An In-Situ LSCM Study on Bainite Formation in a Fe-0.2C-1.5Mn-2.0Cr Alloy
Abstract
:1. Introduction
2. Experimental Procedure and Microstructure Measurement
3. Results
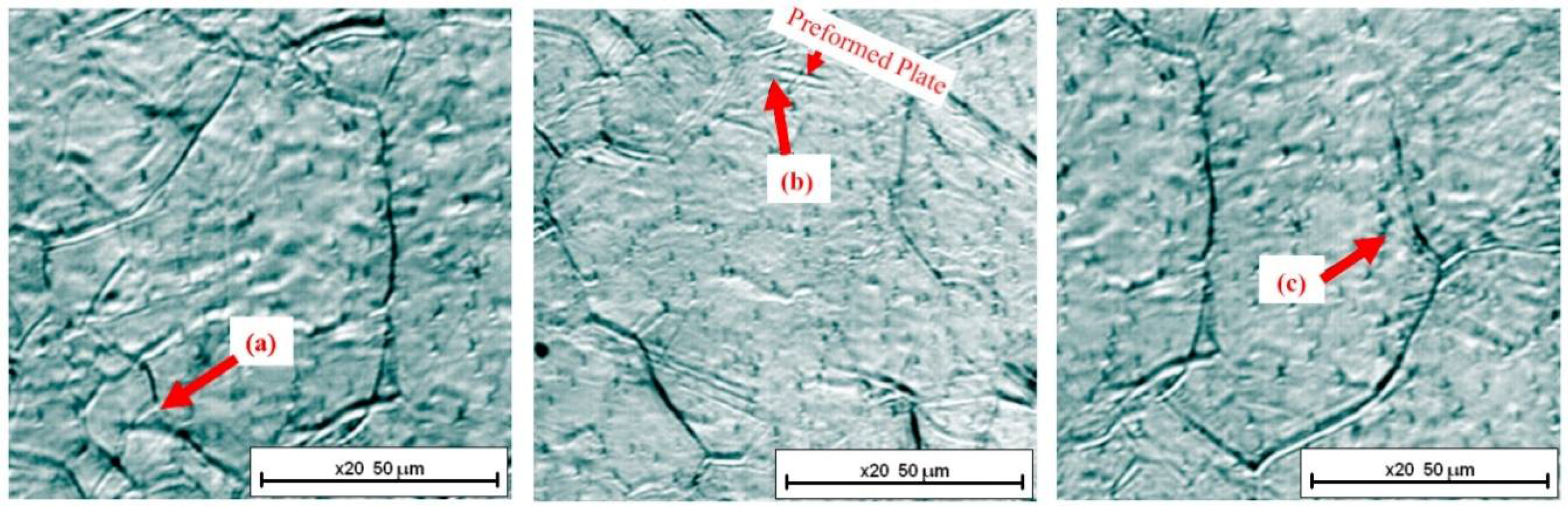
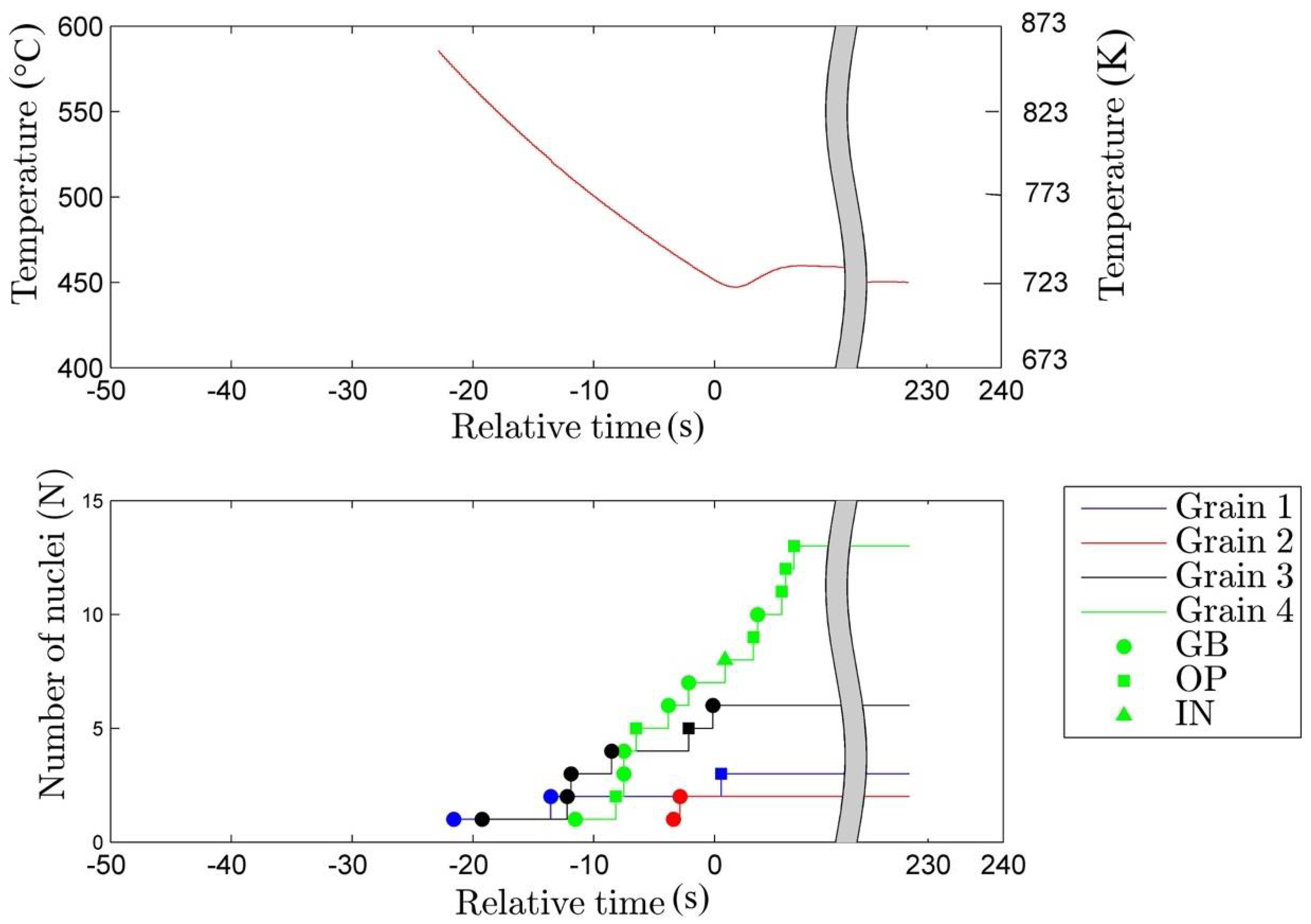
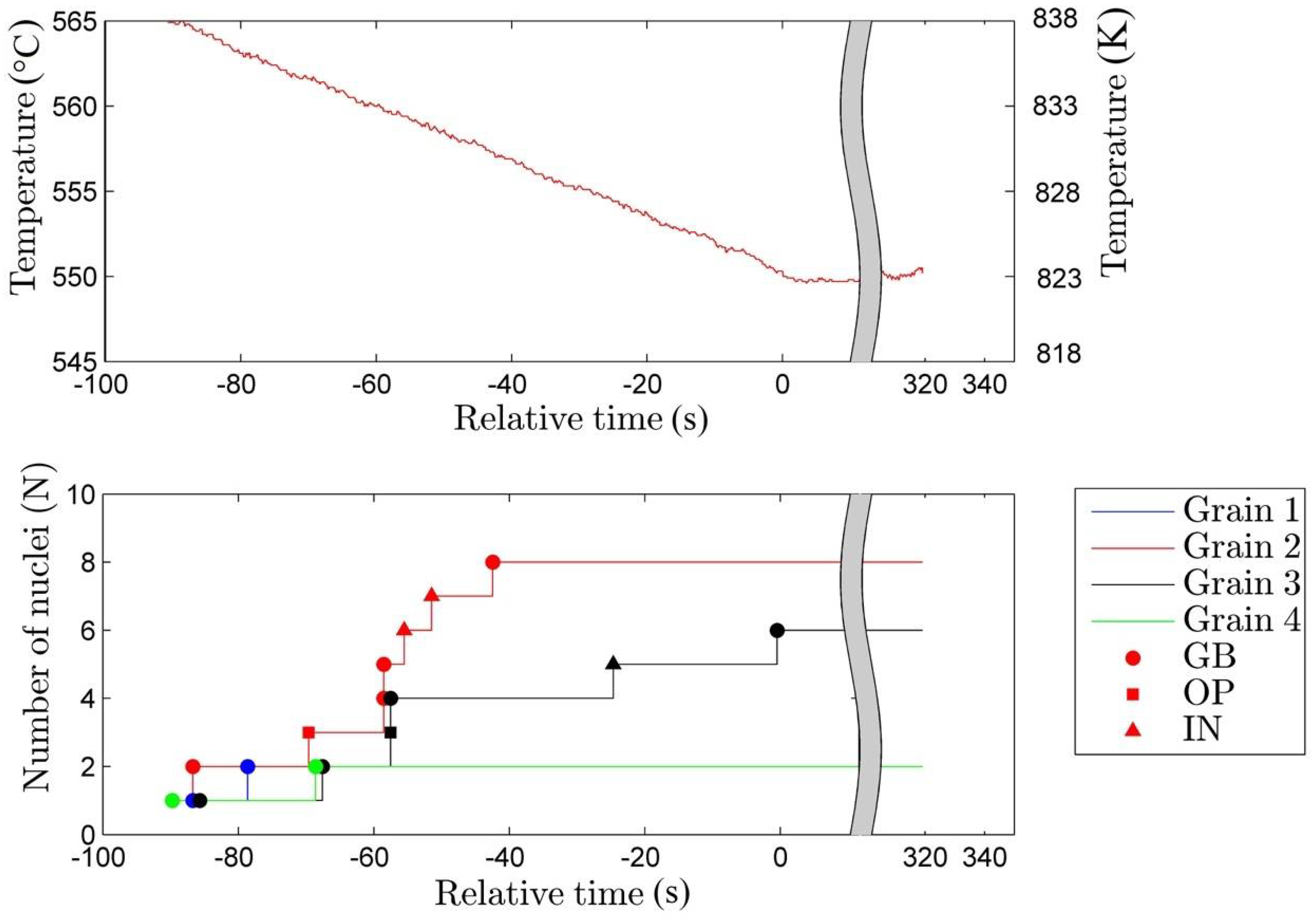
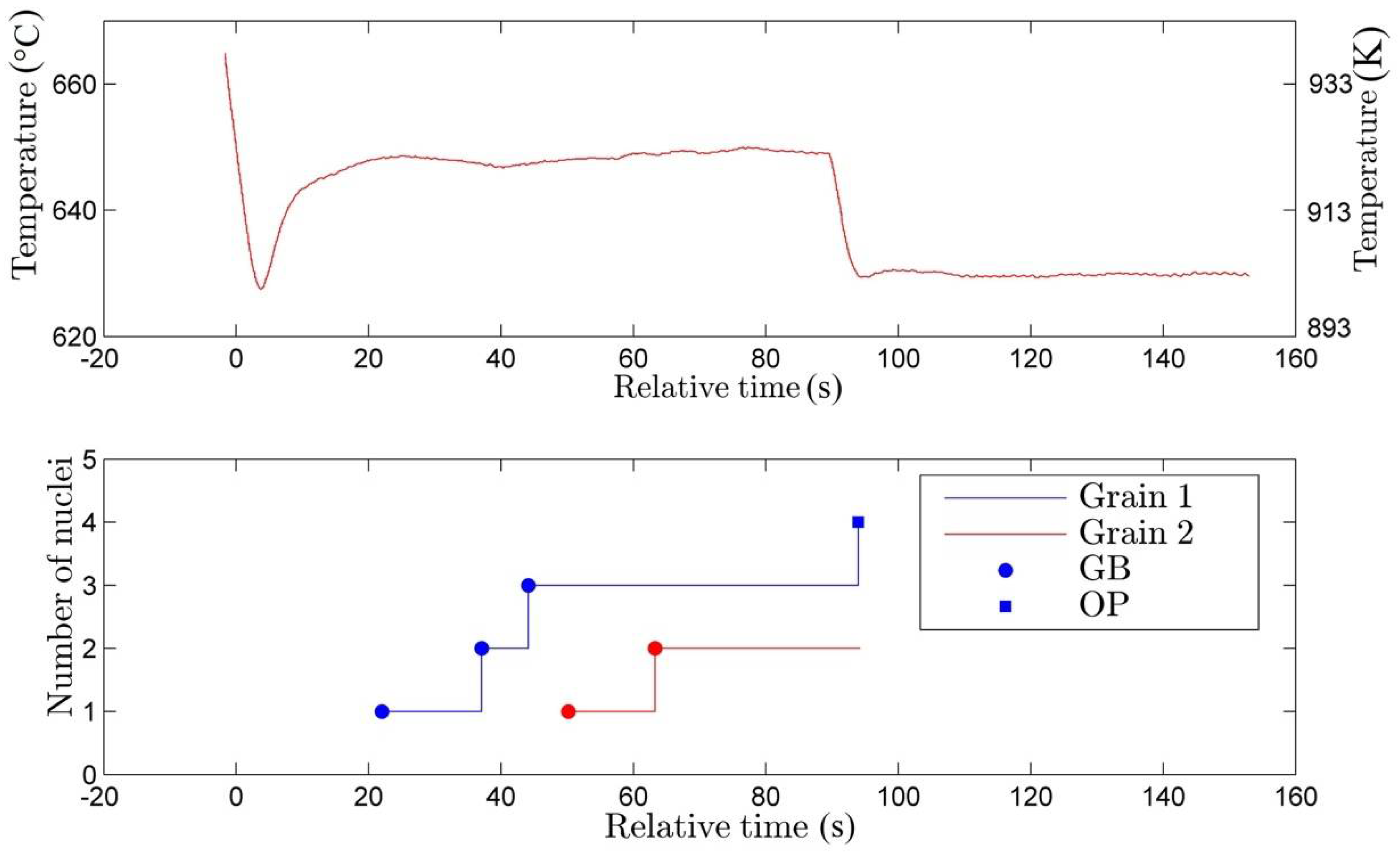
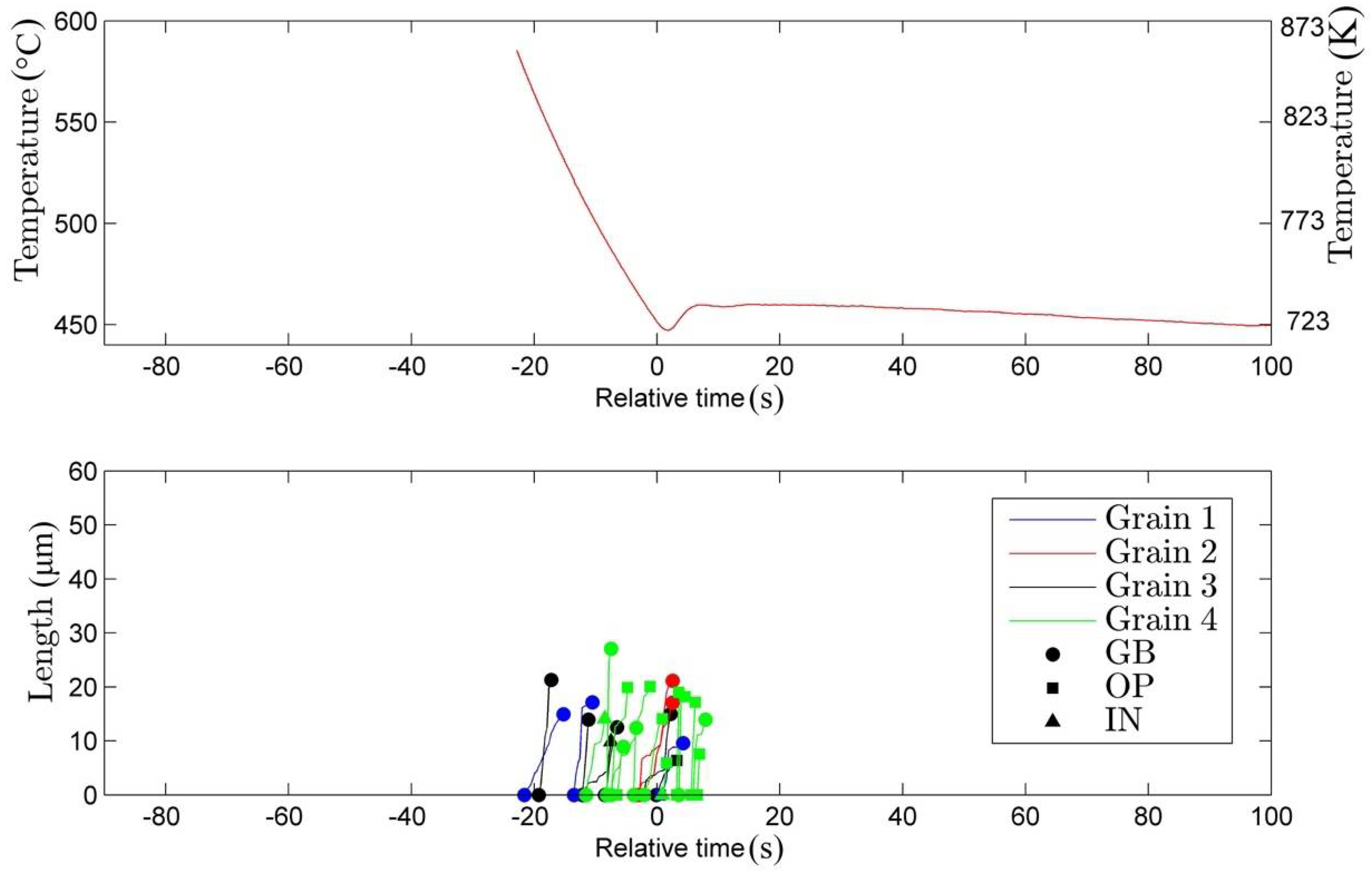
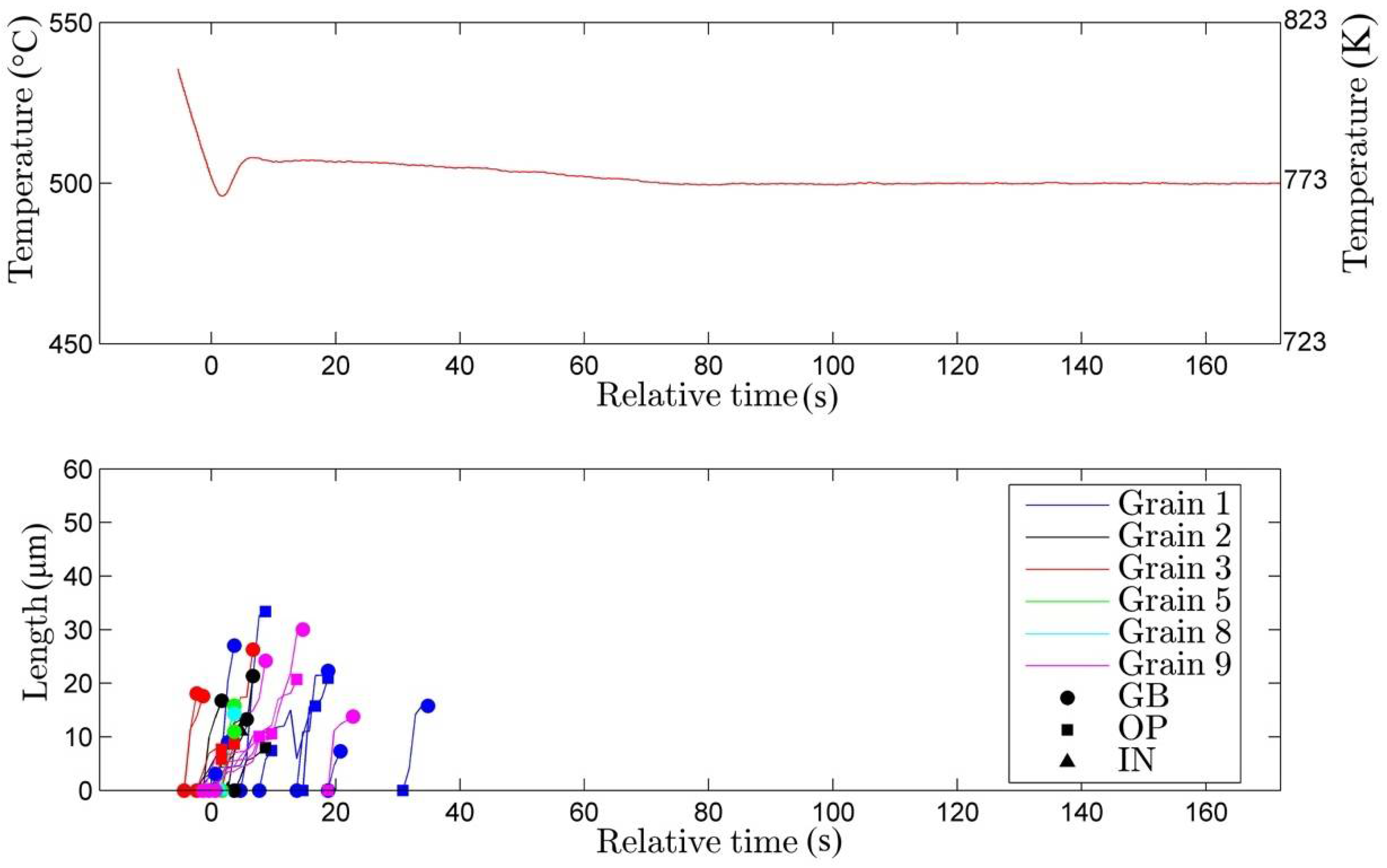
3.1. Nucleation
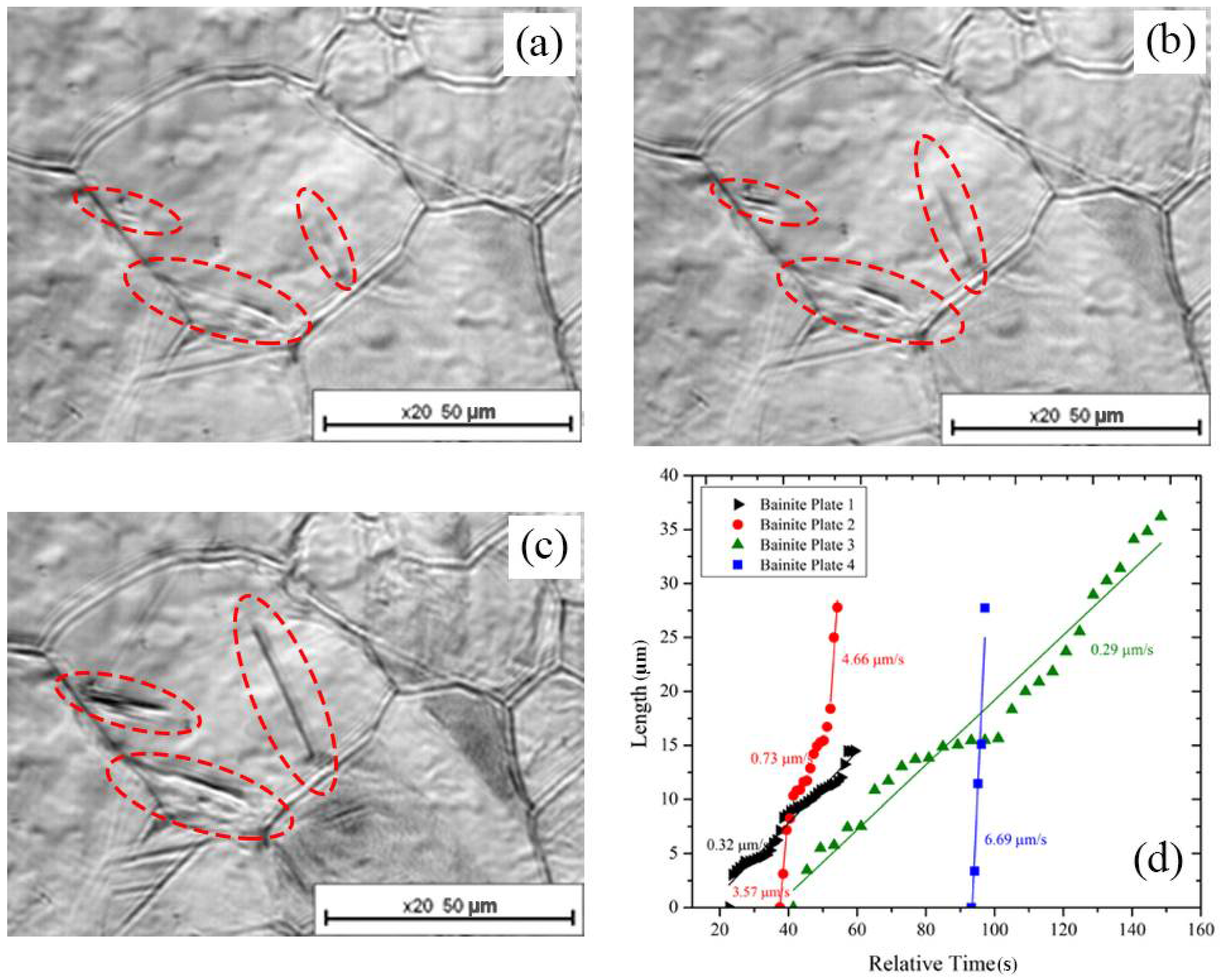
- (i)
- nucleation on the grain boundary (●)
- (ii)
- nucleation on the surface of an existing bainite plate (■)
- (iii)
- nucleation occurring inside the austenitic grain (▲).
3.2. Growth
4. Discussion
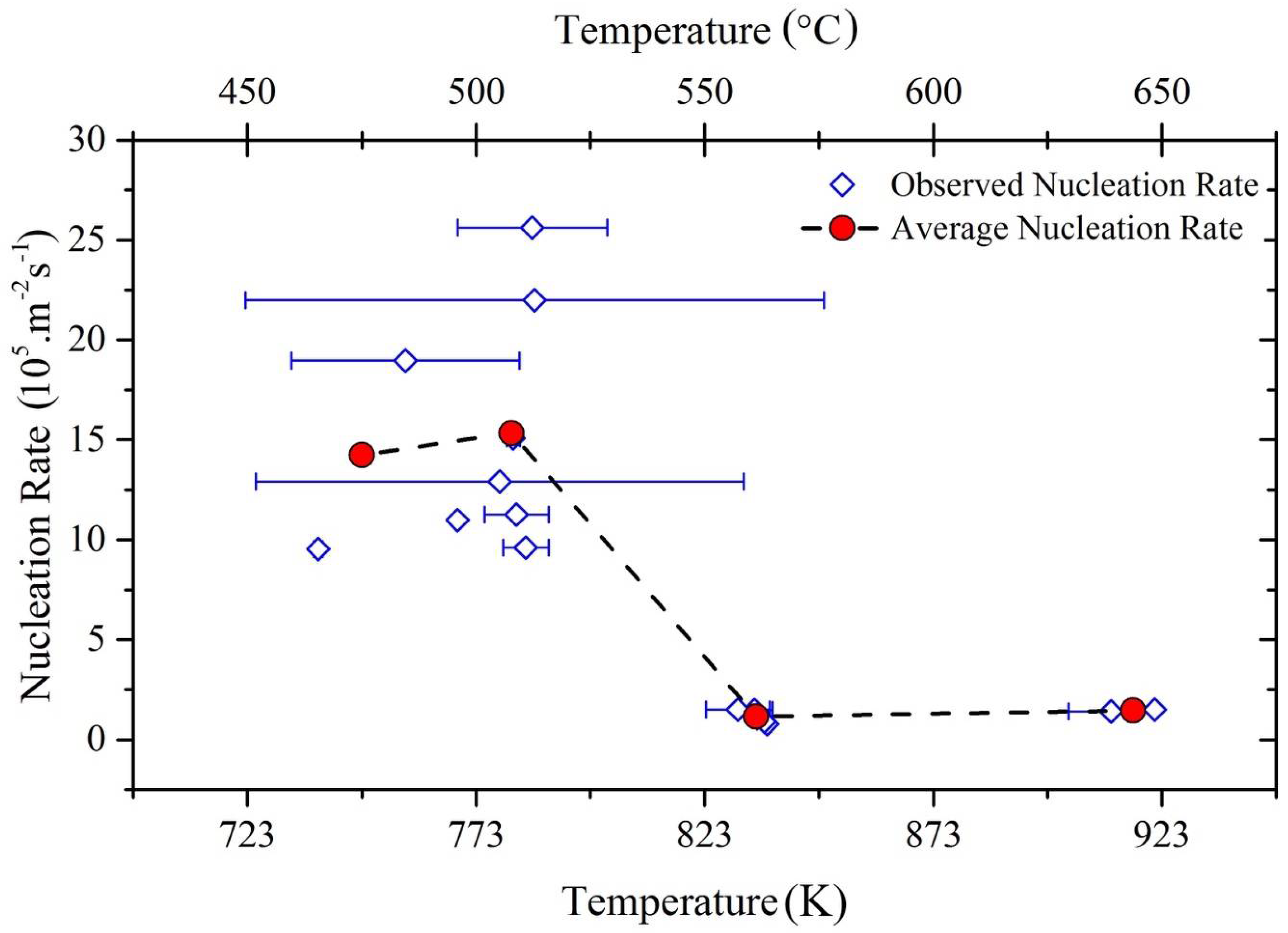
4.1. Nucleation
4.2. Growth
5. Conclusions
- The rate of nucleation (initiation) of bainitic plates is observed to be rather different in different austenite grains.
- The majority of the bainitic plates nucleate on austenite grain boundaries.
- The observed average nucleation rate for bainitic plates is found to be in qualitative agreement with the classical nucleation theory.
- No special relationship is found between the lengthening rate of bainite plates and their nucleation site.
- The predicted values of the maximum lengthening rate by means of an improved diffusional model considering 400 J mol−1 of growth barrier energy are in agreement with experimentally-measured growth rates of bainite plates at high temperatures.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhadeshia, H.K.D.H.; Honeycombe, R. Steels: Microstructure and Properties: Microstructure and Properties; Butterworth-Heinemann: Oxford, UK, 2011; ISBN 0080462928. [Google Scholar]
- Aydin, H.; Essadiqi, E.; Jung, I.H.; Yue, S. Development of 3rd generation AHSS with medium Mn content alloying compositions. Mater. Sci. Eng. A 2013, 564, 501–508. [Google Scholar] [CrossRef]
- Matlock, D.K.; Speer, J.G. Third Generation of AHSS: Microstructure Design Concepts. In Microstructure and Texture in Steels; Springer: London, UK, 2009; pp. 185–205. [Google Scholar]
- Capdevila, C.; Cornide, J.; Tanaka, K.; Nakanishi, K.; Urones-Garrote, E. Kinetic transition during ferrite growth in Fe-C-Mn medium carbon steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2011, 42, 3719–3728. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Shi, J.; Wang, C.; Cao, W.; Sun, X.; Dong, H. Experimental and numerical analysis on formation of stable austenite during the intercritical annealing of 5 Mn steel. Acta Mater. 2011, 59, 4002–4014. [Google Scholar] [CrossRef]
- Wang, C.; Cao, W.; Shi, J.; Huang, C.; Dong, H. Deformation microstructures and strengthening mechanisms of an ultrafine grained duplex medium-Mn steel. Mater. Sci. Eng. A 2013, 562, 89–95. [Google Scholar] [CrossRef]
- Zhang, R.; Cao, W.Q.; Peng, Z.J.; Shi, J.; Dong, H.; Huang, C.X. Intercritical rolling induced ultrafine microstructure and excellent mechanical properties of the medium-Mn steel. Mater. Sci. Eng. A 2013, 583, 84–88. [Google Scholar] [CrossRef]
- Bouaziz, O.; Allain, S.; Scott, C.P.; Cugy, P.; Barbier, D. High manganese austenitic twinning induced plasticity steels: A review of the microstructure properties relationships. Curr. Opin. Solid State Mater. Sci. 2011, 15, 141–168. [Google Scholar] [CrossRef]
- De Cooman, B.C. Structure-properties relationship in TRIP steels containing carbide-free bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 285–303. [Google Scholar] [CrossRef]
- Lee, Y.K.; Choi, C. Driving force for γ→ε martensitic transformation and stacking fault energy of γ in Fe-Mn binary system. Metall. Mater. Trans. A 2000, 31, 355–360. [Google Scholar] [CrossRef]
- Zaefferer, S.; Ohlert, J.; Bleck, W. A study of microstructure, transformation mechanisms and correlation between microstructure and mechanical properties of a low alloyed TRIP steel. Acta Mater. 2004, 52, 2765–2778. [Google Scholar] [CrossRef]
- Grajcar, A.; Zalecki, W.; Burian, W.; Kozłowska, A. Phase Equilibrium and Austenite Decomposition in Advanced High-Strength Medium-Mn Bainitic Steels. Metals 2016, 6, 248. [Google Scholar] [CrossRef]
- Grajcar, A.; Radwański, K.; Krztoń, H.J. Microstructural Analysis of a Thermomechanically Processed Si-Al TRIP Steel Characterized by EBSD and X-Ray Techniques. Solid State Phenom. 2013, 203–204, 34–37. [Google Scholar] [CrossRef]
- Radwański, K.; Wrożyna, A.; Kuziak, R. Role of the advanced microstructures characterization in modeling of mechanical properties of AHSS steels. Mater. Sci. Eng. A 2015, 639, 567–574. [Google Scholar] [CrossRef]
- Kamoutsi, H.; Gioti, E.; Haidemenopoulos, G.N.; Cai, Z.; Ding, H. Kinetics of Solute Partitioning During Intercritical Annealing of a Medium-Mn Steel. Metall. Mater. Trans. A 2015, 46, 4841–4846. [Google Scholar] [CrossRef]
- Sugimoto, K.; Tanino, H.; Kobayashi, J. Impact Toughness of Medium-Mn Transformation-Induced Plasticity-Aided Steels. Steel Res. Int. 2015, 86, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Lee, K.; De Cooman, B.C. Observation of the TWIP + TRIP Plasticity-Enhancement Mechanism in Al-Added 6 Wt Pct Medium Mn Steel. Metall. Mater. Trans. A 2015, 46, 2356–2363. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Sourmail, T.; Caballero, F.G.; Capdevila, C.; García de Andrés, C. New approach for the bainite start temperature calculation in steels. Mater. Sci. Technol. 2005, 21, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Farahani, H.; Xu, W.; van der Zwaag, S. Prediction and Validation of the Austenite Phase Fraction upon Intercritical Annealing of Medium Mn Steels. Metall. Mater. Trans. A 2015, 46, 4978–4985. [Google Scholar] [CrossRef] [Green Version]
- Arlazarov, A.; Gouné, M.; Bouaziz, O.; Hazotte, A.; Petitgand, G.; Barges, P. Evolution of microstructure and mechanical properties of medium Mn steels during double annealing. Mater. Sci. Eng. A 2012, 542, 31–39. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, S.S.; Hong, S.; Suh, B.-C.; Kim, S.-K.; Lee, B.-J.; Kim, N.J.; Lee, S. Effects of Mn Addition on Tensile and Charpy Impact Properties in Austenitic Fe-Mn-C-Al-Based Steels for Cryogenic Applications. Metall. Mater. Trans. A 2014, 45, 5419–5430. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.S.; Lee, S.; Lee, B.-J.; Kwak, J.-H. Microstructural Developments and Tensile Properties of Lean Fe-Mn-Al-C Lightweight Steels. JOM 2014, 66, 1857–1867. [Google Scholar] [CrossRef]
- Han, J.; Lee, S.-J.; Jung, J.-G.; Lee, Y.-K. The effects of the initial martensite microstructure on the microstructure and tensile properties of intercritically annealed Fe-9Mn-0.05C steel. Acta Mater. 2014, 78, 369–377. [Google Scholar] [CrossRef]
- Gutierrez-Urrutia, I.; Raabe, D. Influence of Al content and precipitation state on the mechanical behavior of austenitic high-Mn low-density steels. Scr. Mater. 2013, 68, 343–347. [Google Scholar] [CrossRef]
- Davenport, E.; Bain, E. Transformation of Austenite at Constant Subcritical Temperatures. Trans. AIME 1930, 90, 117–144. [Google Scholar] [CrossRef]
- Paxton, H.W. Commentary by: Transformation of austenite at constant subcritical temperatures. Metall. Trans. 1970, 1, 3479–3501. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Furuhara, T.; Rigsbee, J.M.; Reynolds, W.T.; Howe, J.M. Crystallographic and mechanistic aspects of growth by shear and by diffusional processes. Metall. Trans. A 1990, 21, 2369–2409. [Google Scholar] [CrossRef]
- Hillert, M. The Nature of Bainite. ISIJ Int. 1995, 35, 1134–1140. [Google Scholar] [CrossRef]
- Hillert, M.; Höglund, L.; Ågren, J. Role of carbon and alloying elements in the formation of bainitic ferrite. Metall. Mater. Trans. A 2004, 35, 3693–3700. [Google Scholar] [CrossRef]
- Caballero, F.G.; Bhadeshia, H.K.D.H. Very strong bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Fang, H. An overview on bainite formation in steels. Curr. Opin. Solid State Mater. Sci. 2005, 9, 277–286. [Google Scholar] [CrossRef]
- Aaronson, H.I.; Reynolds, W.T.; Purdy, G.R. The incomplete transformation phenomenon in steel. Metall. Mater. Trans. A 2006, 37, 1731–1745. [Google Scholar] [CrossRef]
- Borgenstam, A.; Hillert, M.; Agren, J. Metallographic evidence of carbon diffusion in the growth of bainite. Acta Mater. 2009, 57, 3242–3252. [Google Scholar] [CrossRef]
- Caballero, F.G.; Garcia-Mateo, C.; Santofimia, M.J.; Miller, M.K.; García de Andrés, C. New experimental evidence on the incomplete transformation phenomenon in steel. Acta Mater. 2009, 57, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Di, X.; Chen, C.; Guo, X.; Xue, Z. A bainite transformation kinetics model and its application to X70 pipeline steel. J. Mater. Sci. 2015, 50, 5079–5090. [Google Scholar] [CrossRef]
- Gong, W.; Tomota, Y.; Harjo, S.; Su, Y.; Aizawa, K. Effect of prior martensite on bainite transformation in nanobainite steel. Acta Mater. 2015, 85, 243–249. [Google Scholar] [CrossRef]
- Hillert, M. Diffusion in growth of bainite. Metall. Mater. Trans. A 1994, 25, 1957–1966. [Google Scholar] [CrossRef]
- Purdy, G.R.; Hillert, M. Overview No. 38: On the nature of the bainite transformation in steels. Acta Metall. 1984, 32, 823–828. [Google Scholar] [CrossRef]
- Hillert, M. The growth of ferrite, bainite and martensite. In Thermodynamics and Phase Transformations: The Selected Works of Mats Hillert; EDP Sciences: Les Ulis, France, 2006; pp. 111–158. [Google Scholar]
- Hehemann, R.F.; Kinsman, K.R.; Aaronson, H.I. A debate on the bainite reaction. Metall. Trans. 1972, 3, 1077–1094. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. The mechanism of bainite formation in steels. Acta Metall. 1980, 28, 1265–1273. [Google Scholar] [CrossRef]
- Reynolds, W.T.; Li, F.Z.; Shui, C.K.; Aaronson, H.I. The Incomplete transformation phenomenon in Fe-C-Mo alloys. Metall. Trans. A 1990, 21, 1433–1463. [Google Scholar] [CrossRef]
- Reynolds, W.T.; Liu, S.K.; Li, F.Z.; Hartfield, S.; Aaronson, H.I. An investigation of the generality of incomplete transformation to bainite in Fe-C-X alloys. Metall. Trans. A 1990, 21, 1479–1491. [Google Scholar] [CrossRef]
- Quidort, D.; Brechet, Y.J.M. Isothermal growth kinetics of bainite in 0.5% C steels. Acta Mater. 2001, 49, 4161–4170. [Google Scholar] [CrossRef]
- Darken, L.S.; Fisher, R.M. Decomposition of Austenite by Diffusional Processes. In Proc. Symposium in Philadelphia; Interscience Publ.: Bucks, UK, 1962; pp. 249–294. [Google Scholar]
- Matsuda, H.; Bhadeshia, H.K.D.H. Kinetics of the bainite transformation. Proc. R. Soc. A Math. Phys. Eng. Sci. 2004, 460, 1707–1722. [Google Scholar] [CrossRef] [Green Version]
- Quidort, D.; Bréchet, Y. The role of carbon on the kinetics of bainite transformation in steels. Scr. Mater. 2002, 47, 151–156. [Google Scholar] [CrossRef]
- Chang, L.C. Microstructures and reaction kinetics of bainite transformation in Si-rich steels. Mater. Sci. Eng. A 2004, 368, 175–182. [Google Scholar] [CrossRef]
- Singh, S.B.; Bhadeshia, H. Estimation of bainite plate-thickness in low-alloy steels. Mater. Sci. Eng. A 1998, 245, 72–79. [Google Scholar] [CrossRef]
- Wu, K.M.; Kagayama, M.; Enomoto, M. Kinetics of ferrite transformation in an Fe-0.28mass%C-3mass%Mo alloy. Mater. Sci. Eng. A 2003, 343, 143–150. [Google Scholar] [CrossRef]
- Enomoto, M.; Maruyama, N.; Wu, K.M.; Tarui, T. Alloying element accumulation at ferrite/austenite boundaries below the time–temperature–transformation diagram bay in an Fe-C-Mo Alloy. Mater. Sci. Eng. A 2003, 343, 151–157. [Google Scholar] [CrossRef]
- Rees, G.I.; Bhadeshia, H.K.D.H. Bainite transformation kinetics Part 2 Non-uniform distribution of carbon. Mater. Sci. Technol. 1992, 8, 994–1003. [Google Scholar] [CrossRef]
- Takahashi, M. Recent progress: kinetics of the bainite transformation in steels. Curr. Opin. Solid State Mater. Sci. 2004, 8, 213–217. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Caballero, F.G.; Capdevila, C.; García-Mateo, C.; de Andrés, C.G. Evaluation of Displacive Models for Bainite Transformation Kinetics in Steels. Mater. Trans. 2006, 47, 1492–1500. [Google Scholar] [CrossRef] [Green Version]
- Van Bohemen, S.M.C.; Sietsma, J. Modeling of isothermal bainite formation based on the nucleation kinetics. Int. J. Mater. Res. 2008, 99, 739–747. [Google Scholar] [CrossRef]
- Ravi, A.M.; Sietsma, J.; Santofimia, M.J. Exploring bainite formation kinetics distinguishing grain-boundary and autocatalytic nucleation in high and low-Si steels. Acta Mater. 2016, 105, 155–164. [Google Scholar] [CrossRef]
- Chen, H.; van der Zwaag, S. Analysis of ferrite growth retardation induced by local Mn enrichment in austenite created by prior interface passages. Acta Mater. 2013, 61, 1338–1349. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, K.; Zhao, L.; Van Der Zwaag, S. Analysis of transformation stasis during the isothermal bainitic ferrite formation in Fe-C-Mn and Fe-C-Mn-Si alloys. Acta Mater. 2013, 61, 5458–5468. [Google Scholar] [CrossRef]
- Chen, H.; Borgenstam, A.; Odqvist, J.; Zuazo, I.; Goune, M.; Ågren, J.; van der Zwaag, S. Application of interrupted cooling experiments to study the mechanism of bainitic ferrite formation in steels. Acta Mater. 2013, 61, 4512–4523. [Google Scholar] [CrossRef]
- Ko, T.; Cottrell, S.A. The formation of bainite. J. Iron Steel Inst. 1952, 172, 307. [Google Scholar]
- Zhang, D.; Terasaki, H.; Komizo, Y. In situ observation of phase transformation in Fe-0.15C binary alloy. J. Alloys Compd. 2009, 484, 929–933. [Google Scholar] [CrossRef]
- Kolmskog, P.; Borgenstam, A.; Hillert, M.; Hedström, P.; Babu, S.S.; Terasaki, H.; Komizo, Y.I. Direct Observation that Bainite can Grow Below MS. Metall. Mater. Trans. A 2012, 43, 4984. [Google Scholar] [CrossRef]
- Yada, H.; Enomoto, M.; Sonoyama, T. Lengthening Kinetics of Bainitic Plates in Iron-Nickel-Carbon Alloys. ISIJ Int. 1995, 35. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, M.-X.; Zhu, M. In situ observation of bainite growth during isothermal holding. Acta Mater. 2006, 54, 2121–2129. [Google Scholar] [CrossRef]
- Xu, G.; Liu, F.; Wang, L.; Hu, H. A new approach to quantitative analysis of bainitic transformation in a superbainite steel. Scr. Mater. 2013, 68, 833–836. [Google Scholar] [CrossRef]
- Wan, X.L.; Wei, R.; Cheng, L.; Enomoto, M.; Adachi, Y. Lengthening kinetics of ferrite plates in high-strength low-carbon low alloy steel. J. Mater. Sci. 2013, 48, 4345–4355. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, G.; Hu, H.; Wang, L.; Xue, Z. In situ measured growth rates of bainite plates in an Fe-C-Mn-Si superbainitic steel. Int. J. Miner. Metall. Mater. 2014, 21, 371–378. [Google Scholar] [CrossRef]
- Agren, J.; Brechet, Y.; Hutchinson, C.; Philibert, J.; Purdy, G. Thermodynamics and Phase Transformations: The Selected Works of Mats Hillert; EDP Sciences: Les Ulis, France, 2006; ISBN 2868838898. [Google Scholar]
- Hillert, M. Role of interfacial energy during solid-state phase transformations. Jernkontorets Ann. 1957, 141, 757–789. [Google Scholar]
- Trivedi, R.; Pound, G.M. Effect of Concentration-Dependent Diffusion Coefficient on the Migration of Interphase Boundaries. J. Appl. Phys. 1967, 38, 3569–3576. [Google Scholar] [CrossRef]
- Tian, J.; Xu, G.; Wang, L.; Zhou, M.; Hu, H. In Situ Observation of the Lengthening Rate of Bainite Sheaves During Continuous Cooling Process in a Fe–C–Mn–Si Superbainitic Steel. Trans. Indian Inst. Met. 2017, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; Gamsjäger, E.; Schider, S.; Khanbareh, H.; van der Zwaag, S. In situ observation of austenite–ferrite interface migration in a lean Mn steel during cyclic partial phase transformations. Acta Mater. 2013, 61, 2414–2424. [Google Scholar] [CrossRef]
- The Math Works Inc. MATLAB Image Processing Toolbox Realese 2015b; The Math Works Inc.: Natick, MA, USA, 2007. [Google Scholar]
- Pak, J.; Suh, D.W.; Bhadeshia, H.K.D.H. Displacive Phase Transformation and Surface Effects Associated with Confocal Laser Scanning Microscopy. Metall. Mater. Trans. A 2012, 43, 4520–4524. [Google Scholar] [CrossRef] [Green Version]
- Kalikmanov, V.I. Classical Nucleation Theory; Springer: Dordrecht, The Netherlands, 2013; pp. 17–41. [Google Scholar]
- Callister, W.D. Materials Science and Engineering: An Introduction, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9780471736967. [Google Scholar]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Kučera, J.; Stránský, K. Diffusion in iron, iron solid solutions and steels. Mater. Sci. Eng. 1982, 52, 1–38. [Google Scholar] [CrossRef]
- Offerman, S.E.; van Dijk, N.H.; Sietsma, J.; Grigull, S.; Lauridsen, E.M.; Margulies, L.; Poulsen, H.F.; Rekveldt, M.T.; van der Zwaag, S. Grain Nucleation and Growth During Phase Transformations. Science 2002, 298, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Clemm, P.; Fisher, J. The influence of grain boundaries on the nucleation of secondary phases. Acta Metall. 1955, 3, 70–73. [Google Scholar] [CrossRef]
- Lange, W.F.; Enomoto, M.; Aaronson, H.I. The kinetics of ferrite nucleation at austenite grain boundaries in Fe-C alloys. Metall. Trans. A 1988, 19, 427–440. [Google Scholar] [CrossRef]
- Quidort, D.; Brechet, Y.J.M. A Model of Isothermal and Non Isothermal Transformation Kinetics of Bainite in 0.5% C Steels. ISIJ Int. 2002, 42, 1010–1017. [Google Scholar] [CrossRef]
- Van Dijk, N.H.; Offerman, S.E.; Sietsma, J.; van der Zwaag, S. Barrier-free heterogeneous grain nucleation in polycrystalline materials: The austenite to ferrite phase transformation in steel. Acta Mater. 2007, 55, 4489–4498. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Liu, Y.; Li, C.; Li, H.; Guo, Q. Acicular ferrite formation during isothermal holding in HSLA steel. J. Mater. Sci. 2016, 51, 3555–3563. [Google Scholar] [CrossRef]
- Leach, L.; Hillert, M.; Borgenstam, A. Modeling C-Curves for the Growth Rate of Widmanstätten and Bainitic Ferrite in Fe-C Alloys. Metall. Mater. Trans. A 2016, 47, 19–25. [Google Scholar] [CrossRef]
- Zener, C. Kinetics of the decomposition of austenite. Trans. Aime 1946, 167, 550–595. [Google Scholar]
- Ågren, J. A revised expression for the diffusivity of carbon in binary Fe-C austenite. Scr. Metall. 1986, 20, 1507–1510. [Google Scholar] [CrossRef]
- Yin, J.; Leach, L.; Hillert, M.; Borgenstam, A. C-Curves for Lengthening of Widmanstätten and Bainitic Ferrite. Metall. Mater. Trans. A 2017, 48, 3997–4005. [Google Scholar] [CrossRef] [Green Version]
- Bhadeshia, H. Bainite in Steels: Theory and Practice, 3rd ed.; Maney Publishing: Leeds, UK, 2015; ISBN 1909662747, 9781909662742. [Google Scholar]
- Enomoto, M.; Sonoyama, T.; Yada, H. Kinetics of Austenite to Ferrite Transformation in 3 mass% Mn Low Carbon Steels. Mater. Trans. JIM 1998, 39, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Howe, J.M. Comparison of the atomic structure, composition, kinetics and mechanisms of interfacial motion in martensitic, bainitic, massive and precipitation face-centered cubic-hexagonal close-packed phase transformations. Mater. Sci. Eng. A 2006, 438–440, 35–42. [Google Scholar] [CrossRef]
- Chen, H.; van der Zwaag, S. On the nature of the bainitic and ferritic transformation stasis in steels. In Proceedings of the PTM2015—2015 International Conference on Solid-Solid Phase Transformations in Inorganic Materials, Whistler, BC, Canada, 28 June–3 July 2015; pp. 37–44. [Google Scholar]
- Chen, H.; Yang, Z.; Zhang, C.; Zhu, K.; van der Zwaag, S. On the transition between grain boundary ferrite and bainitic ferrite in Fe–C–Mo and Fe–C–Mn alloys: The bay formation explained. Acta Mater. 2016, 104, 62–71. [Google Scholar] [CrossRef]
- Chen, H.; Van Der Zwaag, S. An experimental study of the stagnant stage in bainite phase transformations starting from austenite-bainite mixtures. In Proceedings of the TMP 2012—4th International Conference on Thermomechanical Processing of Steels, Sheffield, UK, 10–12 September 2012. [Google Scholar]
- Yang, Z.Z.; Xu, W.; Yang, Z.Z.; Zhang, C.; van der Zwaag, S. A 2D analysis of the competition between the equiaxed ferritic and the bainitic morphology based on a Gibbs Energy Balance approach. Acta Mater. 2016, 105, 317–327. [Google Scholar] [CrossRef]









| Steel Composition (mass%) | Austenitizing Temperature | Isothermal Treatment Temperature | ||
|---|---|---|---|---|
| C | Mn | Cr | ||
| 0.2 | 1.5 | 2.0 | 1223 K (950 °C) | 723 K (450 °C) |
| 1233 K (960 °C) | 773 K (500 °C) | |||
| 1233 K (960 °C) | 823 K (550 °C) | |||
| 1373 K (1100 °C) | 923 K (650 °C) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sainis, S.; Farahani, H.; Gamsjäger, E.; Van der Zwaag, S. An In-Situ LSCM Study on Bainite Formation in a Fe-0.2C-1.5Mn-2.0Cr Alloy. Metals 2018, 8, 498. https://doi.org/10.3390/met8070498
Sainis S, Farahani H, Gamsjäger E, Van der Zwaag S. An In-Situ LSCM Study on Bainite Formation in a Fe-0.2C-1.5Mn-2.0Cr Alloy. Metals. 2018; 8(7):498. https://doi.org/10.3390/met8070498
Chicago/Turabian StyleSainis, Salil, Hussein Farahani, Ernst Gamsjäger, and Sybrand Van der Zwaag. 2018. "An In-Situ LSCM Study on Bainite Formation in a Fe-0.2C-1.5Mn-2.0Cr Alloy" Metals 8, no. 7: 498. https://doi.org/10.3390/met8070498





