The Bovine Tuberculoid Granuloma
Abstract
:1. Introduction
2. Granuloma Staging
3. Early Lesion Development
4. Epithelioid Macrophages, Foamy Macrophages and Multinucleated Giant Cells
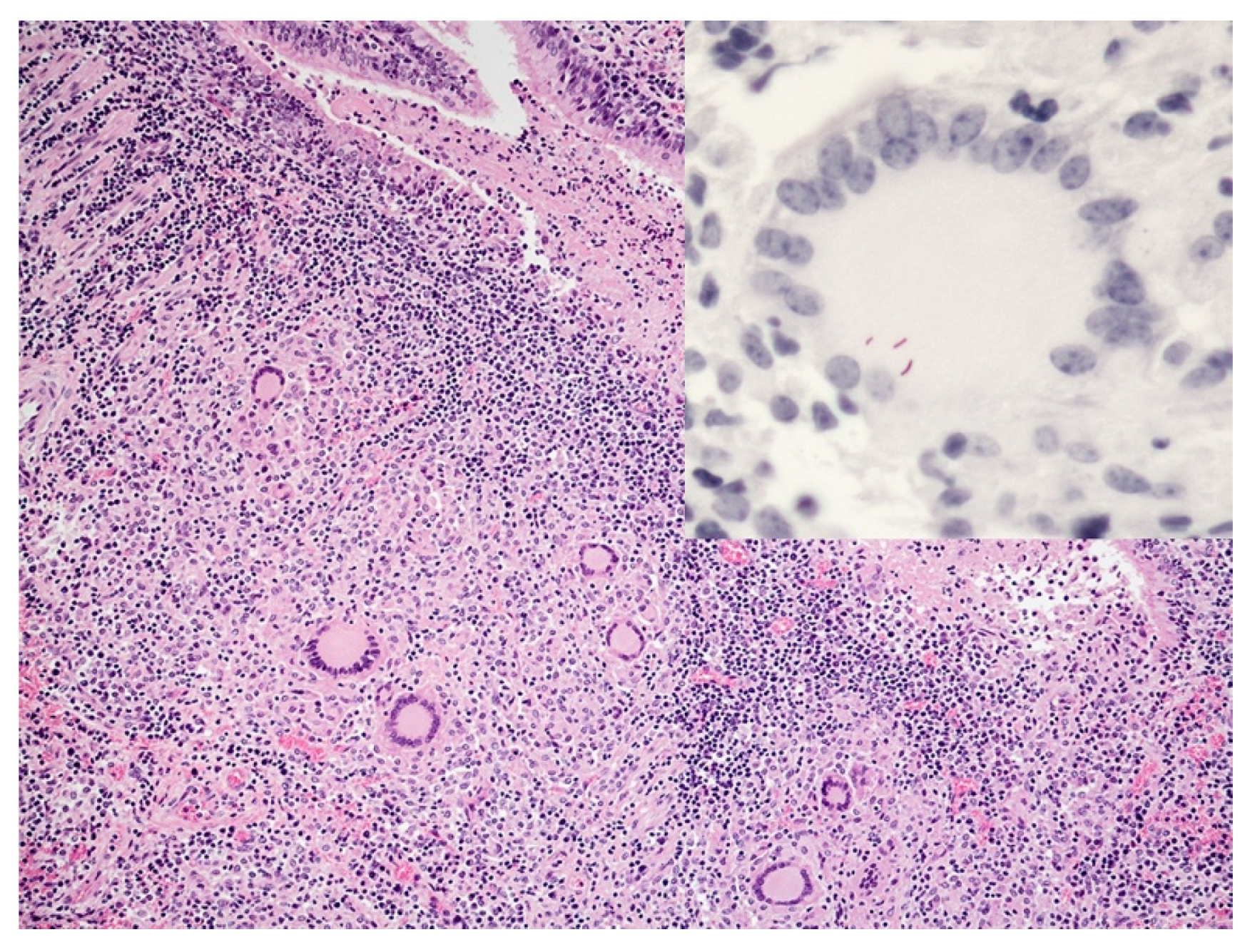
5. Lymphocyte Response
6. Neutrophils
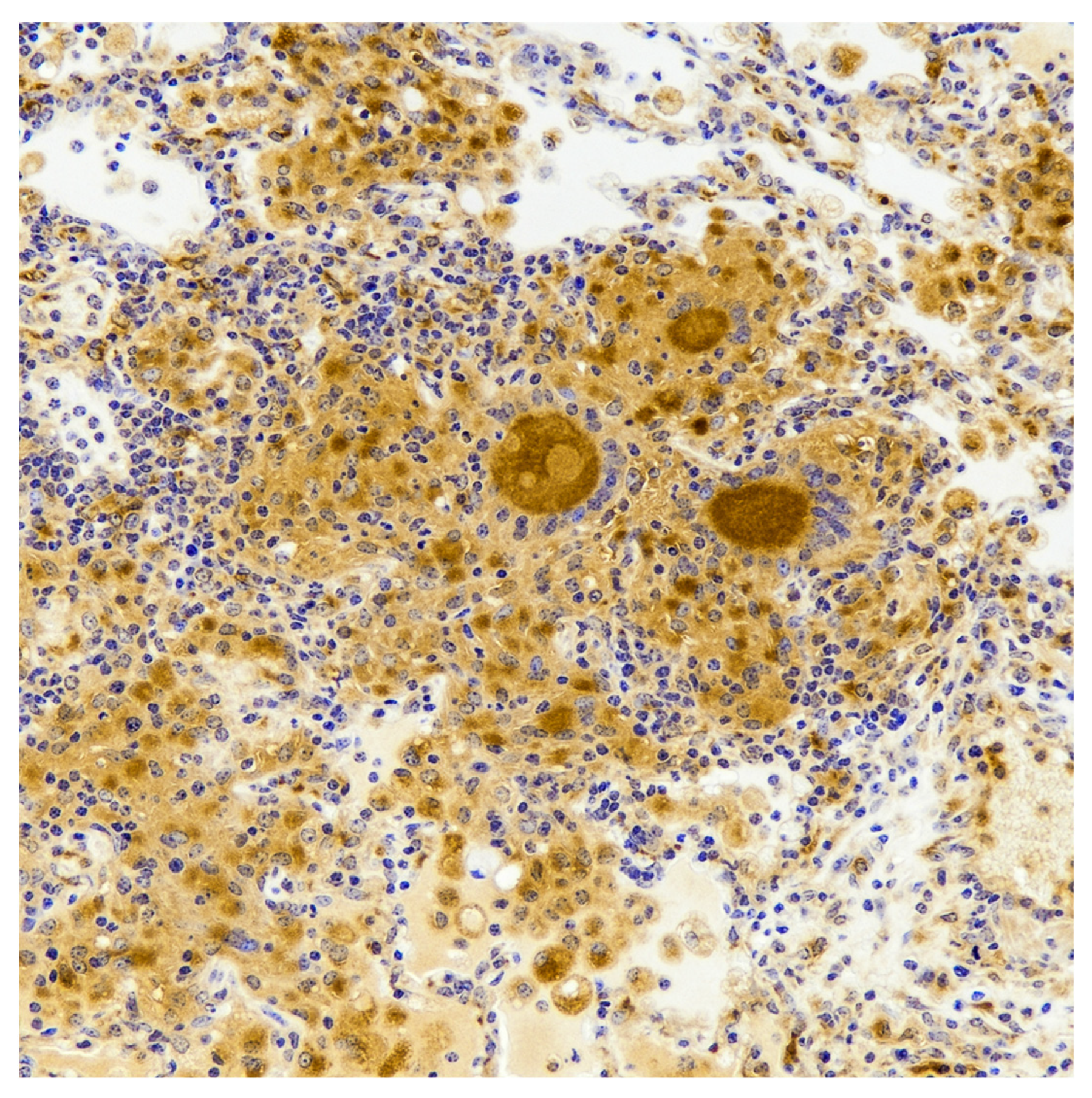
7. Cytokine Response and Bacterial Burdens
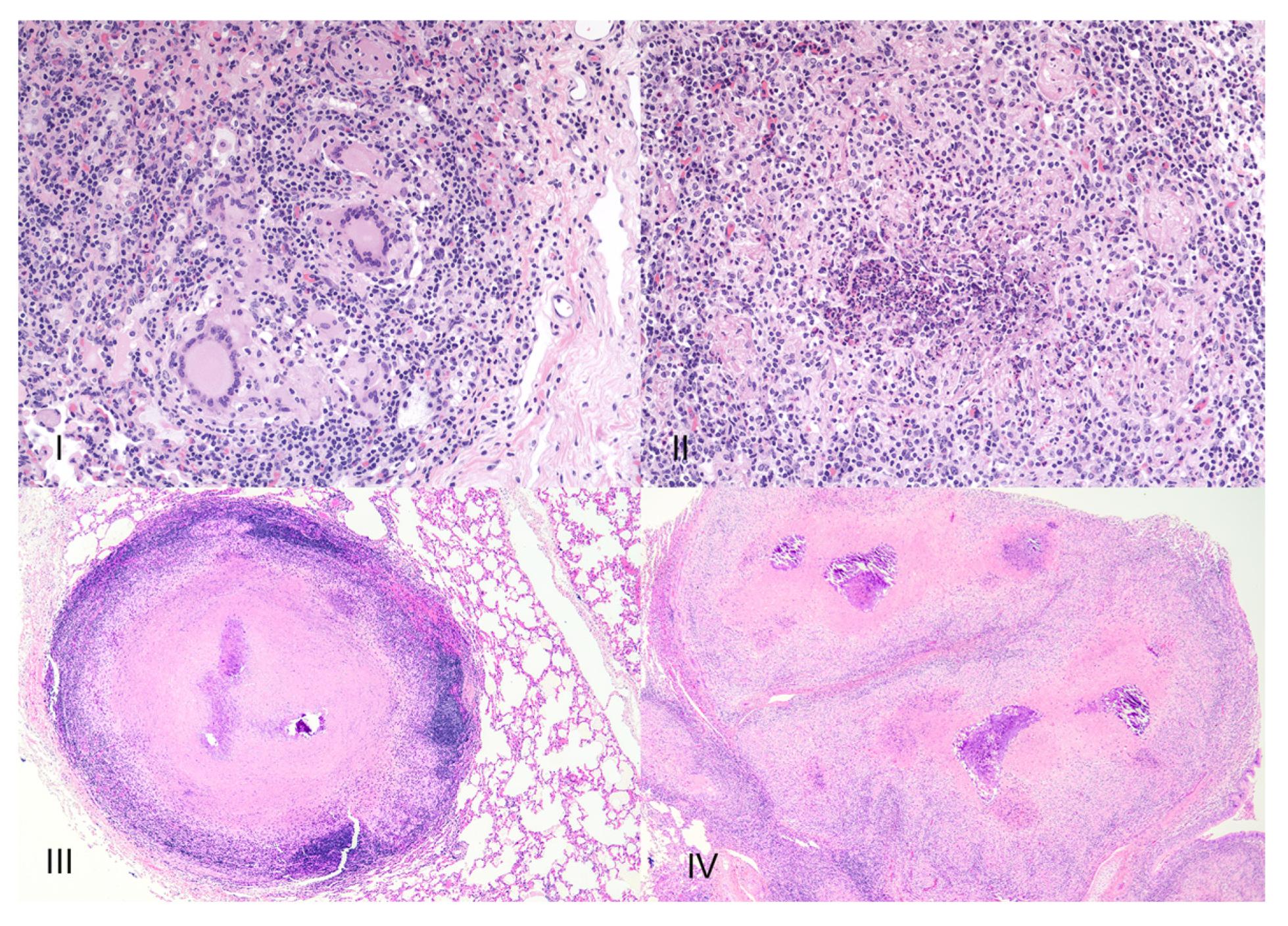
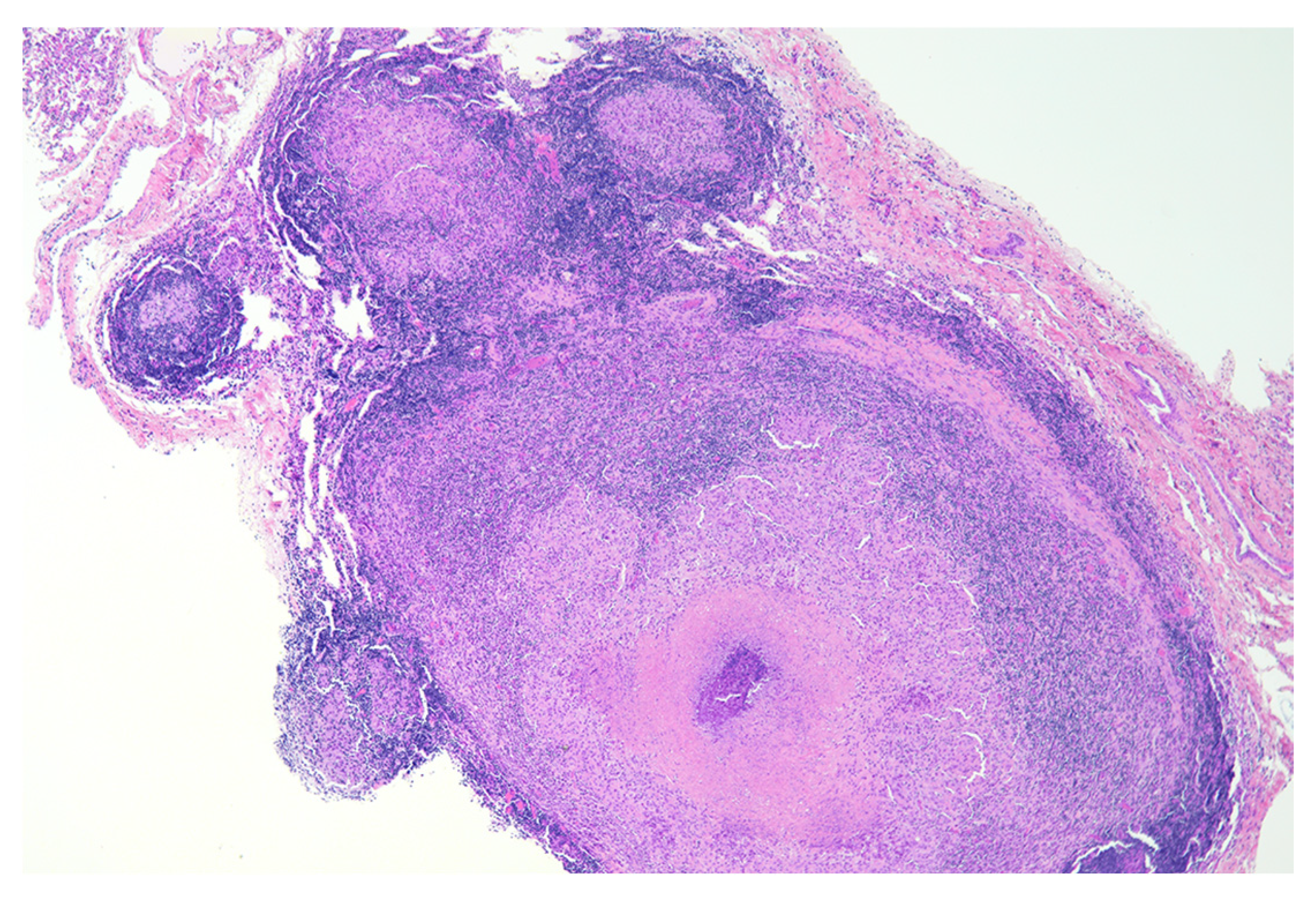
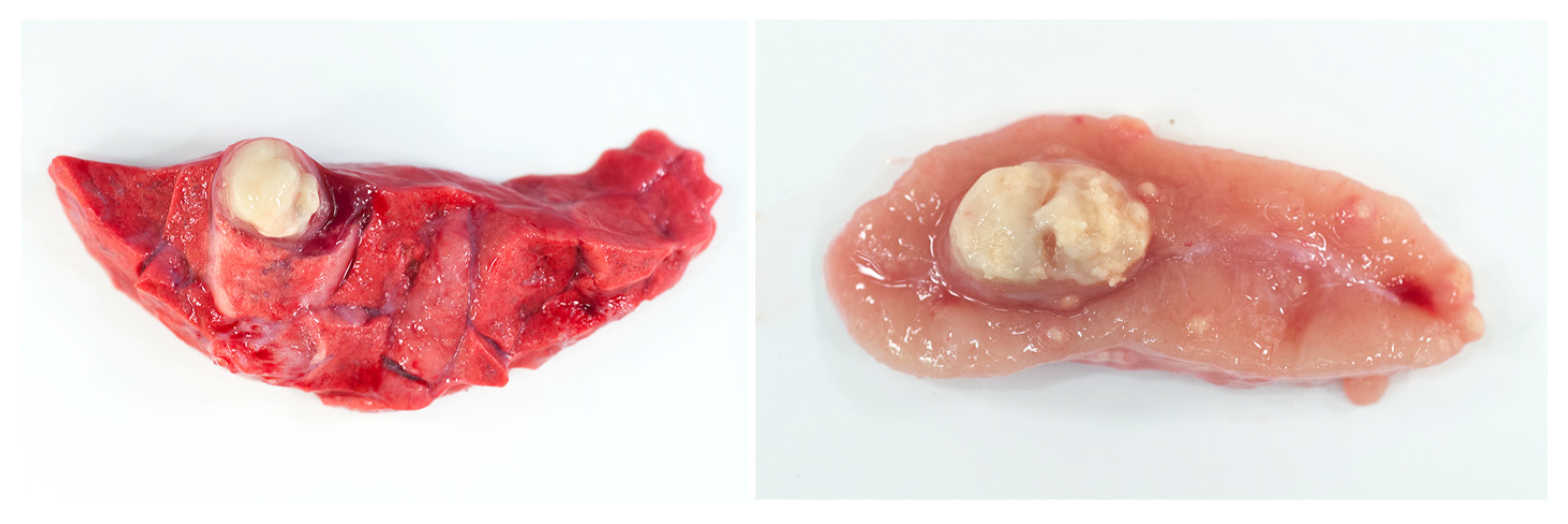
8. Necrosis and Distinct Spatial Arrangement
9. Mineralization
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.N.; Prasad, T.S.; Narayan, P.A.; Muruganandhan, J. Granuloma with Langhans giant cells: An overview. J. Oral Maxillofac. Pathol. 2013, 17, 420–423. [Google Scholar] [CrossRef]
- Pagan, A.J.; Ramakrishnan, L. The formation and function of granulomas. Annu. Rev. Immunol. 2018, 36, 639–665. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; James, D.G. Granulomatous infections: Etiology and classification. Clin. Infect. Dis. 1996, 23, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 2012, 12, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Campos, S.; Smith, N.H.; Boniotti, M.B.; Aranaz, A. Overview and phylogeny of Mycobacterium tuberculosis complex organisms: Implications for diagnostics and legislation of bovine tuberculosis. Res. Vet. Sci. 2014, 97, S5–S19. [Google Scholar] [CrossRef] [PubMed]
- Coscolla, M.; Gagneux, S. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin. Immunol. 2014, 26, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Cousins, D.V.; Bastida, R.; Cataldi, A.; Quse, V.; Redrobe, S.; Dow, S.; Duignan, P.; Murray, A.; Dupont, C.; Ahmed, N.; et al. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int. J. Syst. Evol. Microbiol. 2003, 53, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Cousins, D.V.; Francis, B.R.; Gow, B.L.; Collins, D.M.; McGlashan, C.H.; Gregory, A.; Mackenzie, R.M. Tuberculosis in captive seals: Bacteriological studies on an isolate belonging to the Mycobacterium tuberculosis complex. Res. Vet. Sci. 1990, 48, 196–200. [Google Scholar] [CrossRef]
- Cousins, D.V.; Peet, R.L.; Gaynor, W.T.; Williams, S.N.; Gow, B.L. Tuberculosis in imported hyrax (Procavia capensis) caused by an unusual variant belonging to the Mycobacterium tuberculosis complex. Vet. Microbiol. 1994, 42, 135–145. [Google Scholar] [CrossRef]
- Parsons, S.D.; Drewe, J.A.; Gey van Pittius, N.C.; Warren, R.M.; van Helden, P.D. Novel cause of tuberculosis in meerkats, South Africa. Emerg. Infect. Dis. 2013, 19, 2004–2007. [Google Scholar] [CrossRef]
- Alexander, K.A.; Laver, P.N.; Michel, A.L.; Williams, M.; van Helden, P.D.; Warren, R.M.; Gey van Pittius, N.C. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg. Infect. Dis. 2010, 16, 1296–1299. [Google Scholar] [CrossRef]
- Rusk, R.A.; Palmer, M.V.; Waters, W.R.; McGill, J.L. Measuring bovine gammadelta T cell function at the site of Mycobacterium bovis infection. Vet. Immunol. Immunopathol. 2017, 193–194, 38–49. [Google Scholar] [CrossRef]
- Wells, W.F.; Ratcliffe, H.L.; Grumb, C. On the mechanics of droplet nuclei infection; quantitative experimental air-borne tuberculosis in rabbits. Am. J. Hyg. 1948, 47, 11–28. [Google Scholar]
- Loudon, R.G.; Roberts, R.M. Droplet expulsion from the respiratory tract. Am. Rev. Respir. Dis. 1967, 95, 435–442. [Google Scholar]
- Loudon, R.G.; Roberts, R.M. Singing and the dissemination of tuberculosis. Am. Rev. Respir. Dis. 1968, 98, 297–300. [Google Scholar] [PubMed]
- Wells, W.F. On the mechanics of droplet nuclei infection; apparatus for the quantitative study of droplet nuclei infection of animals. Am. J. Hyg. 1948, 47, 1–10. [Google Scholar]
- McClean, C.M.; Tobin, D.M. Macrophage form, function, and phenotype in mycobacterial infection: Lessons from tuberculosis and other diseases. Pathog. Dis. 2016, 74, ftw068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrt, S.; Schnappinger, D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell. Microbiol. 2009, 11, 1170–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, L.S.; Rajni. Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS J. 2010, 277, 2416–2427. [Google Scholar] [CrossRef]
- Russell, D.G. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol. Rev. 2011, 240, 252–268. [Google Scholar] [CrossRef] [Green Version]
- Smyth, A.; Gordon, S.V. Molecular virulence mechanisms of Mycobacterium bovis. In Bovine Tuberculosis; Chambers, M., Gordon, S.V., Olea-Popelka, F., Barrow, P., Eds.; CABI: Croydon, UK, 2018; pp. 106–121. [Google Scholar]
- Cotran, R.S.; Kumar, V.; Robbins, S.L. The Lung. In Robbins Pathologic Basis of Disease, 5th ed.; Schoen, F.J., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1994; pp. 700–701. [Google Scholar]
- McIlroy, S.G.; Neill, S.D.; McCracken, R.M. Pulmonary lesions and Mycobacterium bovis excretion from the respiratory tract of tuberculin reacting cattle. Vet. Rec. 1986, 118, 718–721. [Google Scholar] [CrossRef]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R. Differential cytokine gene expression in granulomas from lungs and lymph nodes of cattle experimentally infected with aerosolized Mycobacterium bovis. PLoS ONE 2016, 11, e0167471. [Google Scholar] [CrossRef] [PubMed]
- Wangoo, A.; Johnson, L.; Gough, J.; Ackbar, R.; Inglut, S.; Hicks, D.; Spencer, Y.; Hewinson, G.; Vordermeier, M. Advanced granulomatous lesions in Mycobacterium bovis-infected cattle are associated with increased expression of type I procollagen, gamma delta (WC1+) T cells and CD 68+ cells. J. Comp. Pathol. 2005, 133, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Salguero, F.J.; Gibson, S.; Garcia-Jimenez, W.; Gough, J.; Strickland, T.S.; Vordermeier, H.M.; Villarreal-Ramos, B. Differential cell composition and cytokine expression within lymph node granulomas from BCG-vaccinated and non-vaccinated cattle experimentally infected with Mycobacterium bovis. Transbound. Emerg. Dis. 2017, 64, 1734–1749. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.; Gough, J.; Spencer, Y.; Hewinson, G.; Vordermeier, M.; Wangoo, A. Immunohistochemical markers augment evaluation of vaccine efficacy and disease severity in bacillus Calmette-Guerin (BCG) vaccinated cattle challenged with Mycobacterium bovis. Vet. Immunol. Immunopathol. 2006, 111, 219–229. [Google Scholar] [CrossRef]
- Whelan, A.O.; Coad, M.; Upadhyay, B.; Clifford, D.J.; Hewinson, R.G.; Vordermeier, H.M. Lack of correlation between BCG-induced tuberculin skin test sensitisation and protective immunity in cattle. Vaccine 2011, 29, 5453–5458. [Google Scholar] [CrossRef] [PubMed]
- Dean, G.S.; Clifford, D.; Whelan, A.O.; Tchilian, E.Z.; Beverley, P.C.; Salguero, F.J.; Xing, Z.; Vordermeier, H.M.; Villarreal-Ramos, B. Protection induced by simultaneous subcutaneous and endobronchial vaccination with BCG/BCG and BCG/Adenovirus expressing antigen 85A against Mycobacterium bovis in cattle. PLoS ONE 2015, 10, e0142270. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, C.; Bianco, M.V.; Blanco, F.C.; Soria, M.; Gravisaco, M.J.; Montenegro, V.; Vagnoni, L.; Buddle, B.; Garbaccio, S.; Delgado, F.; et al. Vaccination with a BCG strain overexpressing Ag85B protects cattle against Mycobacterium bovis challenge. PLoS ONE 2012, 7, e51396. [Google Scholar] [CrossRef]
- Dean, G.; Whelan, A.; Clifford, D.; Salguero, F.J.; Xing, Z.; Gilbert, S.; McShane, H.; Hewinson, R.G.; Vordermeier, M.; Villarreal-Ramos, B. Comparison of the immunogenicity and protection against bovine tuberculosis following immunization by BCG-priming and boosting with adenovirus or protein based vaccines. Vaccine 2014, 32, 1304–1310. [Google Scholar] [CrossRef]
- Aranday-Cortes, E.; Hogarth, P.J.; Kaveh, D.A.; Whelan, A.O.; Villarreal-Ramos, B.; Lalvani, A.; Vordermeier, H.M. Transcriptional profiling of disease-induced host responses in bovine tuberculosis and the identification of potential diagnostic biomarkers. PLoS ONE 2012, 7, e30626. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.V.; Waters, W.R.; Thacker, T.C. Lesion development and immunohistochemical changes in granulomas from cattle experimentally infected with Mycobacterium bovis. Vet. Pathol. 2007, 44, 863–874. [Google Scholar] [CrossRef]
- Palmer, M.V.; Thacker, T.C.; Kanipe, C.; Boggiatto, P.M. Heterogeneity among pulmonary granulomas in cattle experimentally infected with Mycobacterium bovis. Front. Vet. Sci. 2021, 8, 671460. [Google Scholar] [CrossRef]
- Menin, A.; Fleith, R.; Reck, C.; Marlow, M.; Fernandes, P.; Pilati, C.; Bafica, A. Asymptomatic cattle naturally infected with Mycobacterium bovis present exacerbated tissue pathology and bacterial dissemination. PLoS ONE 2013, 8, e53884. [Google Scholar] [CrossRef]
- Carrisoza-Urbina, J.; Morales-Salinas, E.; Bedolla-Alva, M.A.; Hernandez-Pando, R.; Gutierrez-Pabello, J.A. Atypical granuloma formation in Mycobacterium bovis-infected calves. PLoS ONE 2019, 14, e0218547. [Google Scholar] [CrossRef] [Green Version]
- Liebana, E.; Marsh, S.; Gough, J.; Nunez, A.; Vordermeier, H.M.; Whelan, A.; Spencer, Y.; Clifton-Hardley, R.; Hewinson, G.; Johnson, L. Distribution and activation of T-lymphocyte subsets in tuberculous bovine lymph-node granulomas. Vet. Pathol. 2007, 44, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.V.; Wiarda, J.; Kanipe, C.; Thacker, T.C. Early pulmonary lesions in cattle infected via aerosolized Mycobacterium bovis. Vet. Pathol. 2019, 56, 544–554. [Google Scholar] [CrossRef]
- Cassidy, J.P.; Bryson, D.G.; Pollock, J.M.; Evans, R.T.; Forster, F.; Neill, S.D. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J. Comp. Pathol. 1998, 119, 27–44. [Google Scholar] [CrossRef]
- Aranday-Cortes, E.; Bull, N.C.; Villarreal-Ramos, B.; Gough, J.; Hicks, D.; Ortiz-Pelaez, A.; Vordermeier, H.M.; Salguero, F.J. Upregulation of IL-17A, CXCL9 and CXCL10 in early-stage granulomas induced by Mycobacterium bovis in cattle. Transbound. Emerg. Dis. 2013, 60, 525–537. [Google Scholar] [CrossRef]
- Davis, J.M.; Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 2009, 136, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marakalala, M.J.; Raju, R.M.; Sharma, K.; Zhang, Y.J.; Eugenin, E.A.; Prideaux, B.; Daudelin, I.B.; Chen, P.Y.; Booty, M.G.; Kim, J.H.; et al. Inflammatory signaling in human tuberculosis granulomas is spatially organized. Nat. Med. 2016, 22, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattila, J.T.; Ojo, O.O.; Kepka-Lenhart, D.; Marino, S.; Kim, J.H.; Eum, S.Y.; Via, L.E.; Barry, C.E., 3rd; Klein, E.; Kirschner, D.E.; et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 2013, 191, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Suarez, A.L.; Estrada-Chavez, C.; Arriaga-Diaz, C.; Espinosa-Cueto, P.; Mancilla, R. Coexpression of NRAMP1, iNOS, and nitrotyrosine in bovine tuberculosis. Vet. Pathol. 2006, 43, 709–717. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, Y.; Liu, D.; Dai, X.; Wang, M.; Dai, Y. Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis induces transition of macrophages into epithelioid macrophages by downregulating iNOS / NO-mediated H3K27 trimethylation in macrophages. Mol. Immunol. 2020, 117, 189–200. [Google Scholar] [CrossRef]
- Adams, D.O. The structure of mononuclear phagocytes differentiating in vivo. Am. J. Pathol. 1974, 76, 17–48. [Google Scholar]
- Cotran, R.S.; Kumar, V.; Robbins, S.L. Inflammation and Repair. In Robbins Pathologic Basis of Disease, 5th ed.; Schoen, F.J., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1994; pp. 51–92. [Google Scholar]
- Cronan, M.R.; Beerman, R.W.; Rosenberg, A.F.; Saelens, J.W.; Johnson, M.G.; Oehlers, S.H.; Sisk, D.M.; Jurcic Smith, K.L.; Medvitz, N.A.; Miller, S.E.; et al. Macrophage epithelial reprogramming underlies mycobacterial granuloma formation and promotes infection. Immunity 2016, 45, 861–876. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.O. The granulomatous inflammatory response. A review. Am. J. Pathol. 1976, 84, 164–192. [Google Scholar]
- Turk, J.L.; Narayanan, R.B. The origin, morphology, and function of epithelioid cells. Immunobiology 1982, 161, 274–282. [Google Scholar] [CrossRef]
- Mariano, M.; Malucelli, B.E. Defective phagocytic ability of epithelioid cells reversed by levamisole. J. Pathol. 1980, 130, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, F.; Vermi, W.; Fiorentini, S.; Chilosi, M.; Caruso, A.; Duse, M.; Notarangelo, L.D.; Badolato, R. Expression of inducible nitric oxide synthase in human granulomas and histiocytic reactions. Am. J. Pathol. 1999, 154, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Russell, D.G.; Cardona, P.J.; Kim, M.J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, R.L. Pathology of post primary tuberculosis of the lung: An illustrated critical review. Tuberculosis 2011, 91, 497–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lay, G.; Poquet, Y.; Salek-Peyron, P.; Puissegur, M.P.; Botanch, C.; Bon, H.; Levillain, F.; Duteyrat, J.L.; Emile, J.F.; Altare, F. Langhans giant cells from M. tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J. Pathol. 2007, 211, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Torrelles, J.B.; Sieling, P.A.; Arcos, J.; Knaup, R.; Bartling, C.; Rajaram, M.V.; Stenger, S.; Modlin, R.L.; Schlesinger, L.S. Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J. Biol. Chem. 2011, 286, 35438–35446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.W.; Friedland, J.S. Multinucleate giant cells and the control of chemokine secretion in response to Mycobacterium tuberculosis. Clin. Immunol. 2006, 120, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Waters, W.R.; Palmer, M.V.; Nonnecke, B.J.; Thacker, T.C.; Estes, D.M.; Larsen, M.H.; Jacobs, W.R., Jr.; Andersen, P.; McNair, J.; Minion, F.C.; et al. Signal regulatory protein alpha (SIRPalpha) cells in the adaptive response to ESAT-6/CFP-10 protein of tuberculous mycobacteria. PLoS ONE 2009, 4, e6414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheville, N.F. Healing and Chronic Inflammation, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1999; pp. 135–152. [Google Scholar]
- Neill, S.D.; Pollock, J.M.; Bryson, D.B.; Hanna, J. Pathogenesis of Mycobacterium bovis infection in cattle. Vet. Microbiol. 1994, 40, 41–52. [Google Scholar] [CrossRef]
- Gharun, K.; Senges, J.; Seidl, M.; Losslein, A.; Kolter, J.; Lohrmann, F.; Fliegauf, M.; Elgizouli, M.; Alber, M.; Vavra, M.; et al. Mycobacteria exploit nitric oxide-induced transformation of macrophages into permissive giant cells. EMBO Rep. 2017, 18, 2144–2159. [Google Scholar] [CrossRef]
- Herrtwich, L.; Nanda, I.; Evangelou, K.; Nikolova, T.; Horn, V.; Sagar; Erny, D.; Stefanowski, J.; Rogell, L.; Klein, C.; et al. DNA damage signaling instructs polyploid macrophage fate in granulomas. Cell 2016, 167, 1264–1280. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Budischak, S.A.; Buss, P.; Seguel, M.; Luikart, G.; Jolles, A.E.; Sakamoto, K. Natural resistance to worms exacerbates bovine tuberculosis severity independently of worm coinfection. Proc. Natl. Acad. Sci. USA 2021, 118, e2015080118. [Google Scholar] [CrossRef]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R. Multinucleated giant cell cytokine expression in pulmonary granulomas of cattle experimentally infected with Mycobacterium bovis. Vet. Immunol. Immunopathol. 2016, 180, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, S.H. Tuberculosis: Back on the immunologists’ agenda. Immunity 2006, 24, 351–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, S.H.E. CD8+ T lymphocytes in intracellular microbial infections. Immunol. Today 1988, 9, 168–174. [Google Scholar] [CrossRef]
- Liebana, E.; Johnson, L.; Gough, J.; Durr, P.; Jahans, K.; Clifton-Hadley, R.; Spencer, Y.; Hewinson, R.G.; Downs, S.H. Pathology of naturally occurring bovine tuberculosis in England and Wales. Vet. J. 2007, 176, 354–360. [Google Scholar] [CrossRef] [PubMed]
- McGill, J.L.; Sacco, R.E.; Baldwin, C.L.; Telfer, J.C.; Palmer, M.V.; Waters, W.R. The role of gamma delta T cells in immunity to Mycobacterium bovis infection in cattle. Vet. Immunol. Immunopathol. 2014, 159, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.P.; Bryson, D.G.; Gutierrez Cancela, M.M.; Forster, F.; Pollock, J.M.; Neill, S.D. Lymphocyte subtypes in experimentally induced early-stage bovine tuberculous lesions. J. Comp. Pathol. 2001, 124, 46–51. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Olin, M.R.; Cho, S.N.; Molitor, T.W. T cells in immunity induced by Mycobacterium bovis bacillus Calmette-Guerin vaccination. Infect. Immun. 2004, 72, 1504–1511. [Google Scholar] [CrossRef] [Green Version]
- Palmer, M.V.; Thacker, T.C.; Waters, W.R. Analysis of cytokine gene expression using a novel chromogenic in-situ hybridization method in pulmonary granulomas of cattle infected experimentally by aerosolized Mycobacterium bovis. J. Comp. Pathol. 2015, 153, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, S.; Vordermeier, H.M.; Jones, G.J. CD4+ and gammadelta T Cells are the main producers of IL-22 and IL-17A in lymphocytes from Mycobacterium bovis-infected cattle. Sci. Rep. 2016, 6, 29990. [Google Scholar] [CrossRef] [Green Version]
- Ladero-Auñon, I.; Molina, E.; Holder, A.; Kolakowski, J.; Harris, H.; Urkitza, A.; Anguita, J.; Werling, D.; Elguezabal, N. Bovine neutrophils release extracellular traps and cooperate with macrophages in Mycobacterium avium subsp. paratuberculosis clearance in vitro. Front. Immunol. 2021, 12, 645304. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar] [CrossRef] [Green Version]
- Lyadova, I.V. Neutrophils in Tuberculosis: Heterogeneity Shapes the Way? Mediat. Inflamm. 2017, 2017, 8619307. [Google Scholar] [CrossRef] [Green Version]
- Eum, S.Y.; Kong, J.H.; Hong, M.S.; Lee, Y.J.; Kim, J.H.; Hwang, S.H.; Cho, S.N.; Via, L.E.; Barry, C.E., 3rd. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010, 137, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Shu, D.; Heiser, A.; Wedlock, D.N.; Luo, D.; de Lisle, G.W.; Buddle, B.M. Comparison of gene expression of immune mediators in lung and pulmonary lymph node granulomas from cattle experimentally infected with Mycobacterium bovis. Vet. Immunol. Immunopathol. 2014, 160, 81–89. [Google Scholar] [CrossRef]
- Pritchard, D.G. A century of bovine tuberculosis 1888-1988: Conquest and controversy. J. Comp. Pathol. 1988, 99, 357–399. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, J.P.; Bryson, D.G.; Pollock, J.M.; Evans, R.T.; Forster, F.; Neill, S.D. Lesions in cattle exposed to Mycobacterium bovis-inoculated calves. J. Comp. Pathol. 1999, 121, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.C.; Rice-Ficht, A.C.; Estes, D.M. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 1998, 63, 45–55. [Google Scholar] [CrossRef]
- Spellberg, B.; Edwards, J.E. Type 1/ Type 2 immunity in infectious diseases. Clin. Infect. Dis. 2001, 32, 76–102. [Google Scholar] [CrossRef]
- Tulu, B.; Martineau, H.M.; Zewude, A.; Desta, F.; Jolliffe, D.A.; Abebe, M.; Balcha, T.T.; Belay, M.; Martineau, A.R.; Ameni, G. Cellular and cytokine responses in granulomas of asymptomatic cattle naturally infected with Mycobacterium bovis in Ethiopia. Infect. Immun. 2020, 88, e00507-20. [Google Scholar] [CrossRef] [PubMed]
- Boddu-Jasmine, H.C.; Witchell, J.; Vordermeier, M.; Wangoo, A.; Goyal, M. Cytokine mRNA expression in cattle infected with different dosages of Mycobacterium bovis. Tuberculosis 2008, 88, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Witchell, J.; Maddipatla, S.V.; Wangoo, A.; Vordermeier, M.; Goyal, M. Time dependent expression of cytokines in Mycobacterium bovis infected cattle lymph nodes. Vet. Immunol. Immunopathol. 2010, 138, 79–84. [Google Scholar] [CrossRef]
- Canal, A.M.; Pezzone, N.; Cataldi, A.; Zumarraga, M.; Larzabal, M.; Garbaccio, S.; Fernandez, A.; Dominguez, L.; Aranaz, A.; Rodriguez-Bertos, A. Immunohistochemical detection of pro-inflammatory and anti-inflammatory cytokines in granulomas in cattle with natural Mycobacterium bovis infection. Res. Vet. Sci. 2017, 110, 34–39. [Google Scholar] [CrossRef]
- Johnson, L.; Dean, G.; Rhodes, S.; Hewinson, G.; Vordermeier, M.; Wangoo, A. Low-dose Mycobacterium bovis infection in cattle results in pathology indistinguishable from that of high-dose infection. Tuberculosis 2007, 87, 71–76. [Google Scholar] [CrossRef]
- Wangoo, A.; Cook, H.T.; Taylor, G.M.; Shaw, R.J. Enhanced expression of type 1 procollagen and transforming growth factor-beta in tuberculin induced delayed type hypersensitivity. J. Clin. Pathol. 1995, 48, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Beytut, E. Immunohistochemical evaluation of surfactant proteins and lymphocyte phenotypes in the lungs of cattle with natural tuberculosis. Res. Vet. Sci. 2011, 91, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Widdison, S.; Watson, M.; Coffey, T.J. Correlation between lymph node pathology and chemokine expression during bovine tuberculosis. Tuberculosis 2009, 89, 417–422. [Google Scholar] [CrossRef]
- Palmer, M.V.; Waters, W.R.; Whipple, D.L. Aerosol delivery of virulent Mycobacterium bovis to cattle. Tuberculosis 2002, 82, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Thacker, T.C.; Palmer, M.V.; Waters, W.R. Associations between cytokine gene expression and pathology in Mycobacterium bovis infected cattle. Vet. Immunol. Immunopathol. 2007, 119, 204–213. [Google Scholar] [CrossRef]
- Guirado, E.; Schlesinger, L.S. Modeling the Mycobacterium tuberculosis granuloma—The critical battlefield in host immunity and disease. Front. Immunol. 2013, 4, 98. [Google Scholar] [CrossRef] [Green Version]
- Etna, M.P.; Giacomini, E.; Severa, M.; Coccia, E.M. Pro- and anti-inflammatory cytokines in TB: A two-edged sword in TB pathogenesis. Semin. Immunol. 2014, 26, 543–551. [Google Scholar] [CrossRef]
- Beamer, G.L.; Flaherty, D.K.; Assogba, B.D.; Stromberg, P.; Gonzalez-Juarrero, M.; de Waal Malefyt, R.; Vesosky, B.; Turner, J. Interleukin-10 promotes Mycobacterium tuberculosis disease progression in CBA/J mice. J. Immunol. 2008, 181, 5545–5550. [Google Scholar] [CrossRef] [Green Version]
- Bonecini-Almeida, M.G.; Ho, J.L.; Boechat, N.; Huard, R.C.; Chitale, S.; Doo, H.; Geng, J.; Rego, L.; Lazzarini, L.C.; Kritski, A.L.; et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect. Immun. 2004, 72, 2628–2634. [Google Scholar] [CrossRef] [Green Version]
- Verbon, A.; Juffermans, N.P.; van Deventer, S.J.; Speelman, P.; VanDeutekom, H.V. Serum concentrations of cytokines in patients with active tuberculosis (TB) and after treatment. Clin. Exp. Immunol. 1999, 115, 110–113. [Google Scholar] [CrossRef]
- Waters, W.R.; Maggioli, M.F.; Palmer, M.V.; Thacker, T.C.; McGill, J.L.; Vordermeier, H.M.; Berney-Meyer, L.; Jacobs, W.R.; Larsen, M.H. Interleukin 17A as a biomarker for bovine tuberculosis. Clin. Vaccine Immunol. 2016, 23, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vordermeier, H.M.; Villarreal-Ramos, B.; Cockle, P.J.; McAulay, M.; Rhodes, S.G.; Thacker, T.; Gilbert, S.C.; McShane, H.; Hill, A.V.; Xing, Z.; et al. Viral booster vaccines improve Mycobacterium bovis BCG-induced protection against bovine tuberculosis. Infect. Immun. 2009, 77, 3364–3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddle, B.M.; de Lisle, G.W.; Griffin, J.F.; Hutchings, S.A. Epidemiology, diagnostics, and management of tuberculosis in domestic cattle and deer in New Zealand in the face of a wildlife reservoir. N. Z. Vet. J. 2015, 63, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Ulrichs, T.; Lefmann, M.; Reich, M.; Morawietz, L.; Roth, A.; Brinkmann, V.; Kosmiadi, G.A.; Seiler, P.; Aichele, P.; Hahn, H.; et al. Modified immunohistological staining allows detection of Ziehl-Neelsen-negative Mycobacterium tuberculosis organisms and their precise localization in human tissue. J. Pathol. 2005, 205, 633–640. [Google Scholar] [CrossRef]
- Fayyazi, A.; Eichmeyer, B.; Soruri, A.; Schweyer, S.; Herms, J.; Schwarz, P.; Radzun, H.J. Apoptosis of macrophages and T cells in tuberculosis associated caseous necrosis. J. Pathol. 2000, 191, 417–425. [Google Scholar] [CrossRef]
- Leong, A.S.; Wannakrairot, P.; Leong, T.Y. Apoptosis is a major cause of so-called “caseous necrosis” in mycobacterial granulomas in HIV-infected patients. J. Clin. Pathol. 2008, 61, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Converse, P.J.; Dannenberg, A.M., Jr.; Estep, J.E.; Sugisaki, K.; Abe, Y.; Schofield, B.H.; Pitt, M.L. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect. Immun. 1996, 64, 4776–4787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helke, K.L.; Mankowski, J.L.; Manabe, Y.C. Animal models of cavitation in pulmonary tuberculosis. Tuberculosis 2006, 86, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Medlar, E.M. Pulmonary tuberculosis in cattle. Am. Rev. Tuberc. 1940, 41, 283–306. [Google Scholar]
- Cassidy, J.P. The pathogenesis and pathology of bovine tuberculosis with insights from studies of tuberculosis in humans and laboratory animal models. Vet. Microbiol. 2006, 112, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Van Rhijn, I.; Godfroid, J.; Michel, A.; Rutten, V. Bovine tuberculosis as a model for human tuberculosis: Advantages over small animal models. Microbes Infect. 2008, 10, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Ulrichs, T.; Kaufmann, S.H. New insights into the function of granulomas in human tuberculosis. J. Pathol. 2006, 208, 261–269. [Google Scholar] [CrossRef]
- Saunders, B.; Cooper, A.M. Restraining mycobacteria: Role of granulomas in mycobacterial infections. Immunol. Cell Biol. 2000, 78, 334–341. [Google Scholar] [CrossRef]
- Gonzalez-Juarrero, M.; Turner, O.C.; Turner, J.; Marietta, P.; Brooks, J.V.; Orme, I.M. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 2001, 69, 1722–1728. [Google Scholar] [CrossRef] [Green Version]
- Fujita, J.; O’htsuki, Y.; Suemitsu, I.; Yamadori, I.; Shigeto, E.; Shiode, M.; Nishimura, K.; Hirayama, T.; Masushima, T.; Ishida, T. Immunohistochemical distribution of epithelioid cell, myofibroblast, and transforming growth factor-B1 in the granuloma caused by Mycobacterium avium intracellulare complex pulmonary infection. Microbiol. Immunol. 2002, 46, 67–74. [Google Scholar] [CrossRef]
- Flynn, J.L.; Klein, E. Pulmonary tuberculosis in monkeys. In A Color Atlas of Comparative Pathology of Pulmonary Tuberculosis; Leong, F.J., Dartois, V., Dick, T., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 83–105. [Google Scholar]
- Basaraba, R.J. Experimental tuberculosis: The role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis 2008, 88, S35–S47. [Google Scholar] [CrossRef]
- Canetti, G. The Tubercle bacillus in the Pulmonary Lesion of Man; Springer Publishing Co: New York, NY, USA, 1955; p. 245. [Google Scholar]
- Basaraba, R.J.; Hunter, R.L. Pathology of tuberculosis: How the pathology of human tuberculosis informs and directs animal models. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Myers, R.K.; McGavin, M.D.; Zachary, J.F. Cellular adaptations, injury, and death: Morphologic, biochemical, and genetic bases. In Pathologic Basis of Veterinary Disease, 5th ed.; Zachary, J.F., McGavin, M.D., Eds.; Elsevier Mosby: St. Louis, MO, USA, 2012; pp. 122–124. [Google Scholar]
- Bendayan, D.; Barziv, Y.; Kramer, M.R. Pulmonary calcifications: A review. Respir. Med. 2000, 94, 190–193. [Google Scholar] [CrossRef] [Green Version]
- Neill, S.D.; Bryson, D.G.; Pollock, J.M. Pathogenesis of tuberculosis in cattle. Tuberculosis 2001, 81, 79–86. [Google Scholar] [CrossRef]
- Lin, P.L.; Coleman, T.; Carney, J.P.; Lopresti, B.J.; Tomko, J.; Fillmore, D.; Dartois, V.; Scanga, C.; Frye, L.J.; Janssen, C.; et al. Radiologic responses in cynomolgous macaques for assessing tuberculosis chemotherapy regimens. Antimicrob. Agents Chemother. 2013, 57, 4237–4244. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.L.; Capuano, S.V.; Croix, D.; Pawar, S.; Myers, A.; Zinovik, A.; Klein, E. Non-human primates: A model for tuberculosis research. Tuberculosis 2003, 83, 116–118. [Google Scholar] [CrossRef]
- Lin, P.L.; Ford, C.B.; Coleman, M.T.; Myers, A.J.; Gawande, R.; Ioerger, T.; Sacchettini, J.; Fortune, S.M.; Flynn, J.L. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat. Med. 2014, 20, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.T.; Maiello, P.; Tomko, J.; Frye, L.J.; Fillmore, D.; Janssen, C.; Klein, E.; Lin, P.L. Early changes by (18)Fluorodeoxyglucose positron emission tomography coregistered with computed tomography predict outcome after Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 2014, 82, 2400–2404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gideon, H.P.; Hughes, T.K.; Wadsworth, M.H.; Tu, A.A.; Gierahn, T.M.; Hopkins, F.F.; Wei, J.-R.; Kummerlowe, C.; Grant, N.L.; Nargan, K.; et al. Multimodal profiling of lung granulomas reveals cellular correlates of tuberculosis control. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ehlers, S.; Schaible, U.E. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front. Immunol. 2012, 3, 411. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.L.; Gideon, H.P.; Mattila, J.T.; Lin, P.L. Immunology studies in non-human primate models of tuberculosis. Immunol. Rev. 2015, 264, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Route of Infection | Dose (CFU) | Days after Infection | Age of Animal | Histopathology Description | Granuloma Staging | Cellular Identification | Cytokine Analysis | Reference |
|---|---|---|---|---|---|---|---|---|
| Natural | Unknown | Unknown | variable | Yes | Yes | No | No | [91] |
| Natural | Unknown | Unknown | 18–30 months | Yes | Yes | NA | IHC | [88] |
| Natural | Unknown | Unknown | 2.5–9 years | Yes | Yes | Yes | IHC | [85] |
| Natural | Unknown | Unknown | 49–91 months | Yes | No | NA | IHC | [45] |
| Intratracheal | 5 × 103 | 28 weeks | 15–16 months | Yes | Yes | qPCR | [79] | |
| Intratracheal | 70–6.2 × 104 | 29 weeks | 5 months | Yes | Yes | Yes | IHC/ISH | [25] |
| Intratracheal | 104 | 5–12 weeks | 8 months | Yes | Yes | Yes | No | [37] |
| Intranasal | 1.3 × 104 | 12 weeks | 3 months | No | No | NA | qPCR | [92] |
| Endotracheal | 2 × 103 | 13 weeks | 5–7 months | Yes | Yes | Yes | IHC | [26] |
| Endobronchial | 2 × 103 | 13 weeks | 12 months | Yes | Yes | Yes | IHC/ISH | [40] |
| Aerosol | 103–105 | 21 weeks | 4 months | Yes | No | No | No | [93] |
| Aerosol | 104 | 21 weeks | 3 months | Yes | Yes | No | ISH | [24] |
| Aerosol | 104 | 12 weeks | 6 months | Yes | Yes | Yes | ISH | [12] |
| Aerosol | 104 | 21 weeks | 6 months | Yes | Yes | No | ISH | [72] |
| Aerosol | 104 | 30, 90, 180, 270 days | 9 months | Yes | Yes | No | ISH | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmer, M.V.; Kanipe, C.; Boggiatto, P.M. The Bovine Tuberculoid Granuloma. Pathogens 2022, 11, 61. https://doi.org/10.3390/pathogens11010061
Palmer MV, Kanipe C, Boggiatto PM. The Bovine Tuberculoid Granuloma. Pathogens. 2022; 11(1):61. https://doi.org/10.3390/pathogens11010061
Chicago/Turabian StylePalmer, Mitchell V., Carly Kanipe, and Paola M. Boggiatto. 2022. "The Bovine Tuberculoid Granuloma" Pathogens 11, no. 1: 61. https://doi.org/10.3390/pathogens11010061








