External Sulphate Attack on Alkali-Activated Slag and Slag/Fly Ash Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixing, Casting, Curing, and Sample Preparation
3. Results and Discussions
3.1. Analysis of Solutions
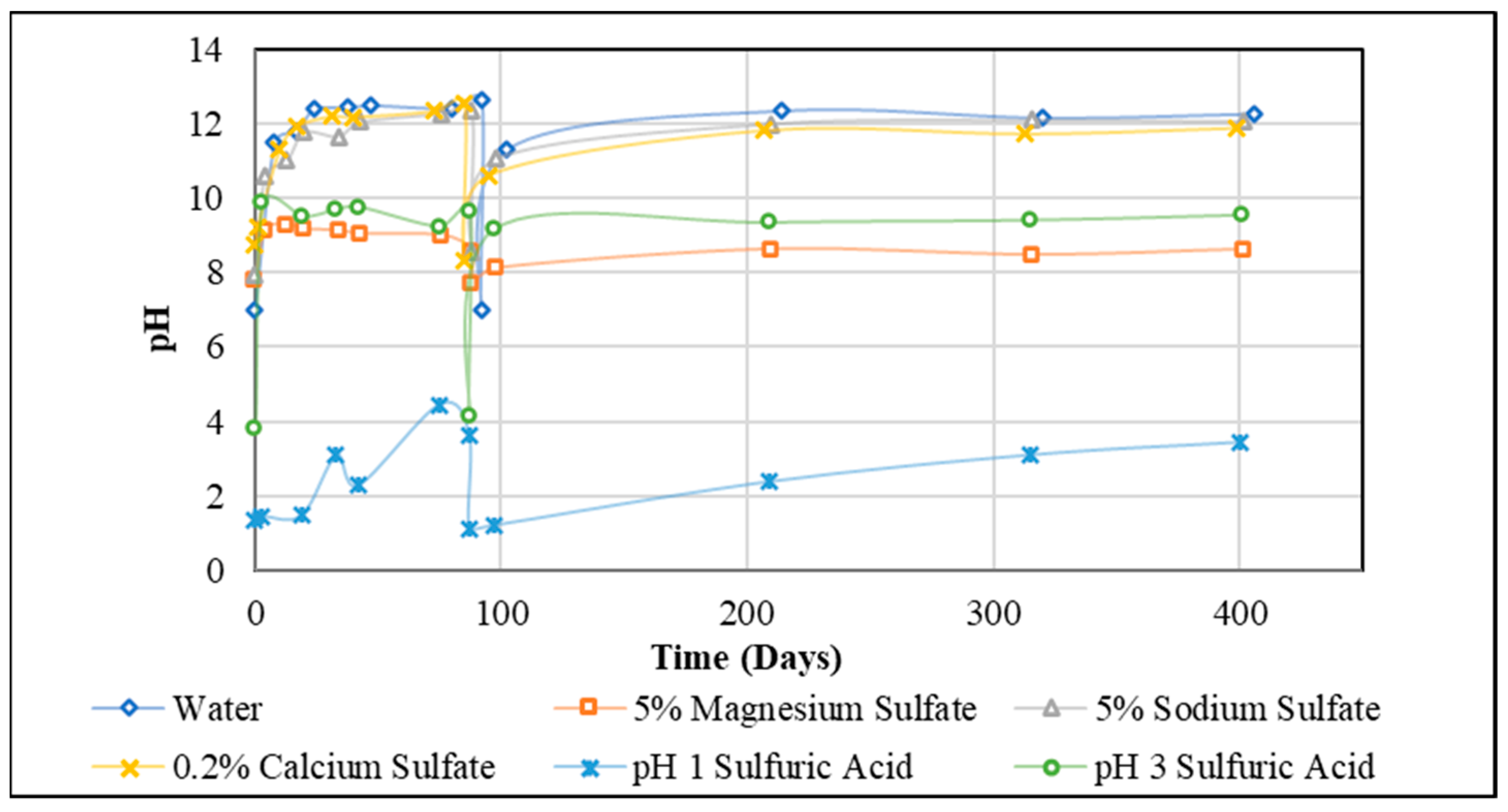
3.1.1. pH of Solutions
- ▪
- Water and salt solutions:
- ▪
- Sulphuric acid solutions:
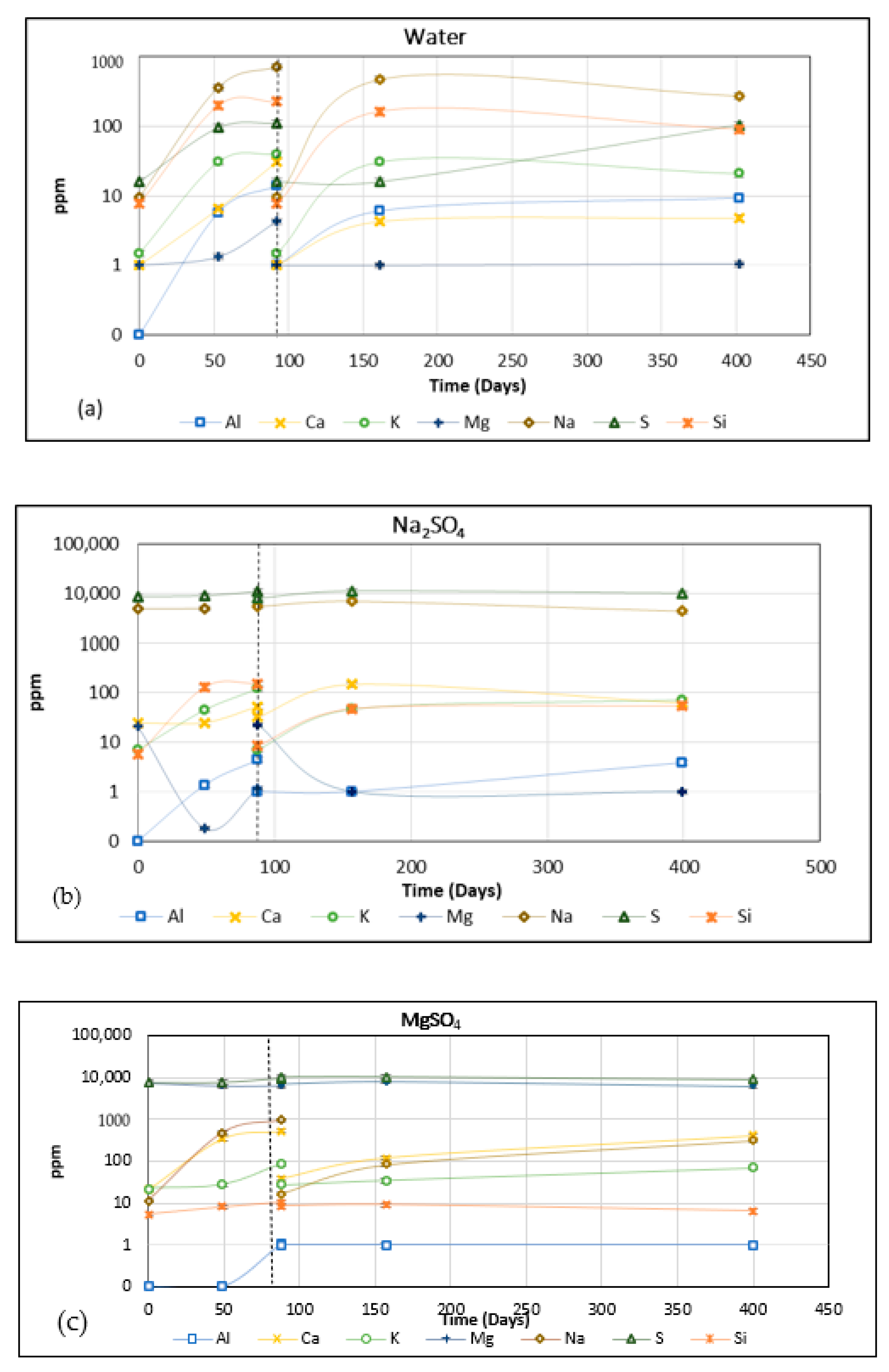
3.1.2. Ionic Characterisation of the Exposure Solutions
- ▪
- Water:
- ▪
- Na2SO4 solution:
- ▪
- MgSO4 solution:
- ▪
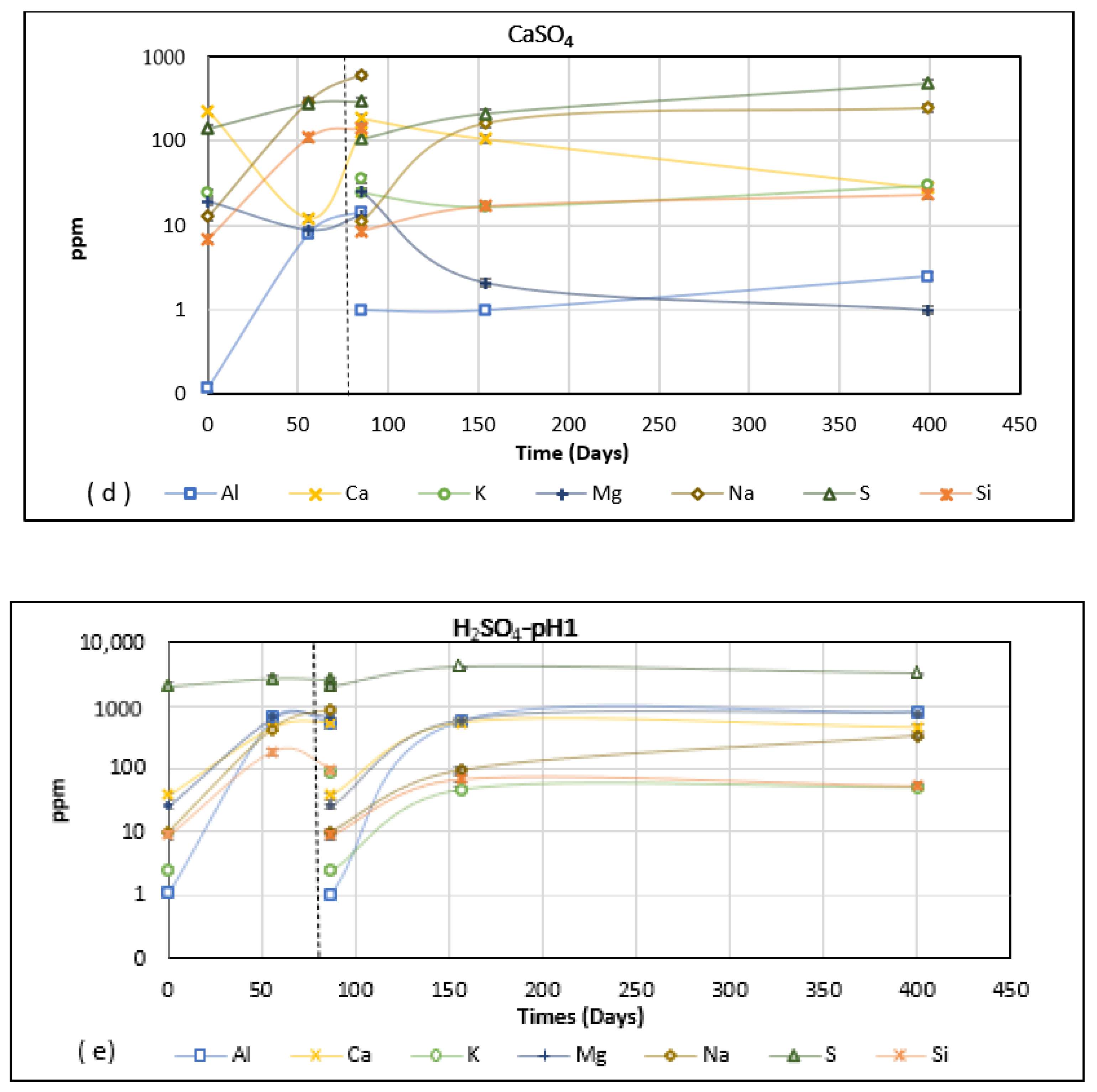
- CaSO4 solution:
- ▪
- Sulphuric acid (H2SO4):
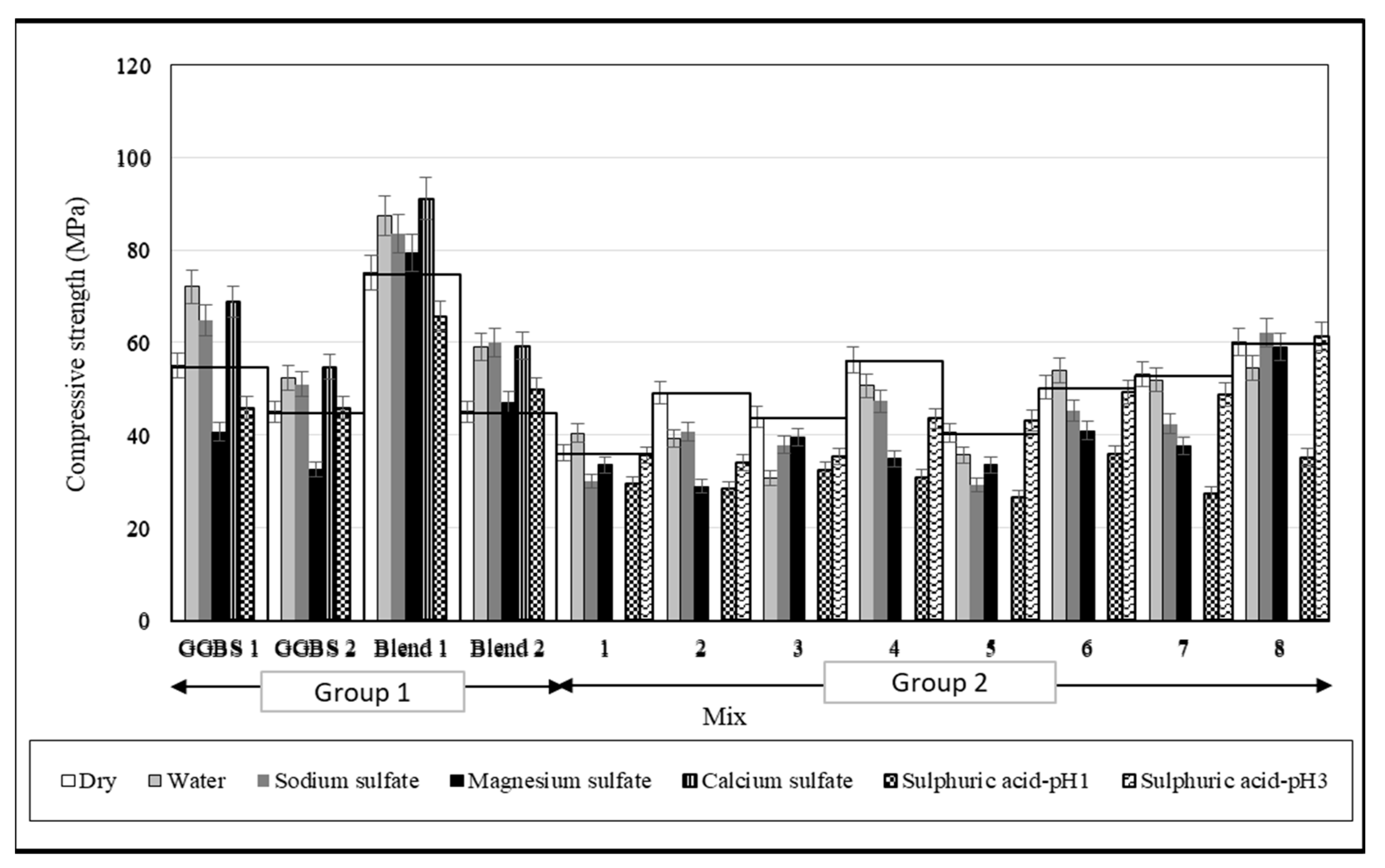
3.2. Fresh Properties and Compressive Strength
3.3. Strength Changes
3.4. Visual Appearance
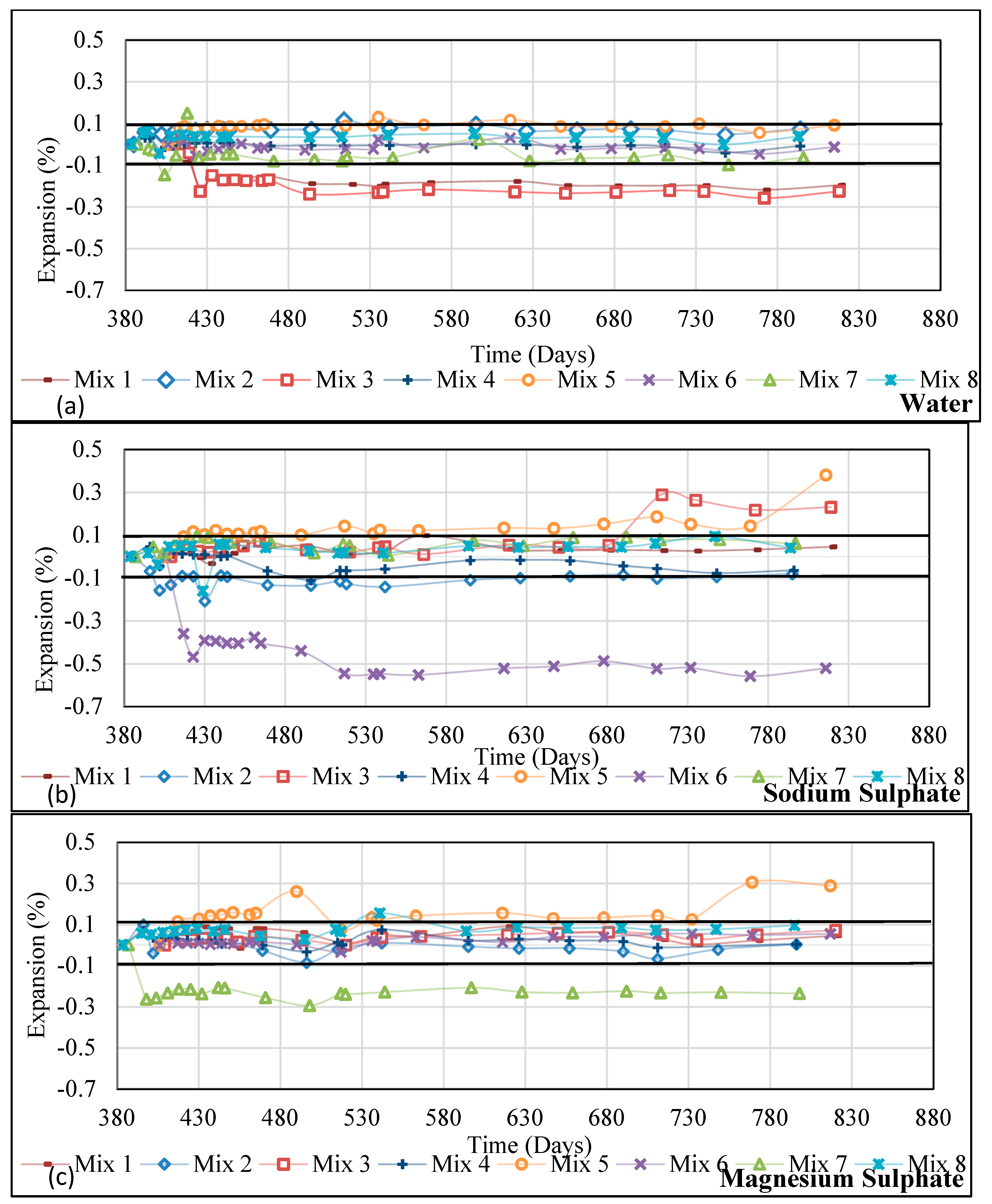
3.5. Expansion/Shrinkage Results
- 1.
- Dry environment:
- 2.
- Immersion in Water:
- 3.
- Sodium sulphate solution:
- 4.
- Magnesium sulphate solutions:
- 5.
- Calcium sulphate solutions:
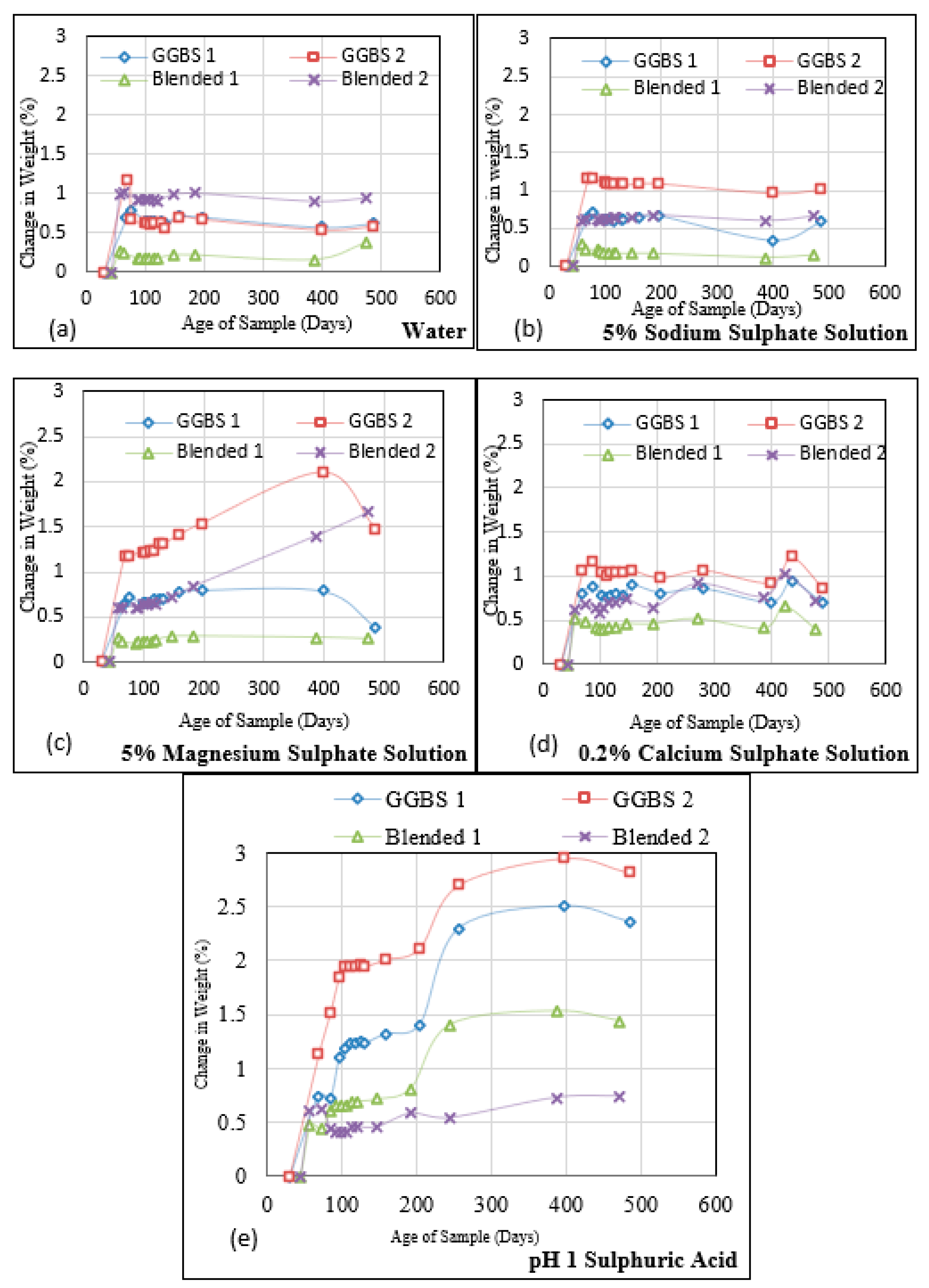
3.6. Change in Mass
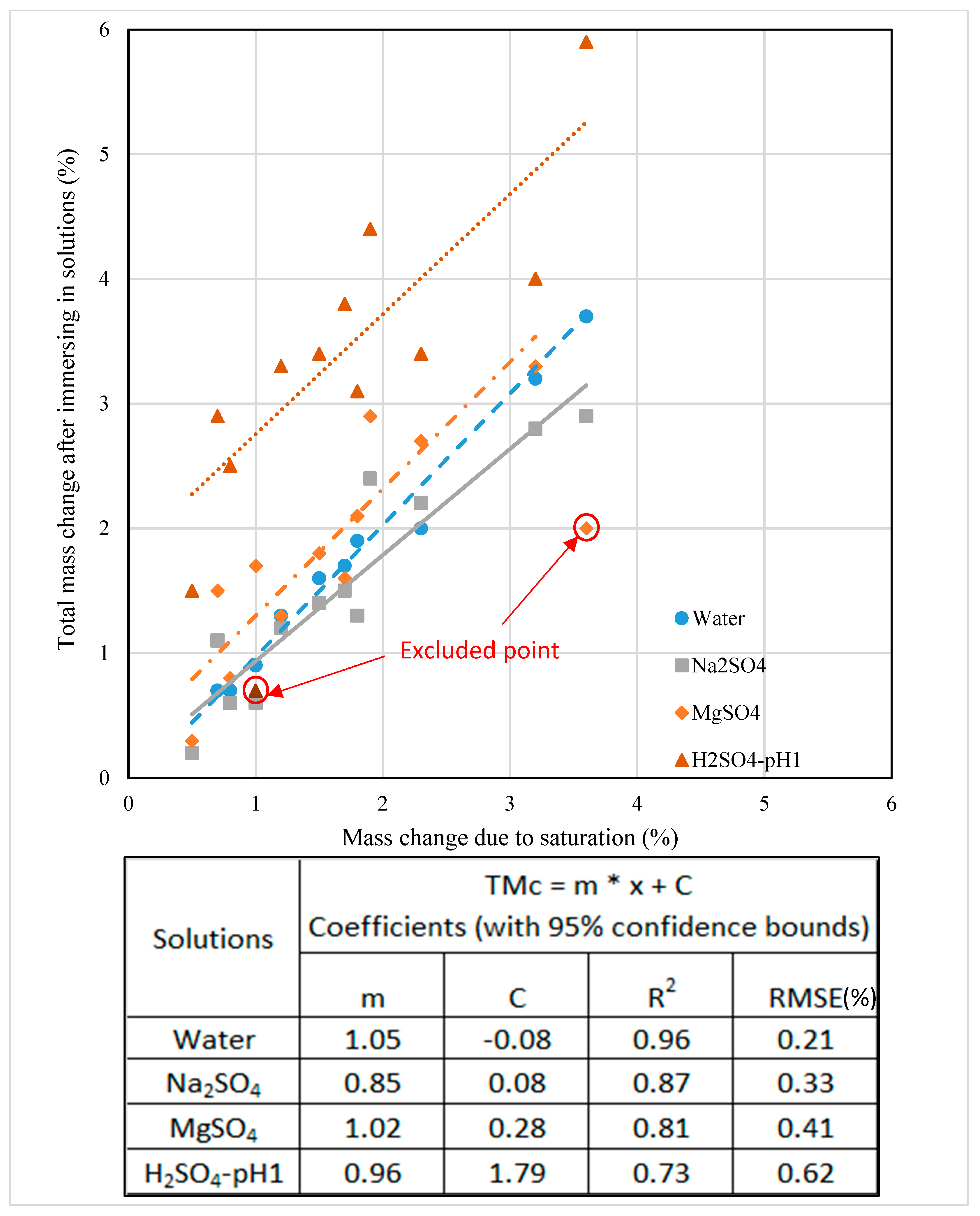
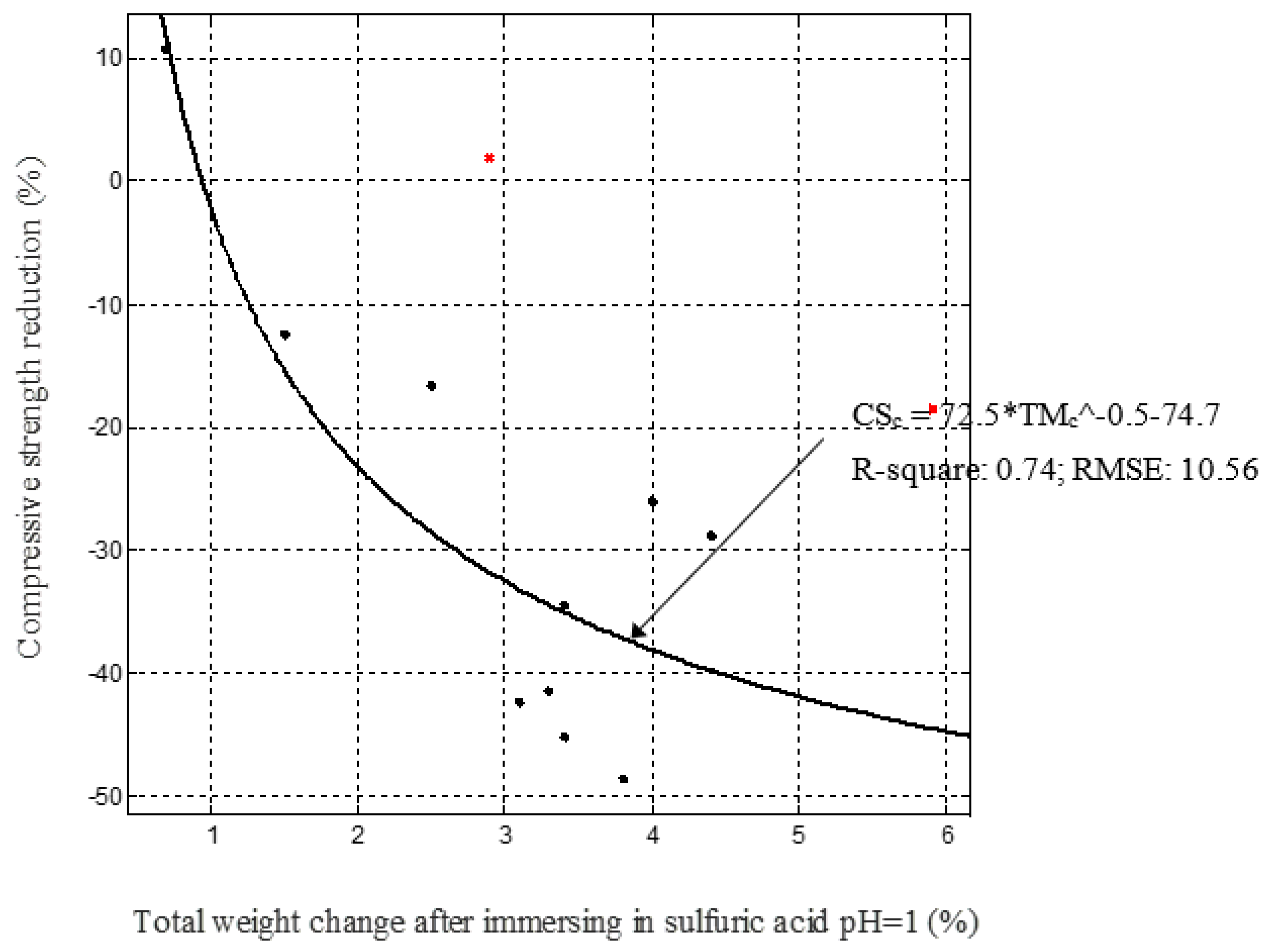
3.7. Strength Loss Modelling Due to Sulphate Ingress
4. Conclusions
- (1)
- Exposure to water led to the egress of Na, Si, and S ions from the specimens. The source of the first two ions was the unreacted sodium hydroxide and silicate activator, whereas the S ions were from sources that existed in GGBS. During exposure, the pH increased from 7 to 12.4 within 24 days. Group 1 mixes were exposed after just 28 days of curing, and these specimens showed an increase in mass and compressive strength. Group 2 mixes, which were cured for a year before exposure, showed mass gain, but no strength gain was noticed. Specimens showed expansion or shrinkage depending on the quantity of Si modulus, Na2O%, and permeability. Whilst it is not possible to discern the exact influence of these variables, longer curing seems to limit the length change to a nominal 0.1% bandwidth allowed for structural applications.
- (2)
- Exposure to Na2SO4 led to leaching of Ca and K in addition to Na, Si, and S ions from the specimens initially. Further, there was a transfer of ions from the solution to the specimen, as expected, in the ionic exposure test. During exposure, the pH increased from 7.92 to 12.04 within 43 days. Group 1 concrete mixes that were exposed to Na2SO4 solution after just 28 days of curing gained strength due to the continued polymerisation of the mixes. The AA-S/F mix with a slag/fly ash ratio of 4 (Blend 1) appeared to perform better as it resisted the ingress/egress of ions and had minimal changes in mass and length. AAS mixes in Group 2 mostly showed strength reduction and expansion. However, those mixes with a high silicate modulus and low alkali dosage showed higher shrinkage.
- (3)
- Exposure to MgSO4 led to the movement of Na from the gel structure and Ca from the decalcification of the Ca-rich gel phases into the exposure solution, which contributed to the weakening of the microstructure. During exposure, the pH increased from 7.82 to 9.13 within 4 days and did not increase any further. All AAS concretes in Groups 1 and 2 exposed to magnesium sulphate solution showed a reduction in strength. The Blend 2 mix with a slag/fly ash ratio of 0.67 had a length change of ≤0.1%, which is within the allowable limit based on ASTM C1012/C1012M-18a.
- (4)
- Exposure to CaSO4 showed no transfer of SO42− from the solution to the samples to form extra sulphate salts while there was a visible transfer of Ca ions into the concrete, which could form new compounds, such as CASH gels, and further strengthen the matrix. During exposure, the pH increased from 8.72 to 12.19 within 30 days. All AAS-AA-S/F concretes exposed to calcium sulphate solutions gained strength and none of the mixes showed any expansion/shrinkage up to 100 days.
- (5)
- Exposure to H2SO4 led to preferential leaching of other elements (Na+, Mg2+, Ca2+ and Al3+) before silicon, which helps to protect tetrahedral silicate and AAM concrete perform better against sulphuric acid attack. During exposure, for a solution with a pH of 3, the pH value increased to 9.87 after just 3 days and for a pH of 1, it took one month for the pH value to change from 1 to 3. The sulphuric acid led to severe strength reduction in both groups of concrete mixes, except for the AA-S/F mix with a slag/fly ash ratio of 0.67 (Blend 2), which appeared to perform best based on the gain in strength and the minimum change in mass. The average mass change of the AASC and (AA-S/F) concrete specimens exposed to sulphuric acid with a pH of 1 was 3.4% and 1.1%, respectively.
- (6)
- Empirical model in Section 3.7 of the article is proposed for predicting changes in compressive strength as a function of mass change due to saturation. A simple performance criterion is proposed, indicating that the mass gain in specimens exposed to salt solutions should be less than 1.3% for a negligible change in compressive strength. It should be noted that the strength reduction (or gain) will not be uniform across the cross-section; the proposed model covers the average strength change and is valid for the outer zone of the concrete.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Provis, J.; van Deventer, J. (Eds.) Alkali Activated Materials, State-of-the-Art Report, RILEM TC-224-AAM, 1st ed.; Springer: London, UK, 2014. [Google Scholar]
- Arbi, K.; Nedeljković, M.; Zuo, Y.; Ye, G. A Review on the Durability of Alkali-Activated Fly Ash/Slag Systems: Advances, Issues, and Perspectives. Ind. Eng. Chem. Res. 2016, 55, 5439–5453. [Google Scholar] [CrossRef] [Green Version]
- Skalny, J.; Marchand, J.; Odler, I. Sulphate Attack on Concrete, 1st ed.; Spon Press: London, UK, 2002. [Google Scholar]
- Shi, C.; Krivenko, P.; Roy, D. Alkali-Activated Cements and Concretes, 1st ed.; Taylor & Francis: Chippenham, UK, 2006. [Google Scholar]
- Komljenović, M.; Baščarević, Z.; Marjanović, N.; Nikolic, V. External sulphate attack on alkali-activated slag. Constr. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Huseien, G.F.; Tahir, M.M.; Mirza, J.; Ismail, M.; Wei Shah, K.; Asad, M.A. Effects of POFA replaced with FA on durability properties of GBFS included alkali-activated mortars. Constr. Build. Mater. 2018, 175, 174–186. [Google Scholar] [CrossRef]
- Kwasny, J.; Aiken, T.A.; Soutsos, M.N.; McIntosh, J.A.; Cleland, D.J. Sulphate and acid resistance of lithomarge-based geopolymer mortars. Constr. Build. Mater. 2018, 166, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Džunuzović, N.; Komljenović, M.; Nikolić, V.; Ivanovic, T. External sulphate attack on alkali-activated fly ash-blast furnace slag composite. Constr. Build. Mater. 2017, 157, 737–747. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulphuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Fang, Y.; You, D.; Gong, Y.; Zhu, C. Properties and microstructure of alkali-activated slag cement cured at below-and about-normal temperature. Constr. Build. Mater. 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Aliques-Granero, J.; Tognonvi, M.T.; Tagnit-Hamoua, A. Durability study of AAMs: Sulphate attack resistance. Constr. Build. Mater. 2019, 229, 117100. [Google Scholar] [CrossRef]
- Bondar, D.; Basheer, M.; Nanukuttan, S. Suitability of alkali-activated slag/fly ash (AA-GGBS/FA) concretes for chloride environments: Characterisation based on mix design and compliance testing. Constr. Build. Mater. 2019, 216, 612–621. [Google Scholar] [CrossRef]
- Bondar, D.; Nanukuttan, S.; Provis, J.; Soutsos, M. Efficient mix design of alkali-activated slag concrete based on packing density of ingredients and paste thickness. J. Clean. Prod. 2019, 218, 438–449. [Google Scholar] [CrossRef]
- BS EN 1097-1. Tests for Mechanical and Physical Properties of Aggregates. Determination of the Resistance to Wear (Micro-Deval); BSI: London, UK, 1911. [Google Scholar]
- Bondar, D.; Ma, Q.; Soutsos, M.; Basheer, M.; Provis, J.; Nanukuttan, S. Alkali activated slag concretes designed for a desired slump, strength and chloride diffusivity. Constr. Build. Mater. 2018, 190, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Chindaprasirta, P.; Sriopas, B.; Phosrib, P.; Yoddumrong, P.; Anantakarn, K.; Kroehong, W. Hybrid high calcium fly ash alkali-activated repair material for concrete exposed to sulfate environment. J. Build. Eng. 2022, 45, 103590. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Sulphate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Allahvedi, A.; Hashemi, H. Investigating the resistance of alkali-activated slag mortar exposed to magnesium sulphate attack. Int. J. Civ. Eng. 2015, 13, 379–387. [Google Scholar]
- Alexander, M.; Bertron, A.; De Belie, N. (Eds.) Performance of Cement-Based Materials in Aqueous Environments, State-of-the-Art Report, RILEM TC 211—PAE; Springer: Dordrecht, The Netherlands, 2013; Volume 10, p. 462. [Google Scholar]
- BS EN 12350-2. Testing Fresh Concrete Part 2: Slump-Test and Part 5: Flow Table Test; BSI: London, UK, 2009. [Google Scholar]
- BS EN 12390-3. Testing Hardened Concrete Part 3: Compressive Strength of Test Specimens; BSI: London, UK, 2009. [Google Scholar]
- Allahverdi, A.; Shaverdi, B.; Najafi Kani, E. Influence of sodium oxide on properties of fresh and hardened paste of alkali-activated blast-furnace slag. Int. J. Civ. Eng. 2010, 8, 304–314. [Google Scholar]
- Giasuddin, H.M.; Sanjayan, J.G.; Ranjith, P.G. Strength of geopolymer cured in saline water in ambient conditions. Fuel 2013, 107, 34–39. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- Kwasny, J.; Aiken, T.A.; Soutsos, M.N.; Cleland, D.J.; McIntosh, J.A. Comparison of lithomarge and cement-based mortars performance in aggressive aqueous environments. In Proceedings of the Sixth International Conference on Durability of Concrete Structures, Leeds, UK, 18–20 July 2018; pp. 160–169. [Google Scholar]
- Gutberlet, T.; Hilbig, H.; Beddoe, R.E. Acid attack on hydrated cement—Effect of mineral acids on the degradation process. Cem. Concr. Res. 2015, 74, 35–43. [Google Scholar] [CrossRef]
- Collins, S.J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Neto, A.A.M.; Cincotto, M.A.; Repette, W. Drying and autogenous shrinkage of pastes and mortars with activated lag cement. Cem. Concr. Res. 2008, 38, 565–574. [Google Scholar] [CrossRef]
- Cartwright, C.; Rajabipour, F.; Radlińska, A. Shrinkage characteristics of alkali-activated slag cement. J. Mater. Civ. Eng. 2015, 27, B4014007. [Google Scholar] [CrossRef]
- Omas, R.J.; Lezama, D.; Peethamparan, S. On drying shrinkage in alkali-activated concrete: Improving dimension and stability by ageing or heat-curing. Cem. Concr. Res. 2017, 91, 13–23. [Google Scholar]
- Ye, C.; Cartwright, F.; Rajabipour, R.A. Effect of drying rate on shrinkage of alkali-activated slag cement. In Proceedings of the 4th International Conference on the Advances in Civil Engineering Durability of Concrete Structures, Lafayette, IN, USA, 21 July 2014. [Google Scholar]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Orosz, K.; Humad, A.; Hedlund, H.; Cwirzen, A. Autogenous Deformation of Alkali-Activated Blast Furnace Slag Concrete Subjected to Variable Curing Temperatures. Adv. Civ. Eng. 2019, 2019, 6903725. [Google Scholar] [CrossRef]
- ASTM International. ASTM C1012/C1012M-18a Standard Test Method for Length Change of Hydraulic-Cement Mortars Exposed to a Sulfate Solution; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]




| Precursor | Component (Mass% as Oxide) | Physical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Others | LOI | Fineness ≥ 45 μm | Particle Density | Water Absorption | |
| GGBS | 35.7 | 11.2 | 43.9 | 0.3 | 6.5 | 2.09 | 0.31 | 7.74% | 2.86 | 35.14% |
| Fly ash | 46.8 | 22.5 | 2.2 | 9.1 | 1.3 | 14.5 | 3.6 | 18.39% | 2.21 | 27% |
| AAM Type | Mix | GGBS/PFA (kg/m3) | Ms (= SiO2/Na2O) | Na2O (%) | W/B | Sand (kg/m3) | Aggregate (kg/m3) | Slump (mm) | 2 Days Comp.St (MPa) | 28 Days Comp.St (MPa) | Dnssd (×10−12 m2/s) [12,15] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: AASC & (AA-S/F) concretes | GGBS 1 | 400/0 | 0.45 | 8 | 0.55 | 841 | 911 | 220 | 29.7 | 51.8 | 2.92 |
| GGBS 2 | 400/0 | 1.0 | 6 | 0.55 | 841 | 911 | 205 | 29.1 | 44.8 | 4.81 | |
| Blend 1 | 340/85 | 1.0 | 8 | 0.47 | 841 | 911 | 220 | 50.7 | 74.9 | 2.37 | |
| Blend 2 | 170/255 | 0.45 | 8 | 0.44 | 841 | 911 | 230 | 14.3 | 45.5 | 5.24 | |
| Group 2: AASC | 1 | 400/0 | 0.45 | 4 | 0.60 | 669 | 1004 | 215 | 15.3 | 26.4 | 4.11 |
| 2 | 400/0 | 0.45 | 4 | 0.55 | 701 | 1051 | 168 | 17.8 | 30 | 5.09 | |
| 3 | 400/0 | 0.45 | 6 | 0.60 | 669 | 1004 | 215 | 21.2 | 35.8 | 4.74 | |
| 4 | 400/0 | 0.45 | 6 | 0.55 | 701 | 1051 | 135 | 24.7 | 44 | 1.87 | |
| 5 | 400/0 | 0.45 | 8 | 0.55 | 701 | 1051 | 225 | 38.4 | 53.6 | 2.92 | |
| 6 | 400/0 | 1.0 | 4 | 0.55 | 701 | 1051 | 160 | 25.8 | 47.8 | 2. 34 | |
| 7 | 400/0 | 1.0 | 6 | 0.55 | 701 | 1051 | 203 | 33.9 | 62.7 | 3.81 | |
| 8 | 400/0 | 1.0 | 8 | 0.55 | 701 | 1051 | 240 | 33.7 | 64.4 | 4.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondar, D.; Nanukuttan, S. External Sulphate Attack on Alkali-Activated Slag and Slag/Fly Ash Concrete. Buildings 2022, 12, 94. https://doi.org/10.3390/buildings12020094
Bondar D, Nanukuttan S. External Sulphate Attack on Alkali-Activated Slag and Slag/Fly Ash Concrete. Buildings. 2022; 12(2):94. https://doi.org/10.3390/buildings12020094
Chicago/Turabian StyleBondar, Dali, and Sreejith Nanukuttan. 2022. "External Sulphate Attack on Alkali-Activated Slag and Slag/Fly Ash Concrete" Buildings 12, no. 2: 94. https://doi.org/10.3390/buildings12020094







