analysis reveals evidence of two novel Vibrio species closely related to V. cholerae. BMC Microbiol. 2010, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Choopun, N. The Population Structure of Vibrio Cholerae in Chesapeake Bay. Ph.D. Thesis, University of Maryland, College Park, MD, USA, June 2004. [Google Scholar]
- Orata, F.D.; Kirchberger, P.C.; Meheust, R.; Barlow, E.J.; Tarr, C.L.; Boucher, Y. The Dynamics of Genetic Interactions between Vibrio metoecus and Vibrio cholerae, Two Close Relatives Co-Occurring in the Environment. Genome Biol. Evol. 2015, 7, 2941–2954. [Google Scholar] [CrossRef] [Green Version]
- Baron, S.; Chevalier, S.; Lesne, J. Vibrio cholerae in the environment: A simple method for reliable identification of the species. J. Health Popul. Nutr. 2007, 25, 312–318. [Google Scholar]
- Colwell, R.R.; Brayton, P.; Herrington, D.; Tall, B.; Huq, A.; Levine, M.M. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J. Microbiol. Biotechnol. 1996, 12, 28–31. [Google Scholar] [CrossRef]
- Colwell, R.R.; Huq, A. Vibrios in the Environment: Viable but Nonculturable Vibrio cholerae. In Vibrio cholerae and Cholera: Molecular to Global Perspectives; Wachsmuth, I.K., Blake, P.A., Olsvik, O., Eds.; American Society for Microbiology: Washington, DC, USA, 1994; pp. 117–133. [Google Scholar] [CrossRef]
- Miller, C.J.; Drasar, B.S.; Feachem, R.G. Response of toxigenic Vibrio cholerae 01 to physico-chemical stresses in aquatic environments. J. Hyg. (Lond.) 1984, 93, 475–495. [Google Scholar] [CrossRef] [Green Version]
- Huq, A.; Xu, B.; Chowdhury, M.A.; Islam, M.S.; Montilla, R.; Colwell, R.R. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl. Environ. Microbiol. 1996, 62, 2508–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobitz, B.; Beck, L.; Huq, A.; Wood, B.; Fuchs, G.; Faruque, A.S.; Colwell, R. Climate and infectious disease: Use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA 2000, 97, 1438–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qadri, F.; Hasan, J.A.; Hossain, J.; Chowdhury, A.; Begum, Y.A.; Azim, T.; Loomis, L.; Sack, R.B.; Albert, M.J. Evaluation of the monoclonal antibody-based kit Bengal SMART for rapid detection of Vibrio cholerae O139 synonym Bengal in stool samples. J. Clin. Microbiol. 1995, 33, 732–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colwell, R.R.; Brayton, P.R.; Grimes, D.J.; Roszak, D.B.; Huq, S.A.; Palmer, L.M. Viable but Non-Culturable Vibrio cholerae and Related Pathogens in the Environment: Implications for Release of Genetically Engineered Microorganisms. Nat. Biotechnol. 1985, 3, 817–820. [Google Scholar] [CrossRef]
- Huq, A.; Colwell, R.R.; Rahman, R.; Ali, A.; Chowdhury, M.A.; Parveen, S.; Sack, D.A.; Russek-Cohen, E. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl. Environ. Microbiol. 1990, 56, 2370–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theron, J.; Cilliers, J.; Du Preez, M.; Brozel, V.S.; Venter, S.N. Detection of toxigenic Vibrio cholerae from environmental water samples by an enrichment broth cultivation-pit-stop semi-nested PCR procedure. J. Appl. Microbiol. 2000, 89, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, G.M.; Nordstrom, J.L.; Bowen, M.D.; Meyer, R.F.; Imbro, P.; DePaola, A. Use of a real time PCR assay for detection of the ctxA gene of Vibrio cholerae in an environmental survey of Mobile Bay. J. Microbiol. Methods 2007, 68, 254–259. [Google Scholar] [CrossRef]
- Neogi, S.B.; Chowdhury, N.; Asakura, M.; Hinenoya, A.; Haldar, S.; Saidi, S.M.; Kogure, K.; Lara, R.J.; Yamasaki, S. A highly sensitive and specific multiplex PCR assay for simultaneous detection of Vibrio cholerae, Vibrio parahaemolyticus and Vibrio vulnificus. Lett. Appl. Microbiol. 2010, 51, 293–300. [Google Scholar] [CrossRef]
- Gubala, A.J. Multiplex real-time PCR detection of Vibrio cholerae. J. Microbiol. Methods 2006, 65, 278–293. [Google Scholar] [CrossRef]
- Haugland, R.A.; Siefring, S.C.; Wymer, L.J.; Brenner, K.P.; Dufour, A.P. Comparison of Enterococcus measurements in freshwater at two recreational beaches by quantitative polymerase chain reaction and membrane filter culture analysis. Water Res. 2005, 39, 559–568. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Turo, C.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Real-Time PCR for Diagnosing and Quantifying Co-infection by Two Globally Distributed Fungal Pathogens of Wheat. Front. Plant. Sci. 2018, 9, 1086. [Google Scholar] [CrossRef]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bliem, R.; Schauer, S.; Plicka, H.; Obwaller, A.; Sommer, R.; Steinrigl, A.; Alam, M.; Reischer, G.H.; Farnleitner, A.H.; Kirschner, A. A novel triplex quantitative PCR strategy for quantification of toxigenic and nontoxigenic Vibrio cholerae in aquatic environments. Appl. Environ. Microbiol. 2015, 81, 3077–3085. [Google Scholar] [CrossRef] [Green Version]
- Lyon, W.J. TaqMan PCR for detection of Vibrio cholerae O1, O139, non-O1, and non-O139 in pure cultures, raw oysters, and synthetic seawater. Appl. Environ. Microbiol. 2001, 67, 4685–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vezzulli, L.; Stauder, M.; Grande, C.; Pezzati, E.; Verheye, H.M.; Owens, N.J.; Pruzzo, C. gbpA as a Novel qPCR Target for the Species-Specific Detection of Vibrio cholerae O1, O139, Non-O1/Non-O139 in Environmental, Stool, and Historical Continuous Plankton Recorder Samples. PLoS ONE 2015, 10, e0123983. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Fullner, K.J.; Clayton, R.; Sexton, J.A.; Rogers, M.B.; Calia, K.E.; Calderwood, S.B.; Fraser, C.; Mekalanos, J.J. Identification of a vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 1999, 96, 1071–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubala, A.J.; Proll, D.F. Molecular-beacon multiplex real-time PCR assay for detection of Vibrio cholerae. Appl. Environ. Microbiol. 2006, 72, 6424–6428. [Google Scholar] [CrossRef] [Green Version]
- Heidelberg, J.F.; Eisen, J.A.; Nelson, W.C.; Clayton, R.A.; Gwinn, M.L.; Dodson, R.J.; Haft, D.H.; Hickey, E.K.; Peterson, J.D.; Umayam, L.; et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 2000, 406, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Rao, K.H.; Sengupta, M.; Bhattacharya, S.K.; Datta, A. Two gene clusters co-ordinate for a functional N-acetylglucosamine catabolic pathway in Vibrio cholerae. Mol. Microbiol. 2011, 80, 1549–1560. [Google Scholar] [CrossRef]
- Stauder, M.; Huq, A.; Pezzati, E.; Grim, C.J.; Ramoino, P.; Pane, L.; Colwell, R.R.; Pruzzo, C.; Vezzulli, L. Role of GbpA protein, an important virulence-related colonization factor, for Vibrio cholerae’s survival in the aquatic environment. Environ. Microbiol. Rep. 2012, 4, 439–445. [Google Scholar] [CrossRef]
- Boucher, Y. Sustained Local Diversity of Vibrio cholerae O1 Biotypes in a Previously Cholera-Free Country. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faruque, S.M.; Ahmed, K.M.; Siddique, A.K.; Zaman, K.; AbdulAlim, A.R.M.; Albert, M.J. Molecular analysis of toxigenic Vibrio cholerae O139 Bengal strains isolated in Bangladesh between 1993 and 1996: Evidence for emergence of a new clone of the Bengal vibrios. J. Clin. Microbiol. 1997, 35, 2299–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalsgaard, A.; Serichantalergs, O.; Forslund, A.; Lin, W.; Mekalanos, J.; Mintz, E.; Shimada, T.; Wells, J.G. Clinical and environmental isolates of Vibrio cholerae serogroup O141 carry the CTX phage and the genes encoding the toxin-coregulated pili. J. Clin. Microbiol. 2001, 39, 4086–4092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekalanos, J.J.; Swartz, D.J.; Pearson, G.D.; Harford, N.; Groyne, F.; de Wilde, M. Cholera toxin genes: Nucleotide sequence, deletion analysis and vaccine development. Nature 1983, 306, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Hoshino, K.; Shimizu, T.; Garg, S.; Shimada, T.; Ho, S.; Bhadra, R.K.; Nair, G.B.; Takeda, Y. Comparative analysis of the gene responsible for lipopolysaccharide synthesis of Vibrio cholerae O1 and O139 and those of non-O1 non-O139 Vibrio cholerae. In Proceedings of the 32nd Joint Conference U.S.-Japan Cooperative Medical Science Program, Cholera and Related Diarrhoeal Diseases Panel, Nagasaki, Japan, 14–16 November 1996; p. 24. [Google Scholar]
- Hoshino, K.; Yamasaki, S.; Mukhopadhyay, A.K.; Chakraborty, S.; Basu, A.; Bhattacharya, S.K.; Nair, G.B.; Shimada, T.; Takeda, Y. Development and evaluation of a multiplex PCR assay for rapid detection of toxigenic Vibrio cholerae O1 and O139. FEMS Immunol. Med. Microbiol. 1998, 20, 201–207. [Google Scholar] [CrossRef]
- Pang, B.; Yan, M.; Cui, Z.; Ye, X.; Diao, B.; Ren, Y.; Gao, S.; Zhang, L.; Kan, B. Genetic diversity of toxigenic and nontoxigenic Vibrio cholerae serogroups O1 and O139 revealed by array-based comparative genomic hybridization. J. Bacteriol. 2007, 189, 4837–4849. [Google Scholar] [CrossRef] [Green Version]
- Faruque, S.M.; Chowdhury, N.; Kamruzzaman, M.; Dziejman, M.; Rahman, M.H.; Sack, D.A.; Nair, G.B.; Mekalanos, J.J. Genetic diversity and virulence potential of environmental Vibrio cholerae population in a cholera-endemic area. Proc. Natl. Acad. Sci. USA 2004, 101, 2123–2128. [Google Scholar] [CrossRef] [Green Version]
- Goel, A.K.; Ponmariappan, S.; Kamboj, D.V.; Singh, L. Single multiplex polymerase chain reaction for environmental surveillance of toxigenic-pathogenic O1 and non-O1 Vibrio cholerae. Folia Microbiol. (Praha) 2007, 52, 81–85. [Google Scholar] [CrossRef]
- Faruque, S.M.; Albert, M.J.; Mekalanos, J.J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 1998, 62, 1301–1314. [Google Scholar] [CrossRef] [Green Version]
- Faruque, S.M.; Abdul Alim, A.R.; Roy, S.K.; Khan, F.; Nair, G.B.; Sack, R.B.; Albert, M.J. Molecular analysis of rRNA and cholera toxin genes carried by the new epidemic strain of toxigenic Vibrio cholerae O139 synonym Bengal. J. Clin. Microbiol. 1994, 32, 1050–1053. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.V.; Isac, S.R.; Colwell, R.R. Development of a hexaplex PCR assay for rapid detection of virulence and regulatory genes in Vibrio cholerae and Vibrio mimicus. J. Clin. Microbiol. 2002, 40, 4321–4324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koskela, K.A.; Matero, P.; Blatny, J.M.; Fykse, E.M.; Olsen, J.S.; Nuotio, L.O.; Nikkari, S. A multiplatform real-time polymerase chain reaction detection assay for Vibrio cholerae. Diagn. Microbiol. Infect. Dis. 2009, 65, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Chua, A.L.; Elina, H.T.; Lim, B.H.; Yean, C.Y.; Ravichandran, M.; Lalitha, P. Development of a dry reagent-based triplex PCR for the detection of toxigenic and non-toxigenic Vibrio cholerae. J. Med. Microbiol. 2011, 60, 481–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashid, R.B.; Ferdous, J.; Tulsiani, S.; Jensen, P.K.M.; Begum, A. Development and Validation of a Novel Real-time Assay for the Detection and Quantification of Vibrio cholerae. Front. Public Health 2017, 5, 109. [Google Scholar] [CrossRef] [Green Version]
- Fykse, E.M.; Skogan, G.; Davies, W.; Olsen, J.S.; Blatny, J.M. Detection of Vibrio cholerae by real-time nucleic acid sequence-based amplification. Appl. Environ. Microbiol. 2007, 73, 1457–1466. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Boucher, Y.; Cordero, O.X.; Takemura, A.; Hunt, D.E.; Schliep, K.; Bapteste, E.; Lopez, P.; Tarr, C.L.; Polz, M.F. Local mobile gene pools rapidly cross species boundaries to create endemicity within global Vibrio cholerae populations. mBio 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Kirchberger, P.C.; Orata, F.D.; Barlow, E.J.; Kauffman, K.M.; Case, R.J.; Polz, M.F.; Boucher, Y. A Small Number of Phylogenetically Distinct Clonal Complexes Dominate a Coastal Vibrio cholerae Population. Appl. Environ. Microbiol. 2016, 82, 5576–5586. [Google Scholar] [CrossRef] [Green Version]
- Carda-Dieguez, M.; Ghai, R.; Rodriguez-Valera, F.; Amaro, C. Wild eel microbiome reveals that skin mucus of fish could be a natural niche for aquatic mucosal pathogen evolution. Microbiome 2017, 5, 162. [Google Scholar] [CrossRef] [Green Version]
- Colwell, R.R. Viable but nonculturable bacteria: A survival strategy. J. Infect. Chemother. 2000, 6, 121–125. [Google Scholar] [CrossRef]
- Sultana, M.; Nusrin, S.; Hasan, N.A.; Sadique, A.; Ahmed, K.U.; Islam, A.; Hossain, A.; Longini, I.; Nizam, A.; Huq, A.; et al. Biofilms Comprise a Component of the Annual Cycle of Vibrio cholerae in the Bay of Bengal Estuary. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, A.; Labbate, M.; Djordjevic, S.P.; Alam, M.; Darling, A.; Melvold, J.; Holmes, A.J.; Johura, F.T.; Cravioto, A.; Charles, I.G.; et al. Indigenous Vibrio cholerae strains from a non-endemic region are pathogenic. Open Biol. 2013, 3, 120181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.; Sultana, M.; Nair, G.B.; Sack, R.B.; Sack, D.A.; Siddique, A.K.; Ali, A.; Huq, A.; Colwell, R.R. Toxigenic Vibrio cholerae in the aquatic environment of Mathbaria, Bangladesh. Appl. Environ. Microbiol. 2006, 72, 2849–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faruque, S.M.; Naser, I.B.; Islam, M.J.; Faruque, A.S.; Ghosh, A.N.; Nair, G.B.; Sack, D.A.; Mekalanos, J.J. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc. Natl. Acad. Sci. USA 2005, 102, 1702–1707. [Google Scholar] [CrossRef] [Green Version]
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, virulence, and infective dose. PLoS Pathog. 2007, 3, 1372–1373. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Islam, A.; Bhuiyan, N.A.; Rahim, N.; Hossain, A.; Khan, G.Y.; Ahmed, D.; Watanabe, H.; Izumiya, H.; Faruque, A.S.; et al. Clonal transmission, dual peak, and off-season cholera in Bangladesh. Infect. Ecol. Epidemiol 2011, 1. [Google Scholar] [CrossRef] [Green Version]
- Davis, B.M.; Kimsey, H.H.; Chang, W.; Waldor, M.K. The Vibrio cholerae O139 Calcutta bacteriophage CTXphi is infectious and encodes a novel repressor. J. Bacteriol. 1999, 181, 6779–6787. [Google Scholar] [CrossRef] [Green Version]
- Trucksis, M.; Michalski, J.; Deng, Y.K.; Kaper, J.B. The Vibrio cholerae genome contains two unique circular chromosomes. Proc. Natl. Acad. Sci. USA 1998, 95, 14464–14469. [Google Scholar] [CrossRef] [Green Version]
- Meibom, K.L.; Li, X.B.; Nielsen, A.T.; Wu, C.Y.; Roseman, S.; Schoolnik, G.K. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA 2004, 101, 2524–2529. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, C.E. The Cholera Years: The United States in 1832, 1849, and 1866; University of Chicago Press: Chicago, IL, USA, 1987; p. 276. [Google Scholar]
- Wright, J.J.; Lee, S.; Zaikova, E.; Walsh, D.A.; Hallam, S.J. DNA extraction from 0.22 microM Sterivex filters and cesium chloride density gradient centrifugation. J. Vis. Exp. 2009. [Google Scholar] [CrossRef] [Green Version]
- Kirchberger, P.C.; Orata, F.D.; Nasreen, T.; Kauffman, K.M.; Tarr, C.L.; Case, R.J.; Polz, M.F.; Boucher, Y.F. Culture-independent tracking of Vibrio cholerae lineages reveals complex spatiotemporal dynamics in a natural population. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stoeckert, C.J., Jr.; Roos, D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Wyckoff, E.E.; Mey, A.R.; Payne, S.M. Iron acquisition in Vibrio cholerae. Biometals 2007, 20, 405–416. [Google Scholar] [CrossRef]
- Butterton, J.R.; Calderwood, S.B. Identification, cloning, and sequencing of a gene required for ferric vibriobactin utilization by Vibrio cholerae. J. Bacteriol. 1994, 176, 5631–5638. [Google Scholar] [CrossRef] [Green Version]
- Menezes, A. Steps for a successful qPCR experiment. Available online: https://www.idtdna.com/pages/education/decoded/article/successful-qpcr (accessed on 27 August 2020).
- Biosystems, A. Creating Standard Curves with Genomic DNA or Plasmid DNA Templates for Use in Quantitative PCR. Available online: http://www.appliedbiosystems.com/support/tutorials/pdf/quant_pcr.pdf (accessed on 27 August 2020).


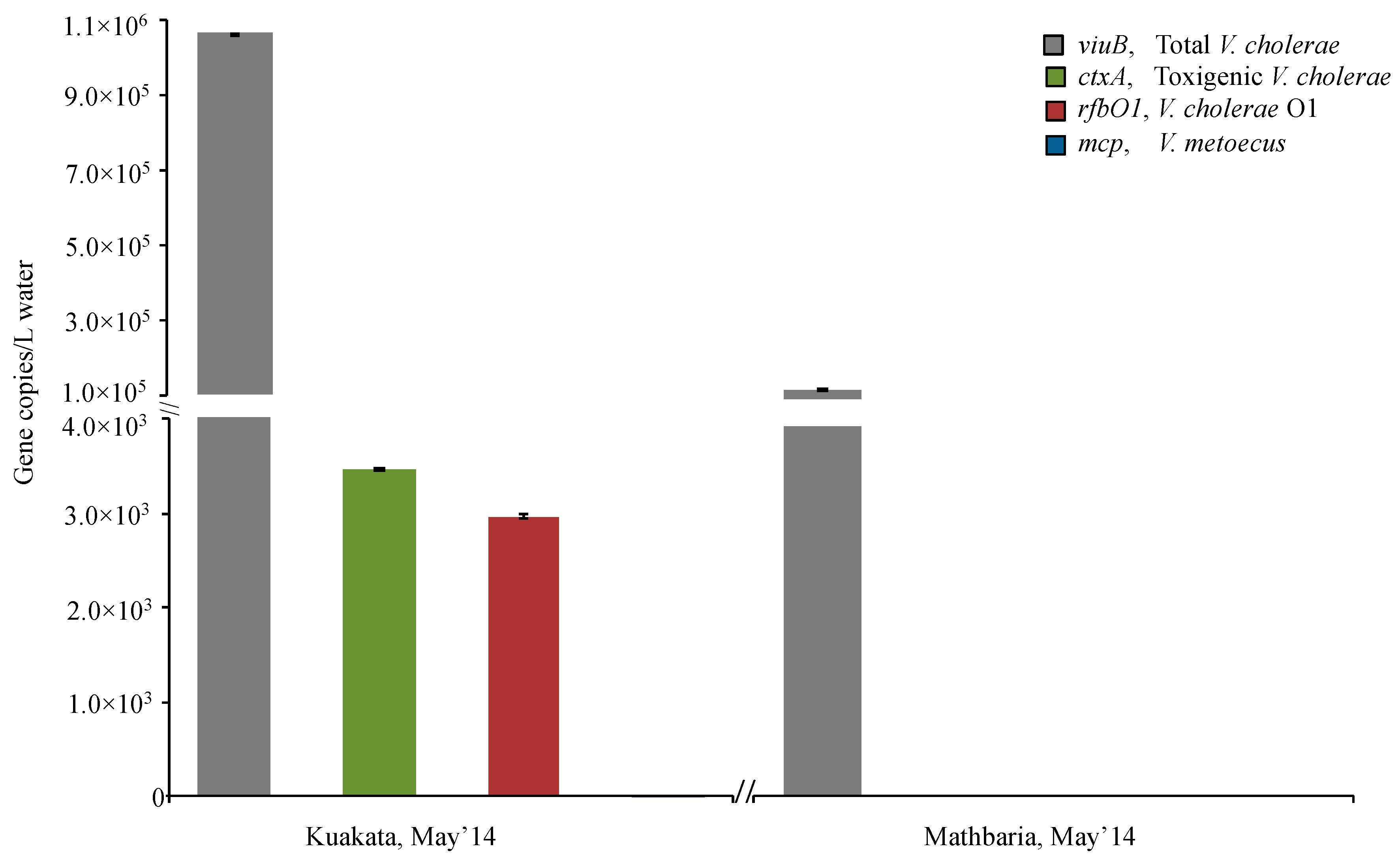


| Target Genes | |||||
|---|---|---|---|---|---|
| Species | No. of Strains | viuB | ctxA | rfbO1 | mcp |
| Vibrio cholerae non O1 | 17 | + | - | - | - |
| Vibrio cholerae O1 CTX- | 2 | + | - | + | - |
| Vibrio cholerae O1 CTX+ | 8 | + | + | + | - |
| Vibrio parahaemolyticus | 1 | - | - | - | - |
| Vibrio vulnificus | 3 | - | - | - | - |
| Vibrio metoecus | 18 | - | - | - | + |
| Vibrio mimicus | 3 | - | - | - | - |
| Escherichia coli | 3 | - | - | - | - |
| Pseudomonas aeruginosa | 3 | - | - | - | - |
| Site | Time | Cultivation | qPCR | ||||
|---|---|---|---|---|---|---|---|
| V. cholerae | V. cholerae O1 | V. metoecus | V. cholerae | V. cholerae O1 | V. metoecus | ||
| Kuakata | May’14 | + | - | - | + | + | - |
| Mathbaria | May’14 | + | - | - | + | - | - |
| Dhaka | Oct’15 | + | + | - | + | + | - |
| Nov’15 | + | - | - | + | + | - | |
| Dec’15 | + | - | - | + | + | - | |
| Jan’16 | + | - | - | + | + | - | |
| Feb’16 | + | - | - | + | + | - | |
| Mar’16 | + | - | - | + | + | - | |
| Oyster Pond | Jun’08 | ND | ND | ND | + | - | - |
| Jul’08 | ND | ND | ND | + | + | - | |
| Aug’08 | ND | ND | ND | + | + | + | |
| Sep’08 | ND | ND | ND | + | - | + | |
| Oyster Pond | Jun’09 | ND | ND | ND | + | - | - |
| Jul’09 | ND | ND | ND | + | + | - | |
| Aug’09 | + | - | + | + | + | + | |
| Sep’09 | + | - | + | + | - | + | |
| Target Gene | Primer and Probe | Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|
| viuB | Probe | 56-FAM/TCATTTGGC/ZEN/CAGAGCATAAACCGGT/3IABkFQ | 77 |
| Forward primer | TCGGTATTGTCTAACGGTAT | ||
| Reverse primer | CGATTCGTGAGGGTGATA | ||
| ctxA | Probe | 5Cy5/AGGACAGAGTGAGTACTTTGACCGAGG/3IAbRQSp | 106 |
| Forward primer | CAGGTGGTCTTATGCCAAG | ||
| Reverse primer | CTAACAAATCCCGTCTGAGTT | ||
| rfbO1 | Probe | 5HEX/AGAAGTGTG/ZEN/TGGGCCAGGTAAAGT/3IABkFQ | 113 |
| Forward primer | GTAAAGCAGGATGGAAACATATTC | ||
| Reverse primer | TGGGCTTACAAACTCAAGTAAG | ||
| mcp | Probe | 5Cy5/TTGTCCGTTTCGACACTGAAAATCA/3IAbRQSp | 81 |
| Forward primer | GCAGTCTCTTGCCGAAACACTA | ||
| Reverse primer | ATGAACAGCTTATCTTGCCATTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasreen, T.; Hussain, N.A.S.; Islam, M.T.; Orata, F.D.; Kirchberger, P.C.; Case, R.J.; Alam, M.; Yanow, S.K.; Boucher, Y.F. Simultaneous Quantification of Vibrio metoecus and Vibrio cholerae with Its O1 Serogroup and Toxigenic Subpopulations in Environmental Reservoirs. Pathogens 2020, 9, 1053. https://doi.org/10.3390/pathogens9121053
Nasreen T, Hussain NAS, Islam MT, Orata FD, Kirchberger PC, Case RJ, Alam M, Yanow SK, Boucher YF. Simultaneous Quantification of Vibrio metoecus and Vibrio cholerae with Its O1 Serogroup and Toxigenic Subpopulations in Environmental Reservoirs. Pathogens. 2020; 9(12):1053. https://doi.org/10.3390/pathogens9121053
Chicago/Turabian StyleNasreen, Tania, Nora A. S. Hussain, Mohammad Tarequl Islam, Fabini D. Orata, Paul C. Kirchberger, Rebecca J. Case, Munirul Alam, Stephanie K. Yanow, and Yann F. Boucher. 2020. "Simultaneous Quantification of Vibrio metoecus and Vibrio cholerae with Its O1 Serogroup and Toxigenic Subpopulations in Environmental Reservoirs" Pathogens 9, no. 12: 1053. https://doi.org/10.3390/pathogens9121053





