Effect of Boron Addition on the Precipitation Behavior of S31254
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
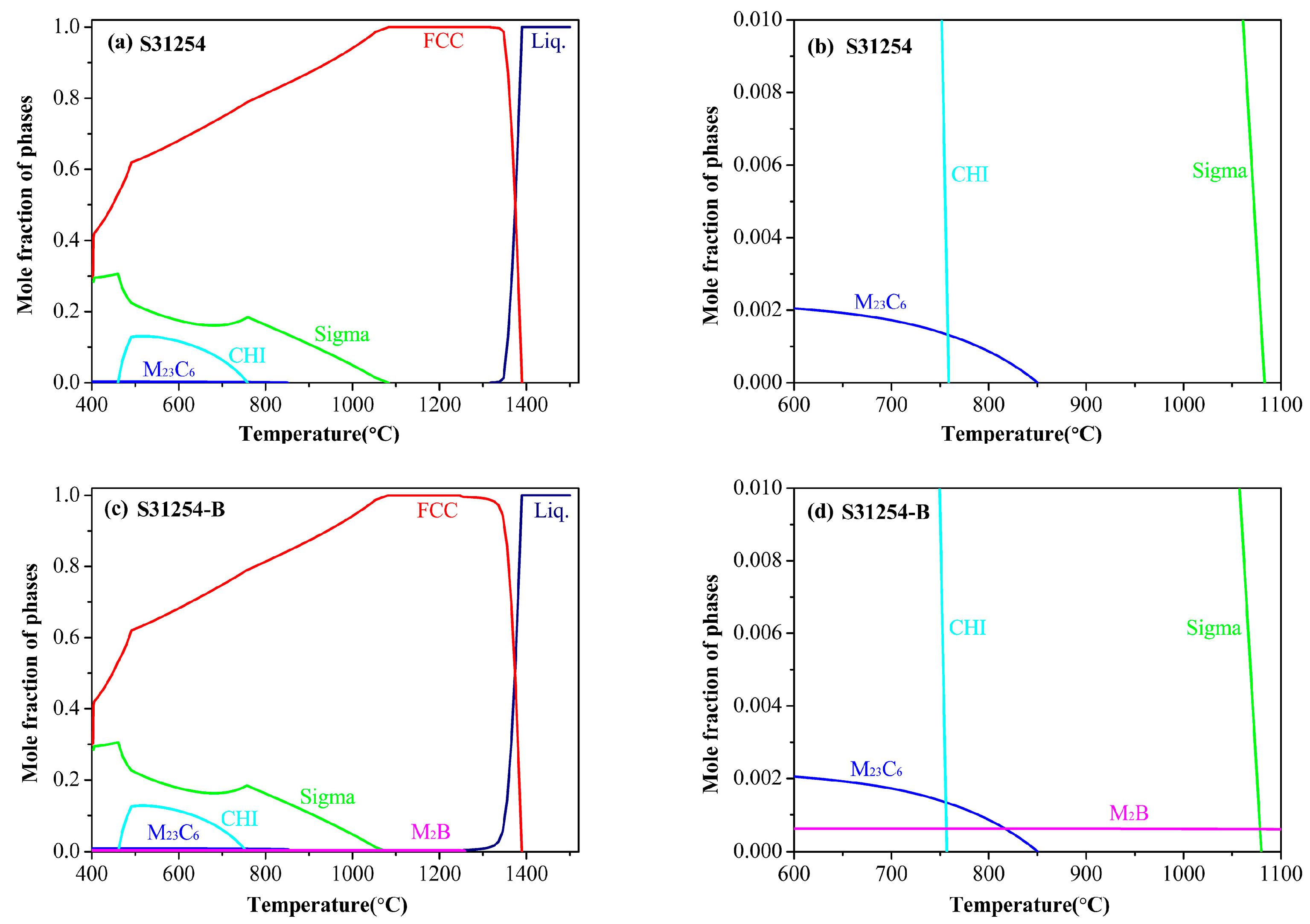
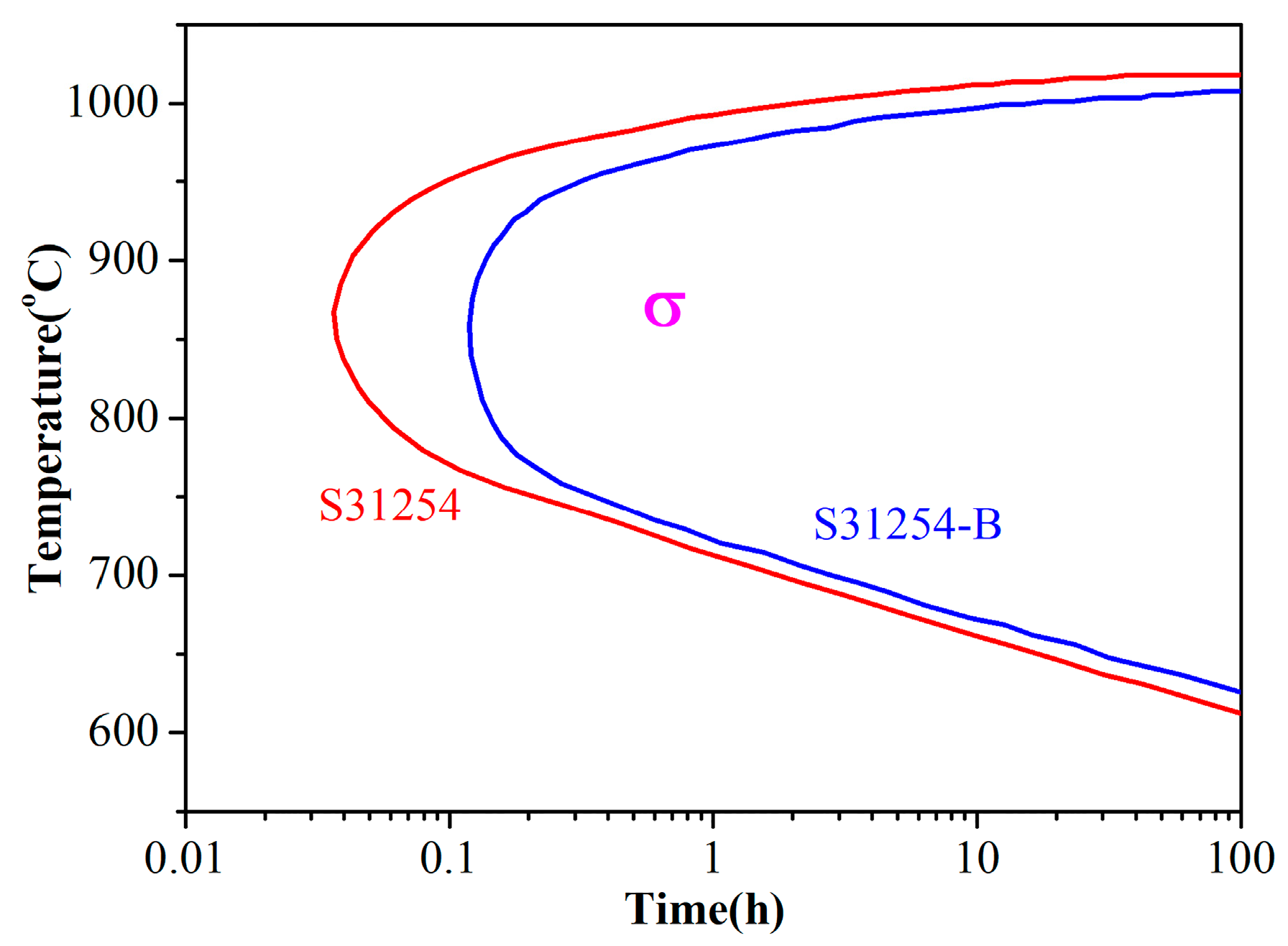
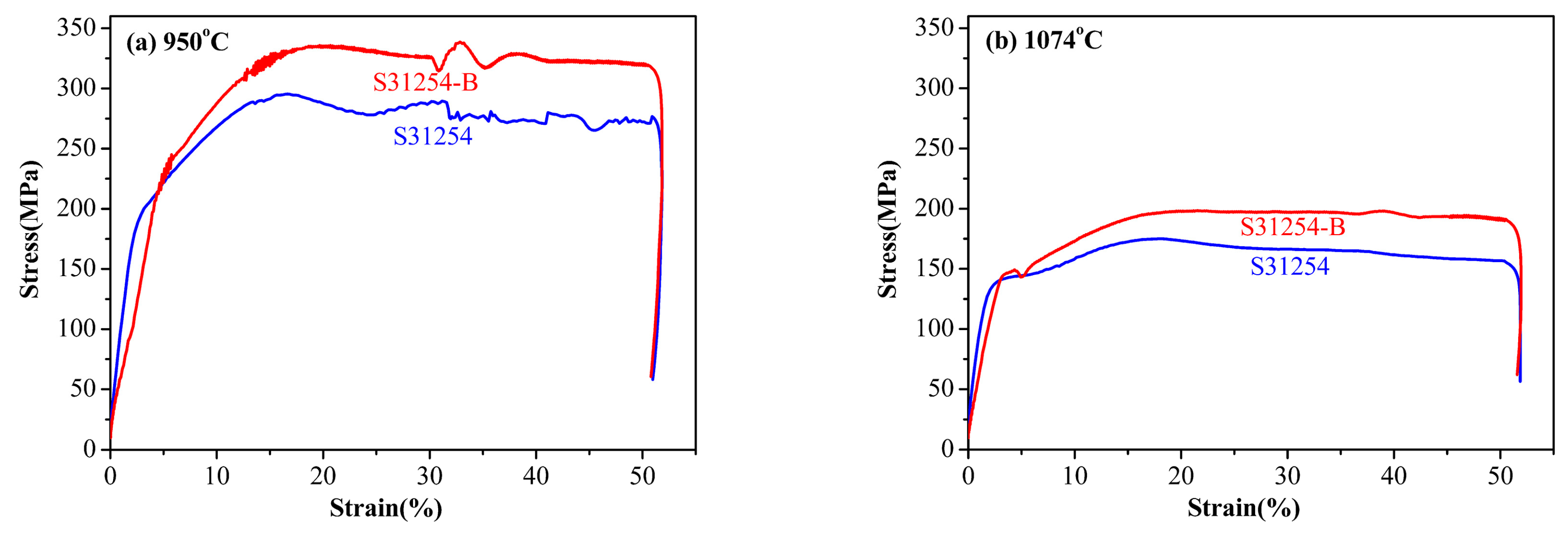
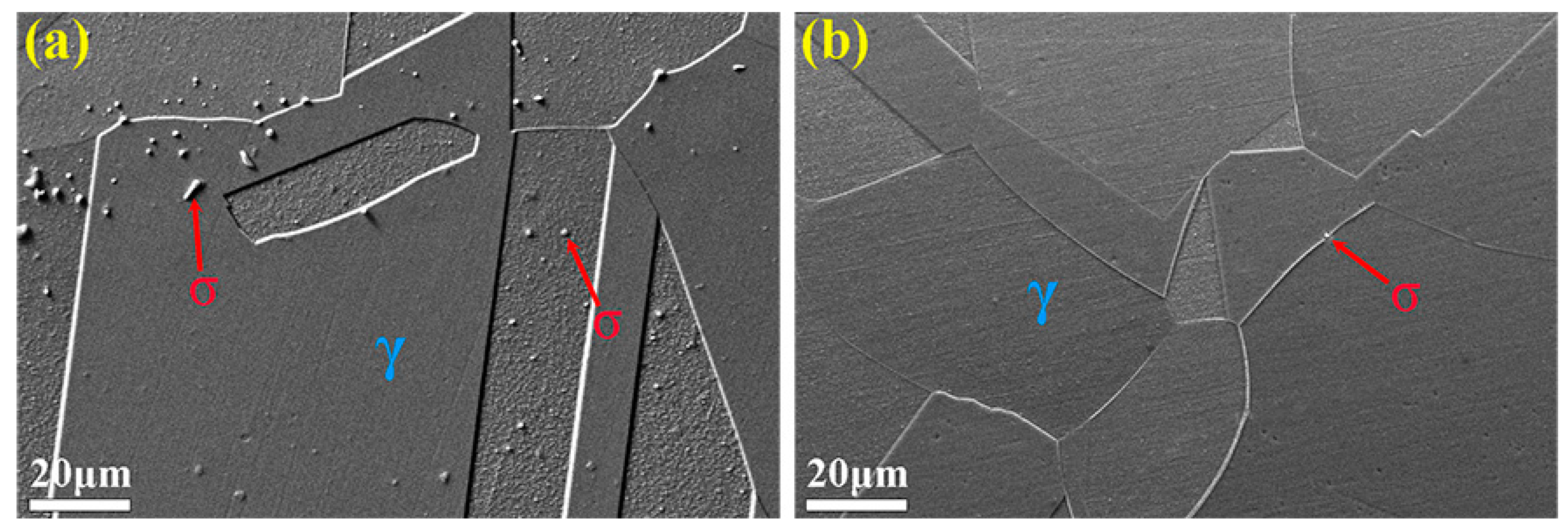
3. Results and Discussion
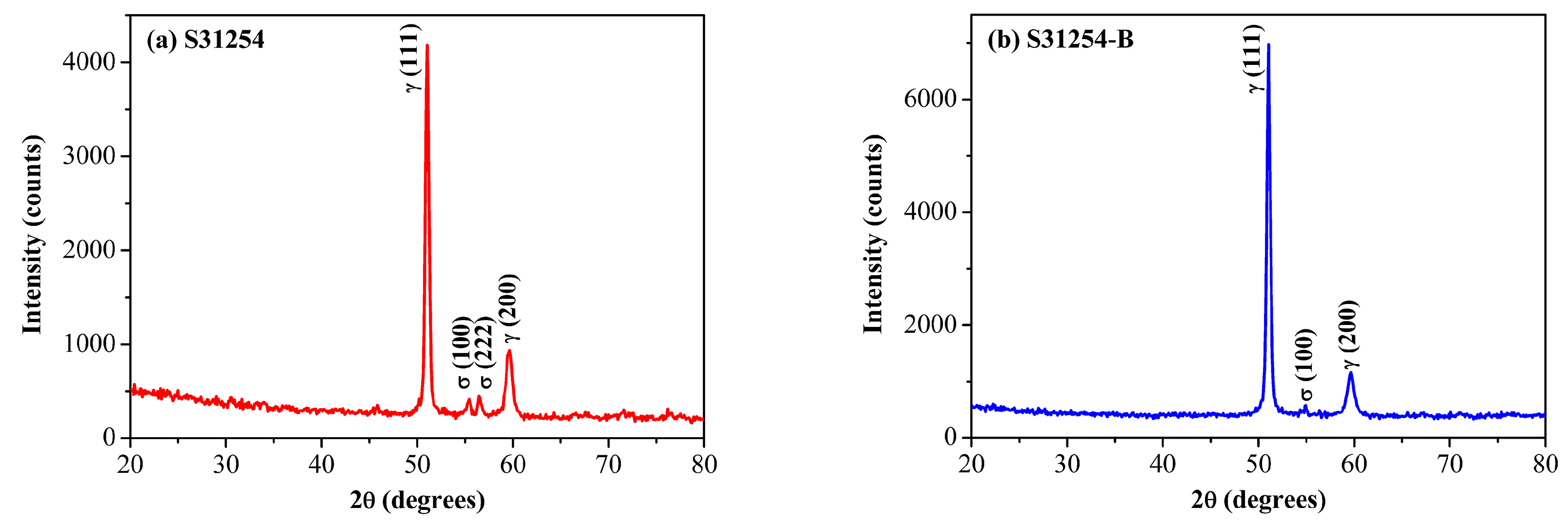
3.1. Kinetics of σ Phase Precipitation
3.2. Stress Strain Curve
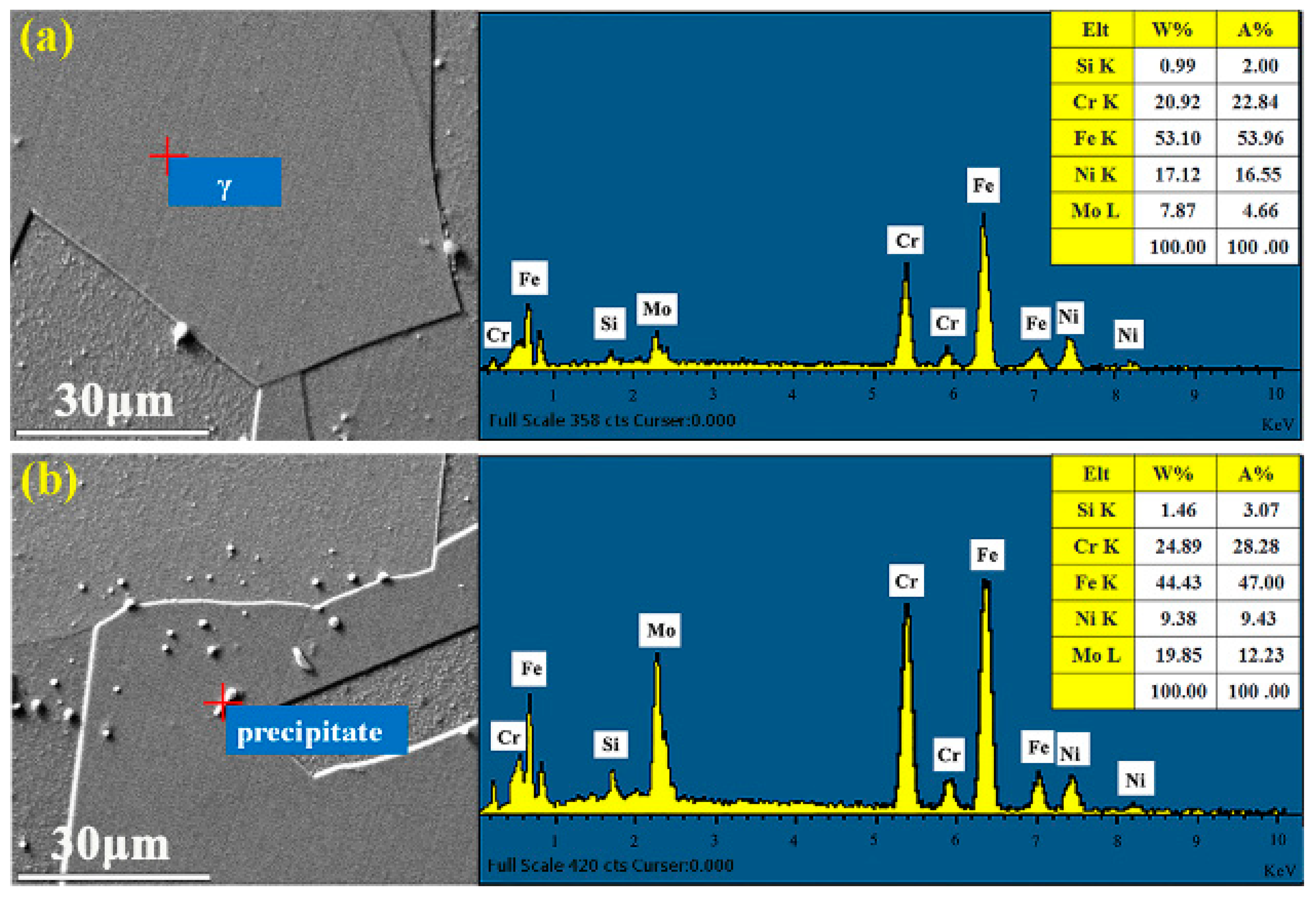
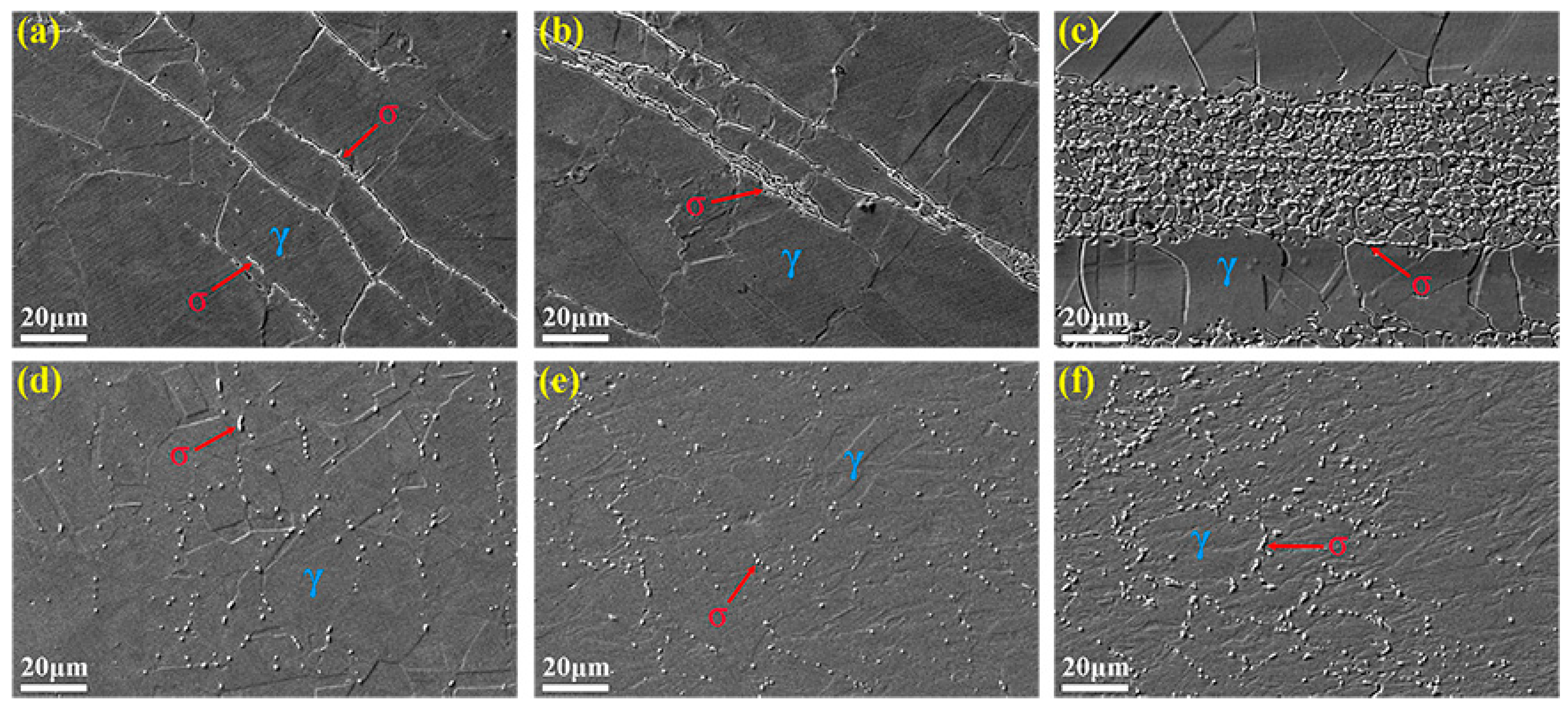
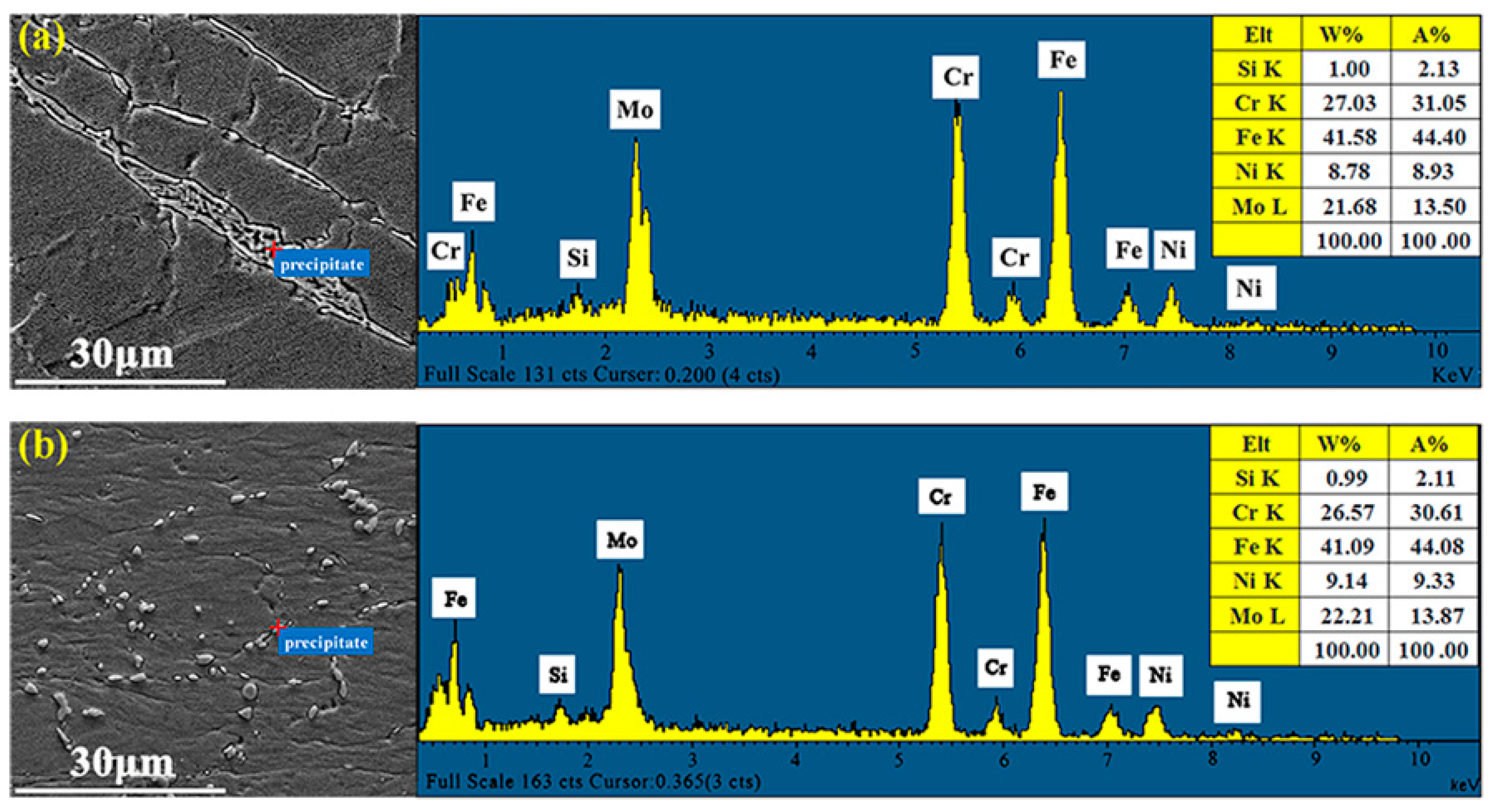
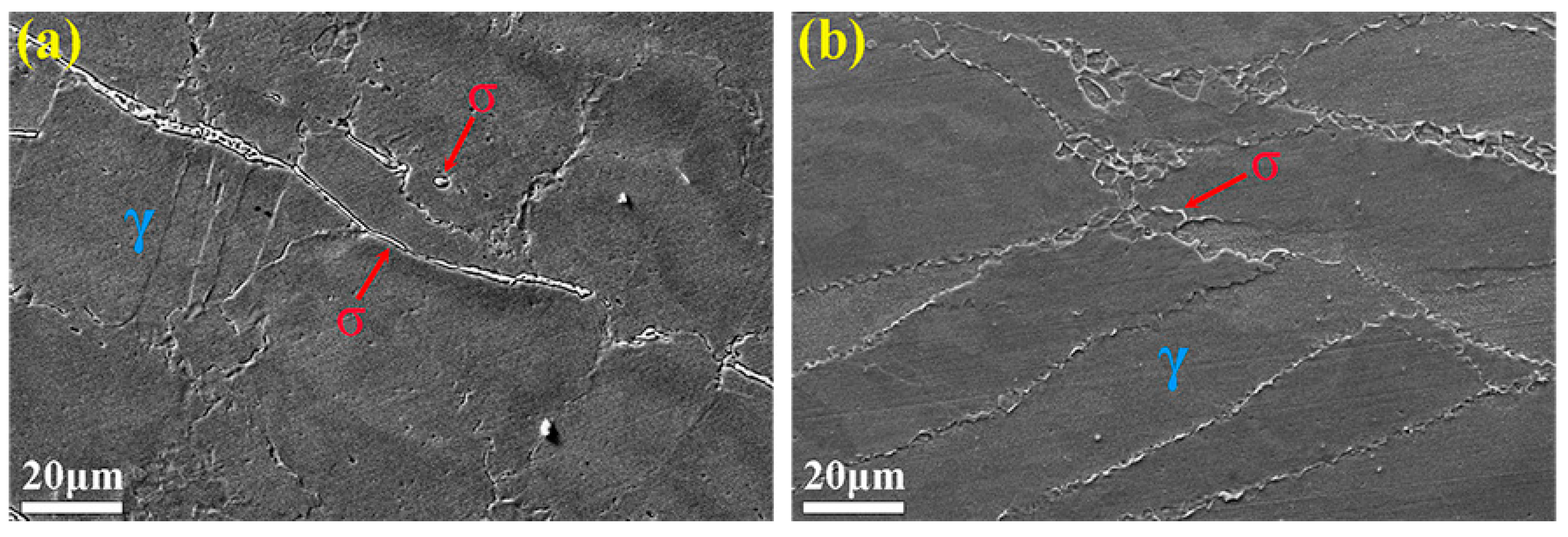
3.3. Microstructure Evolution
3.4. σ Phase Precipitation Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sathiya, P.; Mishra, M.K. Effect of shielding gases on microstructure and mechanical properties of super austenitic stainless steel by hybrid welding. Mater. Des. 2012, 33, 203–212. [Google Scholar] [CrossRef]
- Zambon, A.; Ferro, P. Compositional and residual stress evaluation of CO2 laser welded superaustenitic AISI 904L stainless steel. Mater. Sci. Eng. 2006, 424, 117–127. [Google Scholar] [CrossRef]
- Bonollo, F.; Tiziani, A. Super austenitic stainless steel: The microstructure and fatigue strength a welded joints. Weld. Int. 2004, 18, 24–30. [Google Scholar] [CrossRef]
- Liu, G.; Han, Y. Hot deformation and optimization of process parameters of an as-cast 6Mo superaustenitic stainless steel: A study with processing map. Mater. Des. 2014, 53, 662–672. [Google Scholar] [CrossRef]
- Asli, A.H.; Zarei-Hanzaki, A. Dynamic Recrystallization Behavior of a Fe-Cr-Ni Super-Austenitic Stainless Steel. Mater. Sci. Technol. 2009, 25, 603–606. [Google Scholar]
- Momeni, A.; Dehghani, K. Hot deformation behavior and microstructural evolution of a superaustenitic stainless steel. Mater. Sci. Eng. 2010, 527, 1605–1611. [Google Scholar] [CrossRef]
- Ebrahimi, G.R.; Keshmiri, H. Dynamic recrystallization behavior of a superaustenitic stainless steel containing 16%Cr and 25%Ni. Mater. Sci. Eng. 2011, 528, 7488–7493. [Google Scholar] [CrossRef]
- Zhang, J.; Xuan, F.Z. Effect of plastic deformation on nonlinear ultrasonic response of austenitic stainless steel. Mater. Sci. Eng. 2015, 622, 146–152. [Google Scholar] [CrossRef]
- Wang, S.H.; Wu, C.C. Cyclic deformation and phase transformation of 6Mo superaustenitic stainless steel. Met. Mater. Int. 2017, 13, 275–283. [Google Scholar] [CrossRef]
- Koutsoukis, T.; Redjaïmia, A. Phase transformations and mechanical properties in heat treated superaustenitic stainless steels. Mater. Sci. Eng. 2013, 561, 477–485. [Google Scholar] [CrossRef]
- Han, Y.; Liu, G. Deformation behavior and microstructural evolution of as-cast 904L austenitic stainless steel during hot compression. Mater. Sci. Eng. 2013, 565, 342–350. [Google Scholar] [CrossRef]
- Bradaskja, B.; Pirnar, B. Deformation Behaviour and Microstructural Evolution During Hot Compression of AISI 904L. Steel Res. Int. 2011, 82, 46–51. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Z. Precipitation behavior and phase transformation mechanism of super austenitic stainless steel S32654 during isothermal aging. Mater. Charact. 2018, 137, 244–255. [Google Scholar] [CrossRef]
- Polák, J.; Petráš, R. Low cycle fatigue behavior of Sanicro25 steel at room and at elevated temperature. Mater. Sci. Eng. 2014, 615, 175–182. [Google Scholar] [CrossRef]
- Mallick, P.; Tewary, N.K. Effect of cryogenic deformation on microstructure and mechanical properties of 304 austenitic stainless steel. Mater. Charact. 2017, 133, 77–86. [Google Scholar] [CrossRef]
- Heczko, M.; Polák, J. Microstructure and dislocation arrangements in Sanicro 25 steel fatigued at ambient and elevated temperatures. Mater. Sci. Eng. 2016, 680, 168–181. [Google Scholar] [CrossRef]
- Sun, I.H.; Laird, C. Mechanisms of slip mode modification in F.C.C. solid solutions. Acta Metal. Mater. 1990, 38, 1581–1594. [Google Scholar]
- Lu, J.; Hultman, L. Stacking fault energies in austenitic stainless steels. Acta Mater. 2016, 111, 39–46. [Google Scholar] [CrossRef]
- Korzhavyi, P.A.; Sandström, R. First-principles evaluation of the effect of alloying elements on the lattice parameter of a 23Cr25NiWCuCo austenitic stainless steel to model solid solution hardening contribution to the creep strength. Mater. Sci. Eng. 2015, 626, 213–219. [Google Scholar] [CrossRef]
- Takahashi, J.; Ishikawa, K. Atomic-scale study on segregation behavior at austenite grain boundaries in boron- and molybdenum-added steels. Acta Mater. 2017, 133, 41–45. [Google Scholar] [CrossRef]
- San, X.Y.; Zhang, B. Investigating the effect of Cu-rich phase on the corrosion behavior of Super 304H austenitic stainless steel by TEM. Corros. Sci. 2017, 130, 1609–1616. [Google Scholar] [CrossRef]
- Ueno, M.; Itoh, K. New empirical formula for estimation of hardenability from chemical compositions. Tetsu-to-Hagane 1988, 74, 1073–1080. [Google Scholar] [CrossRef]
- Asahi, H. Effects of Mo addition and austenitizing temperature on hardenability of low alloy B-added steels. ISIJ Int. 2002, 42, 1150–1155. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Z. Detection of susceptibility to intergranular corrosion of aged super austenitic stainless steel S32654 by a modified electrochemical potentiokinetic reactivation method. J. Alloys Compd. 2016, 695, 3083–3093. [Google Scholar] [CrossRef]
- Perron, A.; Ledoux, X. Understanding sigma-phase precipitation in a stabilized austenitic stainless steel (316Nb) through complementary CALPHAD-based and experimental investigations. Acta Mater. 2014, 79, 16–29. [Google Scholar] [CrossRef]
- Djahazi, M. Influence of Boron Distribution on Precipitation and Recrystallization in Hot Worked Austenite. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1989. [Google Scholar]
- Yao, X.X. On the grain boundary hardening in a B-bearing 304 austenitic stainless steel. Mater. Sci. Eng. 1999, 271, 353–359. [Google Scholar] [CrossRef]
- Laha, K.; Kyono, J. Beneficial effect of B segregation on creep cavitation in a type 347 austenitic stainless steel. Scr. Mater. 2005, 52, 675–678. [Google Scholar] [CrossRef]
- Nakajima, H.; Hamada, K. Development of low carbon and boron added 22Mn-13Cr-9Ni-1Mo-0.24N steel (JK2LB) for jacket which undergoes nb3sn heat treatment. IEEE Trans. Appl. Supercon. 2004, 14, 1145–1148. [Google Scholar] [CrossRef]




| No. | C | Si | Mn | P | S | Cr | Ni | Mo | Cu | N | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S31254 | 0.010 | 0.610 | 0.430 | 0.024 | 0.001 | 20.180 | 18.000 | 6.000 | 0.690 | 0.193 | 0.000 |
| S31254-B | 0.014 | 0.620 | 0.940 | 0.014 | 0.006 | 20.150 | 18.110 | 6.120 | 0.720 | 0.200 | 0.040 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Cui, Y.; Wang, J.; Dong, N.; Saqlain Qurashi, M.; Wei, H.; Yang, Y.; Han, P. Effect of Boron Addition on the Precipitation Behavior of S31254. Metals 2018, 8, 497. https://doi.org/10.3390/met8070497
Bai J, Cui Y, Wang J, Dong N, Saqlain Qurashi M, Wei H, Yang Y, Han P. Effect of Boron Addition on the Precipitation Behavior of S31254. Metals. 2018; 8(7):497. https://doi.org/10.3390/met8070497
Chicago/Turabian StyleBai, Jingang, Yishi Cui, Jian Wang, Nan Dong, Muhammad Saqlain Qurashi, Hairui Wei, Yongchao Yang, and Peide Han. 2018. "Effect of Boron Addition on the Precipitation Behavior of S31254" Metals 8, no. 7: 497. https://doi.org/10.3390/met8070497
APA StyleBai, J., Cui, Y., Wang, J., Dong, N., Saqlain Qurashi, M., Wei, H., Yang, Y., & Han, P. (2018). Effect of Boron Addition on the Precipitation Behavior of S31254. Metals, 8(7), 497. https://doi.org/10.3390/met8070497






