Curcumin Reduces Amyloid Fibrillation of Prion Protein and Decreases Reactive Oxidative Stress
Abstract
:1. Introduction
2. Results and Discussion
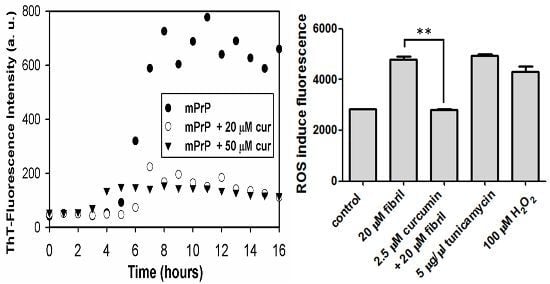
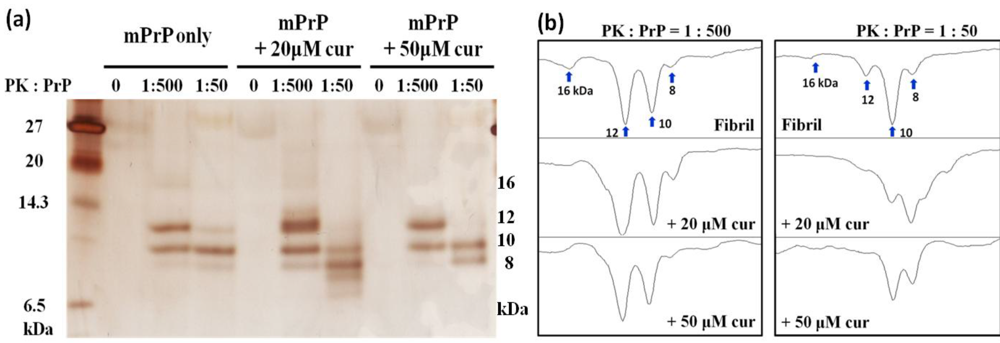
2.1. The Effect of Curcumin on the Cell-Free mPrP Amyloid Formation


2.2. The Effect of Curcumin in Cells

2.2.1. Hemolysis of Mouse Blood
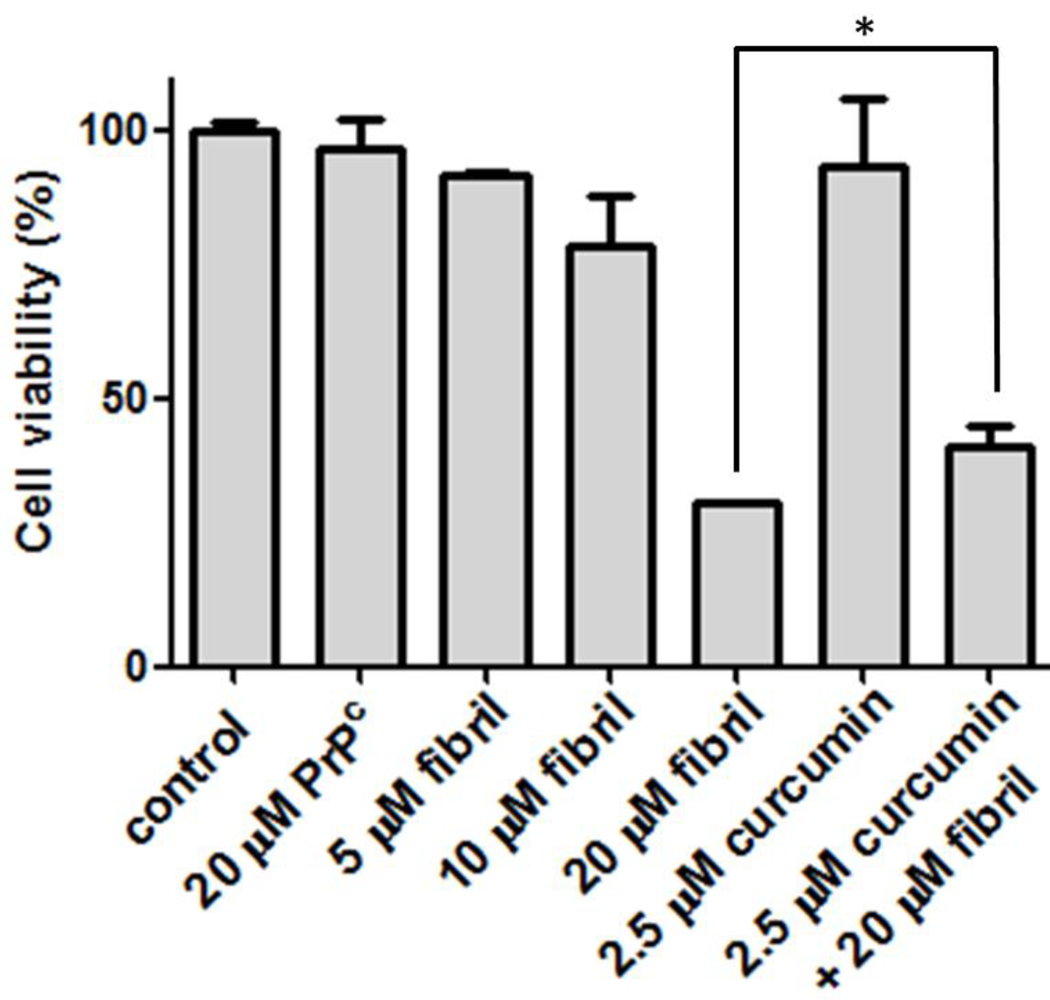
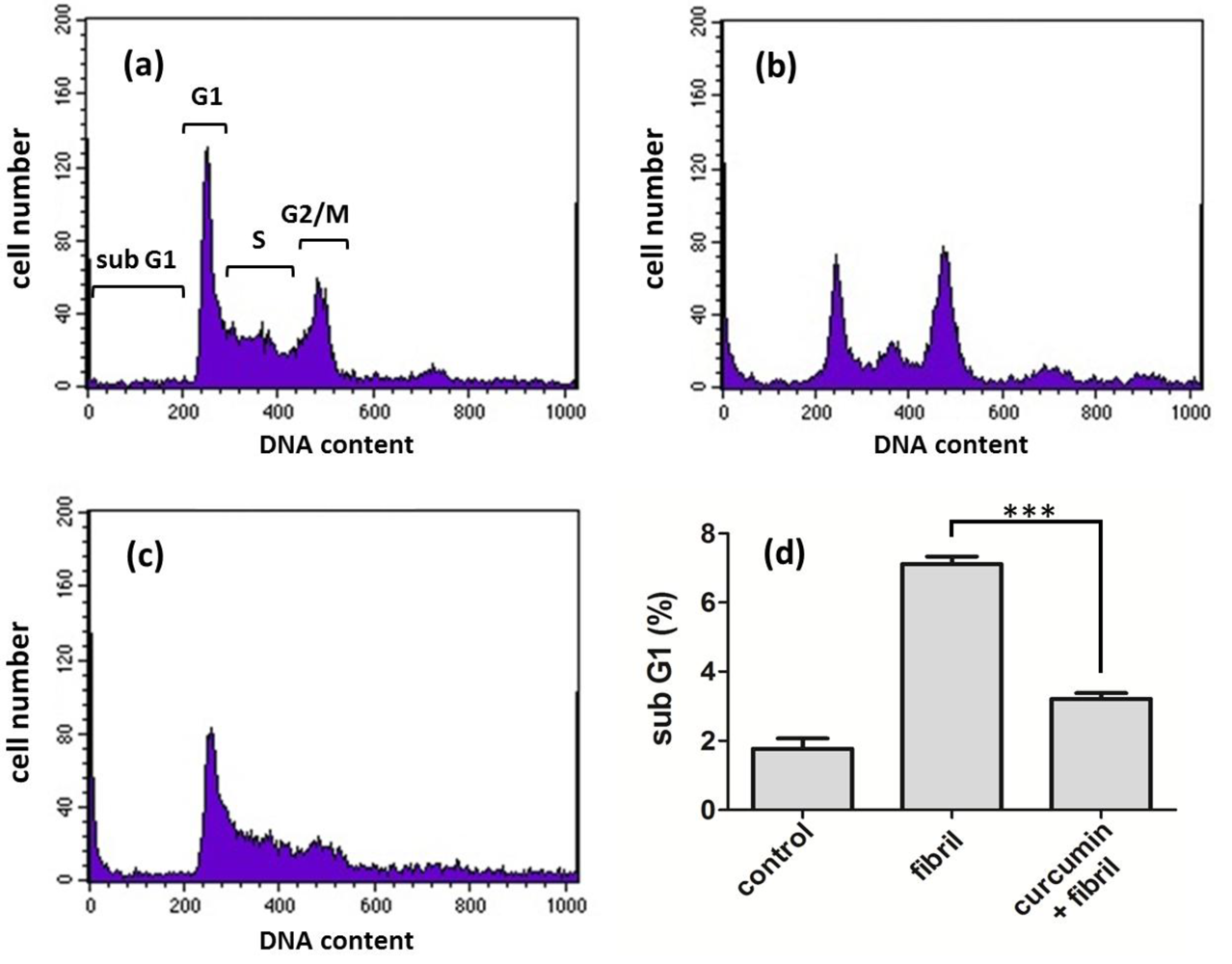
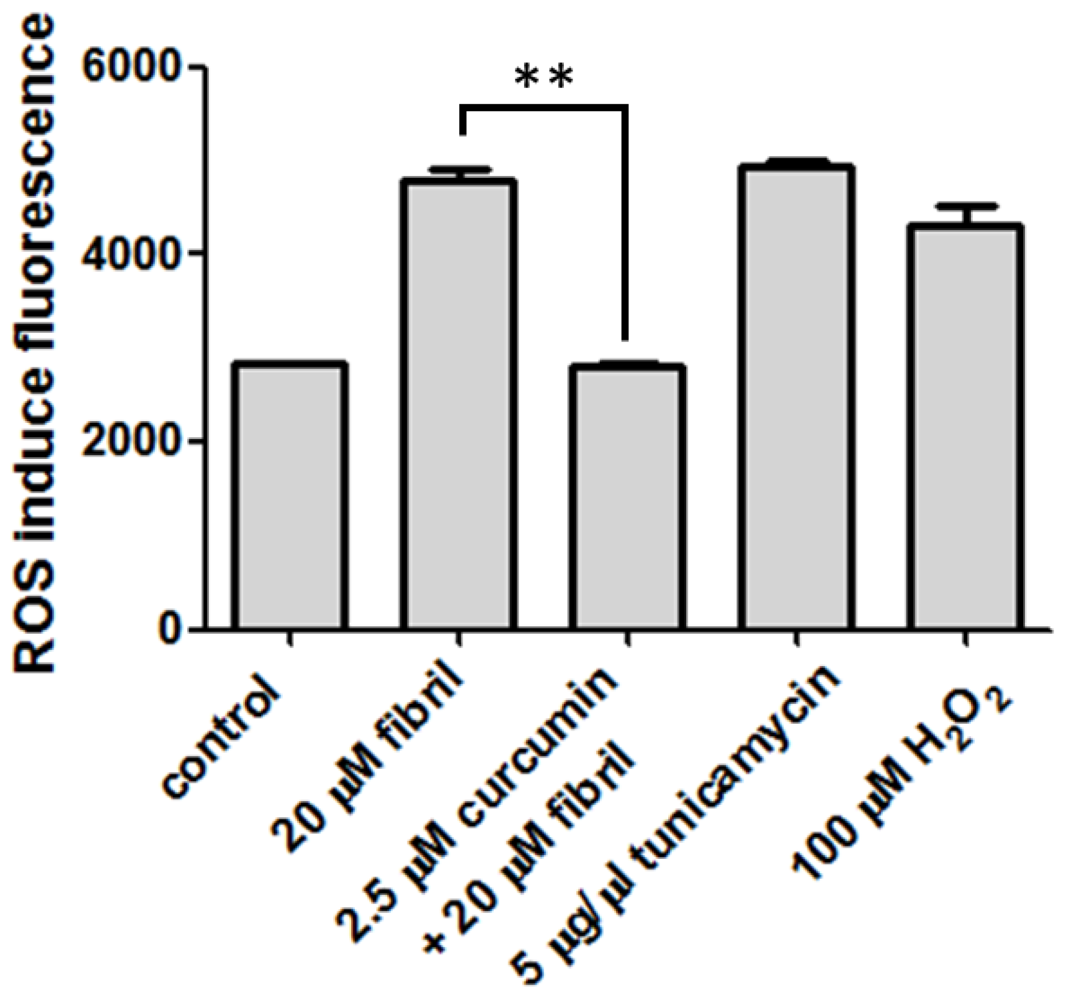
2.2.2. Viability, Apoptosis and ROS Level of N2a Cells
3. Experimental Section
3.1. mPrP Expression and Purification
3.2. Kinetics of Fibril Conversion
3.3. TEM
3.4. Proteinase K Digestion
3.5. Hemolytic Assay
3.6. Cell Culture and Viability Assay
3.7. Cell Apoptosis Analysis
3.8. Cell ROS Measurement
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 1998, 95, 13363–13383. [Google Scholar] [CrossRef]
- Bossy-Wetzel, E.; Schwarzenbacher, R.; Lipton, S.A. Molecular pathways to neurodegeneration. Nat. Med. 2004, 10, S2–S9. [Google Scholar] [CrossRef]
- Brown, P.; Preece, M.; Brandel, J.P.; Sato, T.; McShane, L.; Zerr, I.; Fletcher, A.; Will, R.G.; Pocchiari, M.; Cashman, N.R.; et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 2000, 55, 1075–1081. [Google Scholar] [CrossRef]
- Caughey, B.; Raymond, L.D.; Raymond, G.J.; Maxson, L.; Silveira, J.; Baron, G.S. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 2003, 77, 5499–5502. [Google Scholar] [CrossRef]
- Hafner-Bratkovic, I.; Gaspersic, J.; Smid, L.M.; Bresjanac, M.; Jerala, R. Curcumin binds to the alpha-helical intermediate and to the amyloid form of prion protein - a new mechanism for the inhibition of PrP(Sc) accumulation. J. Neurochem. 2008, 104, 1553–1564. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef]
- Buchanan, M.M.; Hutchinson, M.; Watkins, L.R.; Yin, H. Toll-like receptor 4 in CNS pathologies. J. Neurochem. 2010, 114, 13–27. [Google Scholar]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar]
- Caughey, B.; Race, R.E. Potent inhibition of scrapie-associated PrP accumulation by congo red. J. Neurochem. 1992, 59, 768–771. [Google Scholar] [CrossRef]
- Demaimay, R.; Harper, J.; Gordon, H.; Weaver, D.; Chesebro, B.; Caughey, B. Structural aspects of Congo red as an inhibitor of protease-resistant prion protein formation. J. Neurochem. 1998, 71, 2534–2541. [Google Scholar]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Ollivier Milhavet, S.L. Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res. Rev. 2002, 38, 328–339. [Google Scholar] [CrossRef]
- Bocharova, O.V.; Breydo, L.; Parfenov, A.S.; Salnikov, V.V.; Baskakov, I.V. In vitro conversion of full-length mammalian prion protein produces amyloid form with physical properties of PrP(Sc). J. Mol. Biol. 2005, 346, 645–659. [Google Scholar] [CrossRef]
- Luo, J.C.; Wang, S.C.; Jian, W.B.; Chen, C.H.; Tang, J.L.; Lee, C.I. Formation of amyloid fibrils from beta-amylase. FEBS Lett. 2012, 586, 680–685. [Google Scholar] [CrossRef]
- Schagger, H. Tricine-SDS-PAGE. Nat. Protoc. 2006, 1, 16–22. [Google Scholar] [CrossRef]
- Sunde, M.; Blake, C. The structure of amyloid fibrils by electron microscopy and X-ray diffraction. Adv. Protein Chem. 1997, 50, 123–159. [Google Scholar] [CrossRef]
- Wang, J.B.; Wang, Y.M.; Zeng, C.M. Quercetin inhibits amyloid fibrillation of bovine insulin and destabilizes preformed fibrils. Biochem. Biophys. Res. Commun. 2011, 415, 675–679. [Google Scholar] [CrossRef]
- Williams, T.L.; Serpell, L.C. Membrane and surface interactions of Alzheimer's Abeta peptide--insights into the mechanism of cytotoxicity. FEBS J. 2011, 278, 3905–3917. [Google Scholar] [CrossRef]
- Butterfield, S.M.; Lashuel, H.A. Amyloidogenic protein-membrane interactions: Mechanistic insight from model systems. Angew. Chem. Int. Ed. Engl. 2010, 49, 5628–5654. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol.Pharmaceut. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Institute, N.C. Clinical development plan: Curcumin. J. Cell. Biochem. Suppl. 1996, 26, 72–85. [Google Scholar]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Chandra, V.; Pandav, R.; Dodge, H.H.; Johnston, J.M.; Belle, S.H.; DeKosky, S.T.; Ganguli, M. Incidence of Alzheimer's disease in a rural community in India: The Indo-US study. Neurology 2001, 57, 985–989. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. ix-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Bounhar, Y.; Zhang, Y.; Goodyer, C.G.; LeBlanc, A. Prion protein protects human neurons against Bax-mediated apoptosis. J.Biol. Chem. 2001, 276, 39145–39149. [Google Scholar] [CrossRef]
- Shmerling, D.; Hegyi, I.; Fischer, M.; Blattler, T.; Brandner, S.; Gotz, J.; Rulicke, T.; Flechsig, E.; Cozzio, A.; von Mering, C.; et al. Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 1998, 93, 203–214. [Google Scholar] [CrossRef]
- Kim, J.I.; Ju, W.K.; Choi, J.H.; Choi, E.; Carp, R.I.; Wisniewski, H.M.; Kim, Y.S. Expression of cytokine genes and increased nuclear factor-kappa B activity in the brains of scrapie-infected mice. Brain Res. Mol. Brain Res. 1999, 73, 17–27. [Google Scholar] [CrossRef]
- Perez, M.; Rojo, A.I.; Wandosell, F.; Diaz-Nido, J.; Avila, J. Prion peptide induces neuronal cell death through a pathway involving glycogen synthase kinase 3. Biochem. J. 2003, 372, 129–136. [Google Scholar] [CrossRef]
- Huang, H.C.; Xu, K.; Jiang, Z.F. Curcumin-mediated neuroprotection against amyloid-beta-induced mitochondrial dysfunction involves the inhibition of GSK-3beta. J. Alzheimers. Dis. 2012, 32, 981–996. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, C.-F.; Yu, K.-H.; Jheng, C.-P.; Chung, R.; Lee, C.-I. Curcumin Reduces Amyloid Fibrillation of Prion Protein and Decreases Reactive Oxidative Stress. Pathogens 2013, 2, 506-519. https://doi.org/10.3390/pathogens2030506
Lin C-F, Yu K-H, Jheng C-P, Chung R, Lee C-I. Curcumin Reduces Amyloid Fibrillation of Prion Protein and Decreases Reactive Oxidative Stress. Pathogens. 2013; 2(3):506-519. https://doi.org/10.3390/pathogens2030506
Chicago/Turabian StyleLin, Chi-Fen, Kun-Hua Yu, Cheng-Ping Jheng, Raymond Chung, and Cheng-I Lee. 2013. "Curcumin Reduces Amyloid Fibrillation of Prion Protein and Decreases Reactive Oxidative Stress" Pathogens 2, no. 3: 506-519. https://doi.org/10.3390/pathogens2030506
APA StyleLin, C.-F., Yu, K.-H., Jheng, C.-P., Chung, R., & Lee, C.-I. (2013). Curcumin Reduces Amyloid Fibrillation of Prion Protein and Decreases Reactive Oxidative Stress. Pathogens, 2(3), 506-519. https://doi.org/10.3390/pathogens2030506