Kinetics of Roasting Decomposition of the Rare Earth Elements by CaO and Coal
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Sampling
2.2.2. Mixing
2.2.3. Briquetting
2.2.4. Roasting
2.2.5. Analysis
2.2.6. Analytical Facility
3. Results and Discussion
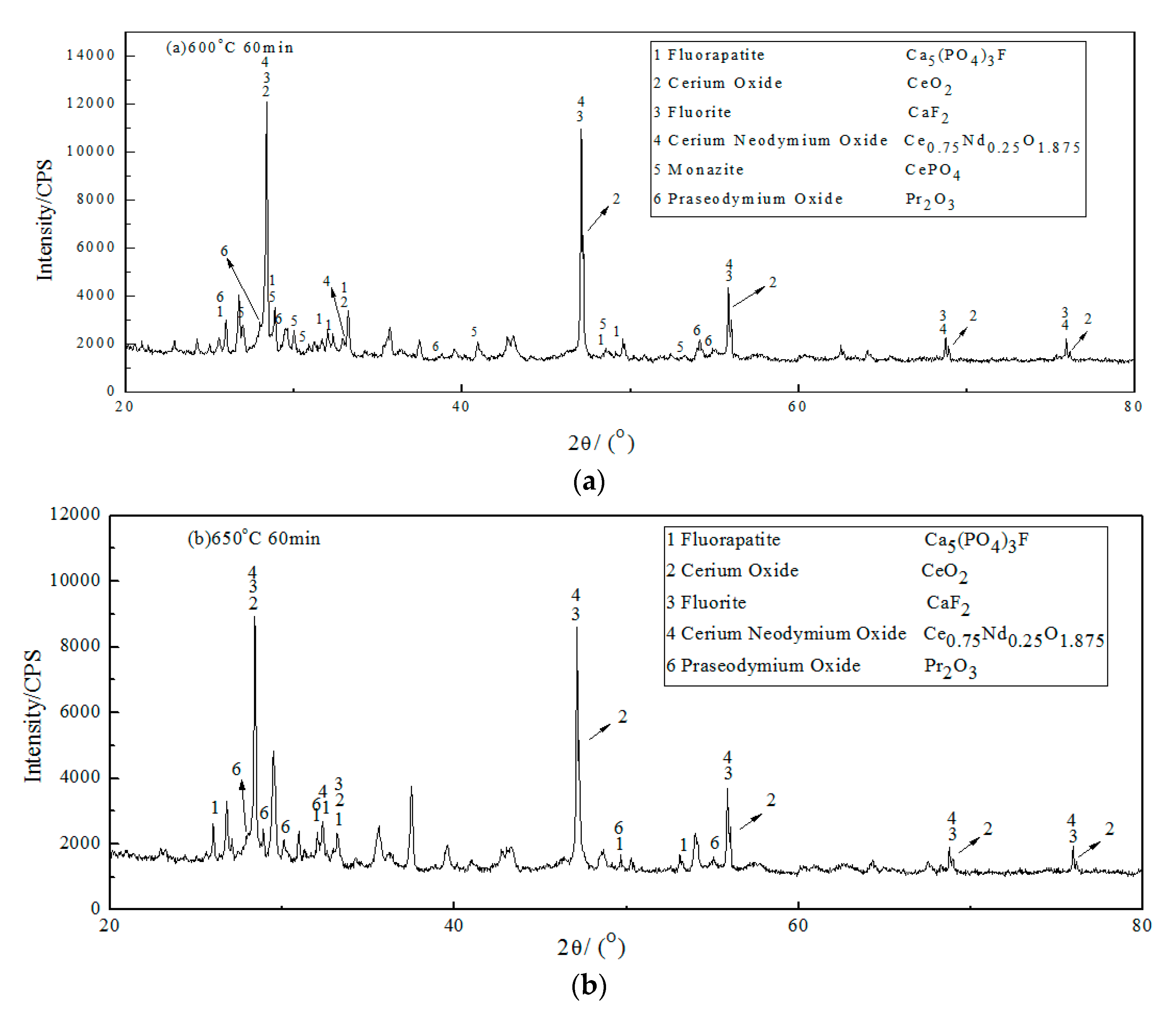
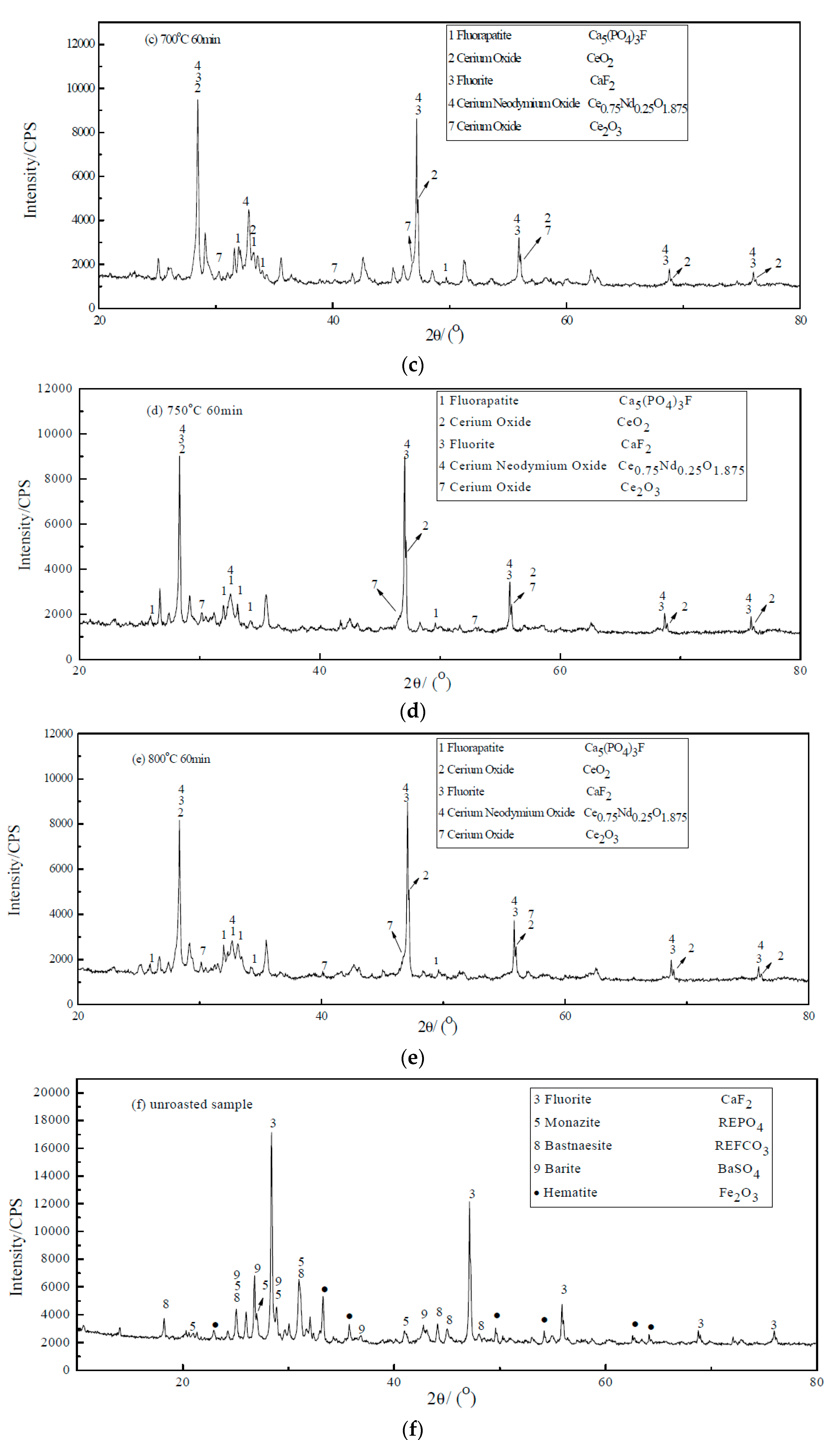
3.1. XRD of Roasted Products
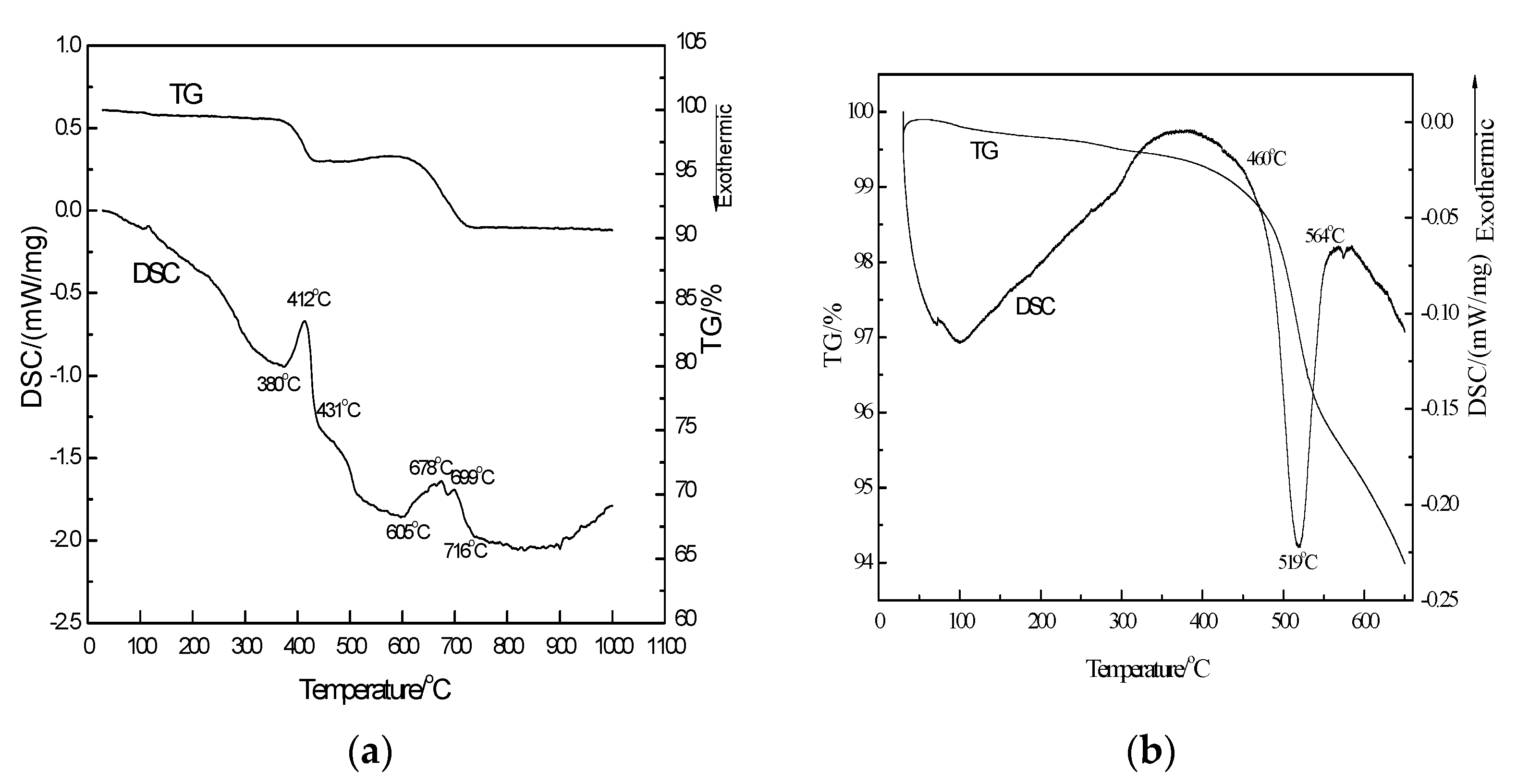
3.2. Analysis of TG-DSC
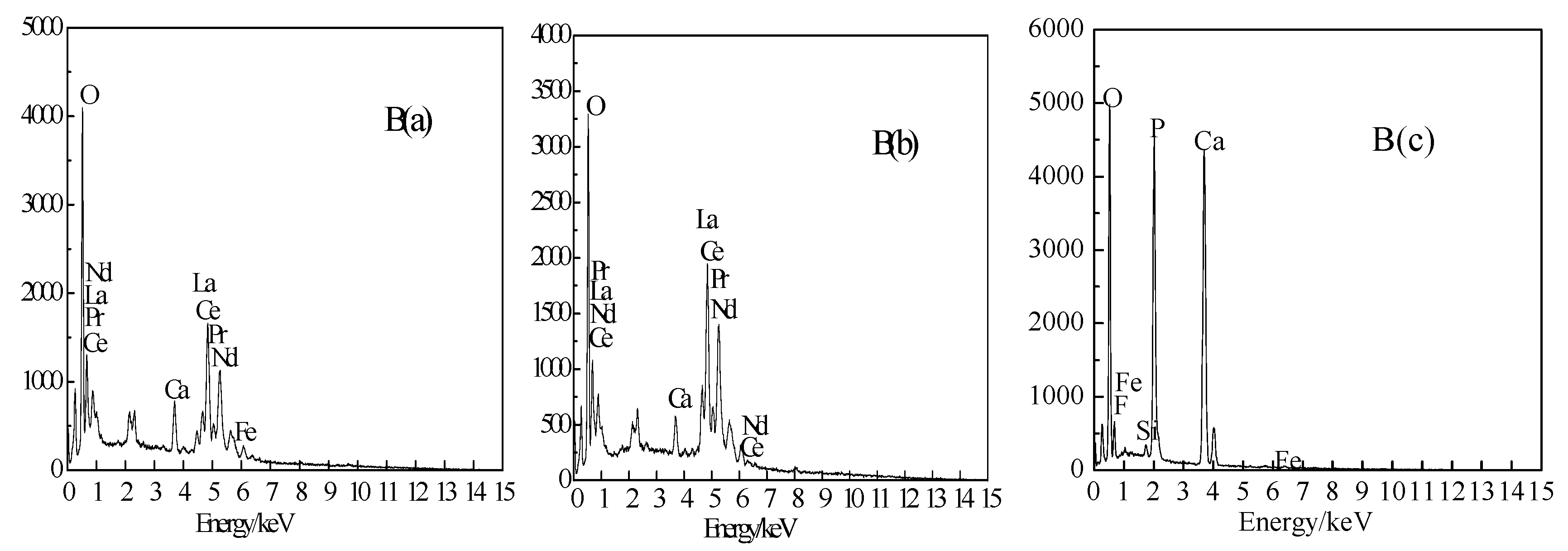
3.3. Analysis Using SEM-EDS
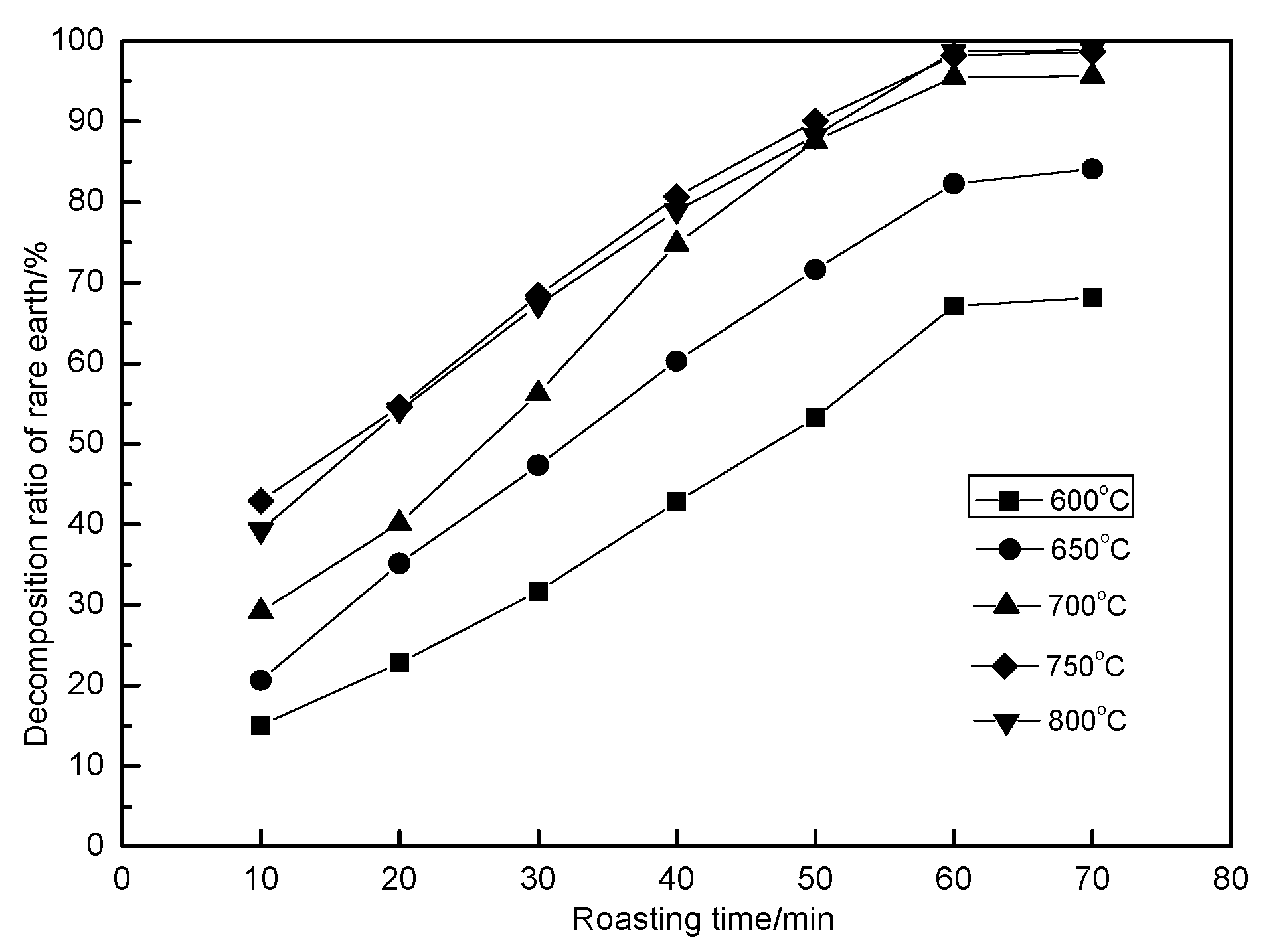
3.4. Effect of Roasting Time
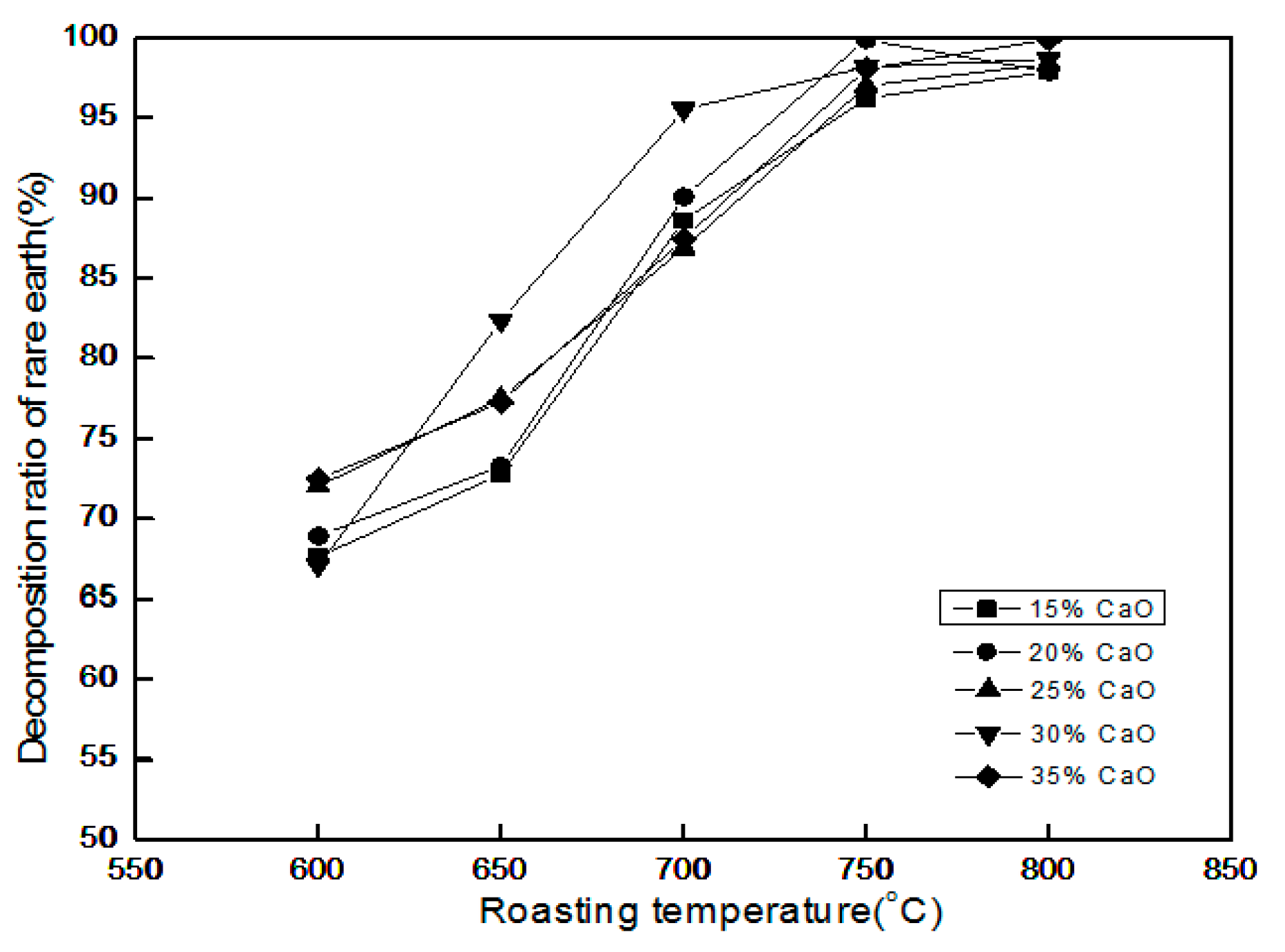
3.5. Effect of Roasting Temperature
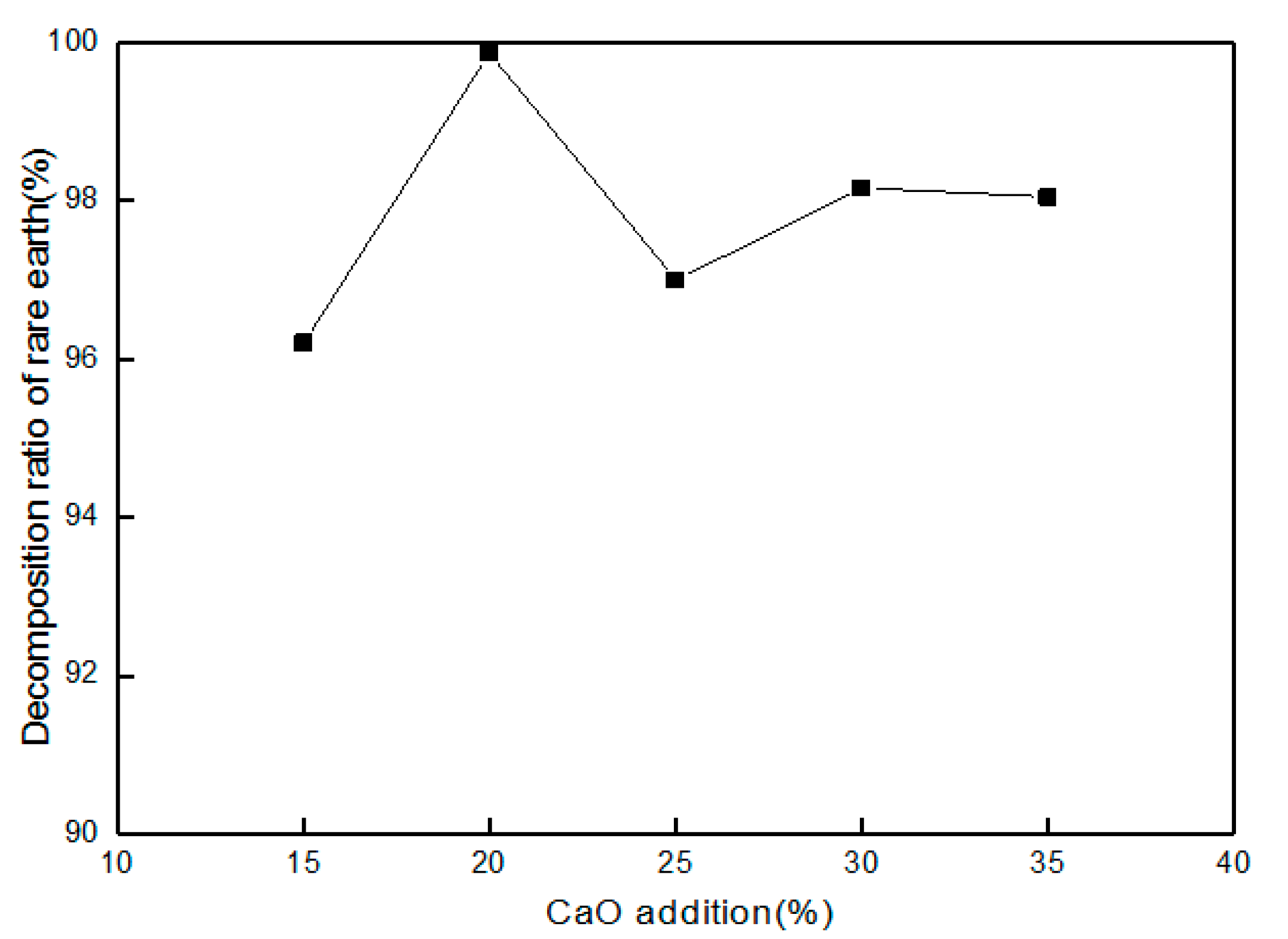
3.6. Effect of CaO Addition
3.7. Analysis of the Effect of Fluorine-Fixing by CaO
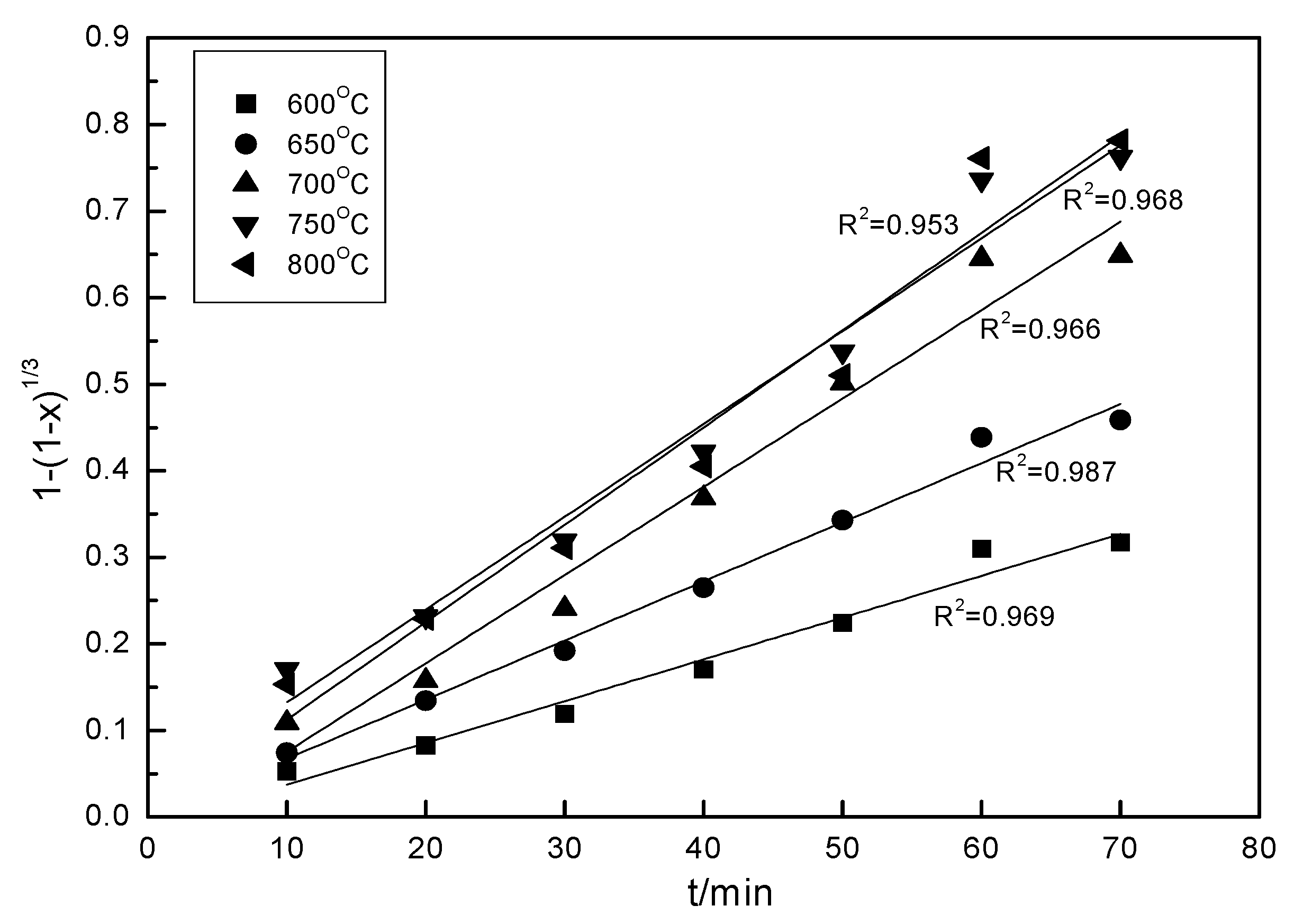
3.8. Kinetics Mode of Mixed Rare Earth Tailing’s Decomposition Process
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.L.; Xie, M.Y.; Wang, H.J.; Xu, S.M. Solvent extraction and separaten of heavy rare earths from chloride media using nonsymmetric (2,3-dimethylbutyl)(2,4,4′-trimethylpentyl) phosphinic acid. Hydrometallurgy 2017, 167, 39–47. [Google Scholar] [CrossRef]
- Chen, J.J.; Yang, Q.S. General Survey of Rare Earth Function Materials. Hunan Nonferr. Met. 2007, 23, 30–33. [Google Scholar]
- Eliseeva, S.V.; Bünzli, J.C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176. [Google Scholar] [CrossRef]
- Jun, F.; Kazuya, T.; Naoko, W.; Yoshio, T. Simultaneous recovery and separaten of rare earth elements in ferromanganese nodules by using Shewanella putrefaciens. Hydrometallurgy 2016, 166, 80–86. [Google Scholar]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Xu, G.X. Rare Earths, 2nd ed.; Metallurgical Industry Press: Beijing, China, 1995. [Google Scholar]
- Radhika, S.; Nagaphari Kumar, B.; Lakshmi Kantam, M.; Damachardra Reddy, B. Solvent extraction and separaten of rare-earths from phosphoric acid solutions with TOPS99. Hydrometallurgy 2011, 110, 50–55. [Google Scholar] [CrossRef]
- Banda, R.; Jeon, H.; Lee, M. Solvent extraction separaten of Pr and Nd from chloride solution containing La using Cyanex 272 and its mixture with other extractants. Sep. Purif. Technol. 2012, 98, 481–487. [Google Scholar] [CrossRef]
- Huang, X.W.; Li, H.W.; Wang, C.F.; Wang, G.Z.; Xue, X.X.; Zhang, G.C. Development status and research progress in rare earth industry in China. Rare Met. 2007, 31, 279–288. [Google Scholar]
- Cai, Z.L.; Cao, M.L.; Chen, L.P.; Yu, Y.F.; Hu, H.Y. Study on the beneficiation process for recovering rare-earth from the LIMS tailing of HIMS rougher concentrate after magnetizing roasting in Baogang concentrator. Metalmine 2009, 7, 155–157. [Google Scholar]
- Wang, Y.; Yu, X.L.; Shu, Y.; Wang, Z.C. Extraction of rare earths from mixed bastnaesite-monazite of concentrate by carbochlorination reaction. Nonferr. Met. 2009, 61, 68–71. [Google Scholar]
- Wu, C. Bayan Obo controversy: Carbonatites versus Iron Oxide-Ca-Au-(REE-U). Resour. Geol. 2008, 58, 348–354. [Google Scholar] [CrossRef]
- Zhang, P.S.; Tao, K.J.; Yang, Z.M.; Yang, X.M. Mineralogy and Geology of Rare Earths in China; Science Press: Beijing, China, 1995; p. 209. [Google Scholar]
- Yang, H.; Wu, J.; Xue, X.X.; Zhang, B.; Li, Y. Roasting and leaching process of mixture of rare earth silicates slag and ammonium sulfate. J. Chin. Soc. Rare Earths 2015, 33, 440–448. [Google Scholar]
- Li, Y.; Lin, N.; He, J.G.; Xue, X.X.; Huang, X.W. Leaching of rare earth and preparaten of cryolite from Baotou magnetic tailings. J. Chin. Soc. Rare Earths 2012, 30, 251–256. [Google Scholar]
- Li, Y.; Wang, M.; Huang, X.W. Alumium roasting-acid leaching of rare earth from Baotou magnetic tailings. J. Chin. Soc. Rare Earths 2015, 33, 81–86. [Google Scholar]
- Yu, X.L.; Wang, Z.C.; Han, Y.X.; Gan, H.M.; Liu, J. Study on process of decomposition of Baosteel concentrator tailings by carbothermic chlorination. Met. Miner. 2007, 9, 113–115. [Google Scholar]
- Yu, X.L.; Wang, Z.C.; Han, Y.X.; Zeng, F.W.; Zhang, Q. Extraction of rare earth from Baogang tailings by carbochlorination reaction tailing AlCl3 as befluorination agent. Chin. Rare Earths 2006, 27, 30–34. [Google Scholar]
- Xu, Y.H.; Liu, H.J.; Cui, J.G.; Meng, Z.J.; Zhao, W.Y.; Li, L.C. Techniques for clean smelting and resource comprehensive recycle of Baotou rare earth concentrates. J. Chin. Soc. Rare Earths 2012, 30, 632. [Google Scholar]
- Lang, X.C.; Yu, X.L. The latest development of Chinese mixed rare earth concentrate processing. Rare Met. Cem. Carbides 2009, 37, 43–47. [Google Scholar]
- Wang, M.H.; Zeng, M.; Wang, L.S.; Zhou, J.H.; Cui, D.L.; Wang, Q.G.; Wen, R.G.; Chen, X.S. Catnlytic leaching process of bastnaesite with hydrochloric. J. Chin. Soc. Rare Earths 2013, 31, 148. [Google Scholar]
- Wang, X.T.; Liu, J.J. Oxidation baking decomposition process for Baotou mixed RE concentrate. Rare Earth 1996, 17, 6–9. [Google Scholar]
- Ji, J.M. Recovery experiment iron and rare earth minerals from tailing of inverse flotation in dressing plant of Baosteel. Met. Mine 2013, 3, 158. [Google Scholar]
- Zhang, Y.; Ma, P.Q.; Che, L.P.; Qian, J.B.; Ma, Y.P.; Ren, J.H. Study on rare earth flotation from Bao Steel’s tailings. Chin. Rare Earths 2010, 31, 93. [Google Scholar]
- Shi, W.Z.; Wang, J.Y.; Zhu, G.C. Kinetics on chlorination rare earth of Baotou mixed concentrate after fixed fluorine treatment. Chin. J. Nonferr. Met. 2004, 14, 1254–1258. [Google Scholar]
- Shi, W.Z.; Zhu, G.C.; Hua, J.; Xu, S.M.; Chi, R.A. Recovery of RE from rare earth concentrate with ammonium chloride roasting. J. Henan Univ. (Nat. Sci.) 2002, 32, 45–48. [Google Scholar]
- Bian, X.; Chen, J.L.; Zhao, Z.H.; Yin, S.H.; Luo, Y.; Zhang, F.Y.; Wu, W.Y. Kinetics of mixed rare earths minerals decomposed by CaO with NaCl-CaCl2 melting salt. J. Rare Earth 2010, 28, 86–89. [Google Scholar] [CrossRef]
- Sun, X.C.; Wu, Z.Y.; Bian, X.; Gao, B.; Wu, W.Y.; Tu, G.F. Influence of NaCl-CaCl2 on decomposing monazite with CaO. J. Rare Earths 2007, 28, 779–782. [Google Scholar]
- Luo, S.Y.; Zhang, J.Y.; Zhou, T.P. Models for kinetics analysis of solid-solid reactions and their Applications. Mater. Rev. 2000, 14, 6–7. [Google Scholar]


| Fetotal | CaO | SiO2 | F | REO | P2O5 | BaO | MgO | SO3 |
|---|---|---|---|---|---|---|---|---|
| 25.40 | 21.56 | 11.26 | 8.66 | 6.84 | 3.10 | 2.64 | 2.42 | 2.42 |
| MnO | Al2O3 | Na2O | TiO2 | K2O | Nb2O5 | ZnO | Cl | lgnition loss |
| 1.60 | 1.18 | 0.87 | 0.56 | 0.46 | 0.23 | 0.06 | 0.05 | 10.69 |
| Ad | Vdaf | Fcad | St,d | Bomb Calorimetric Value/(MJ·kg−1) |
|---|---|---|---|---|
| 8.78 | 9.20 | 82.49 | 0.30 | 28.35 |
| >149 μm | >74 μm, <149 μm | >58 μm, <74 μm | >38 μm, <58 μm | <38 μm |
|---|---|---|---|---|
| 9.29 | 25.05 | 30.71 | 32.10 | 2.85 |
| Analytical Facility | Facility Model and Origin | Purpose |
|---|---|---|
| X ray Fluorescence | ZSX 100e, China | Chemical composition of magnetic tailing |
| industrial analyzer | TRGF-8000,China | Chemical composition of coal |
| laser particle size analyzer | BT9300H, Dandong Bettersize Instrument Co. Ltd. China | particle size distribution |
| briquetting machine | shanghai jingsheng, 769-40C, China | briquetting |
| box resistance furnace | shenyang changcheng, SX_12_16, China | roasting |
| X-ray powder diffraction | X’Pert Pro, Panalytical, The Netherlands | phase transformation and decomposition process |
| thermal gravimetric and differential scanning calorimetry | Netzsch STA 449F3, Germany | phase transformation and decomposition process |
| scanning electron microscope and energy dispersive spectrometry | Ultra Plus, Zeiss, Germany | phase transformation and decomposition process |
| Inductively Coupled Plasma-Atomic Emission Specrometry | Plasma 1000, China | determining content |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Yang, H.; Xue, X.-X.; Zhou, Y. Kinetics of Roasting Decomposition of the Rare Earth Elements by CaO and Coal. Metals 2017, 7, 213. https://doi.org/10.3390/met7060213
Yuan S, Yang H, Xue X-X, Zhou Y. Kinetics of Roasting Decomposition of the Rare Earth Elements by CaO and Coal. Metals. 2017; 7(6):213. https://doi.org/10.3390/met7060213
Chicago/Turabian StyleYuan, Shuai, He Yang, Xiang-Xin Xue, and Yan Zhou. 2017. "Kinetics of Roasting Decomposition of the Rare Earth Elements by CaO and Coal" Metals 7, no. 6: 213. https://doi.org/10.3390/met7060213
APA StyleYuan, S., Yang, H., Xue, X.-X., & Zhou, Y. (2017). Kinetics of Roasting Decomposition of the Rare Earth Elements by CaO and Coal. Metals, 7(6), 213. https://doi.org/10.3390/met7060213






