Influence of Milling Atmosphere on the Controlled Formation of Ultrafine Dispersoids in Al-Based MMCs
Abstract
:1. Introduction
2. Experimental Procedure
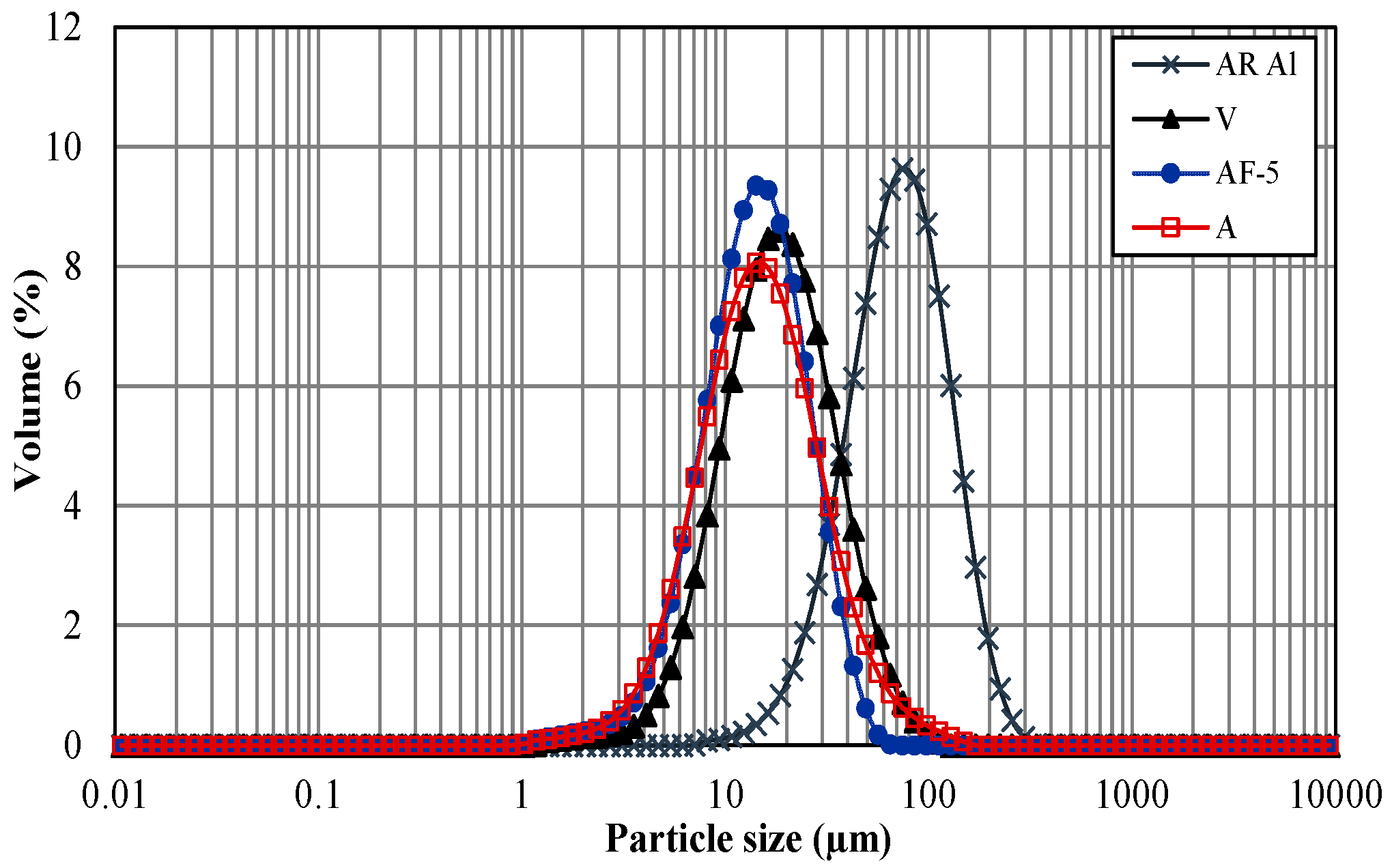
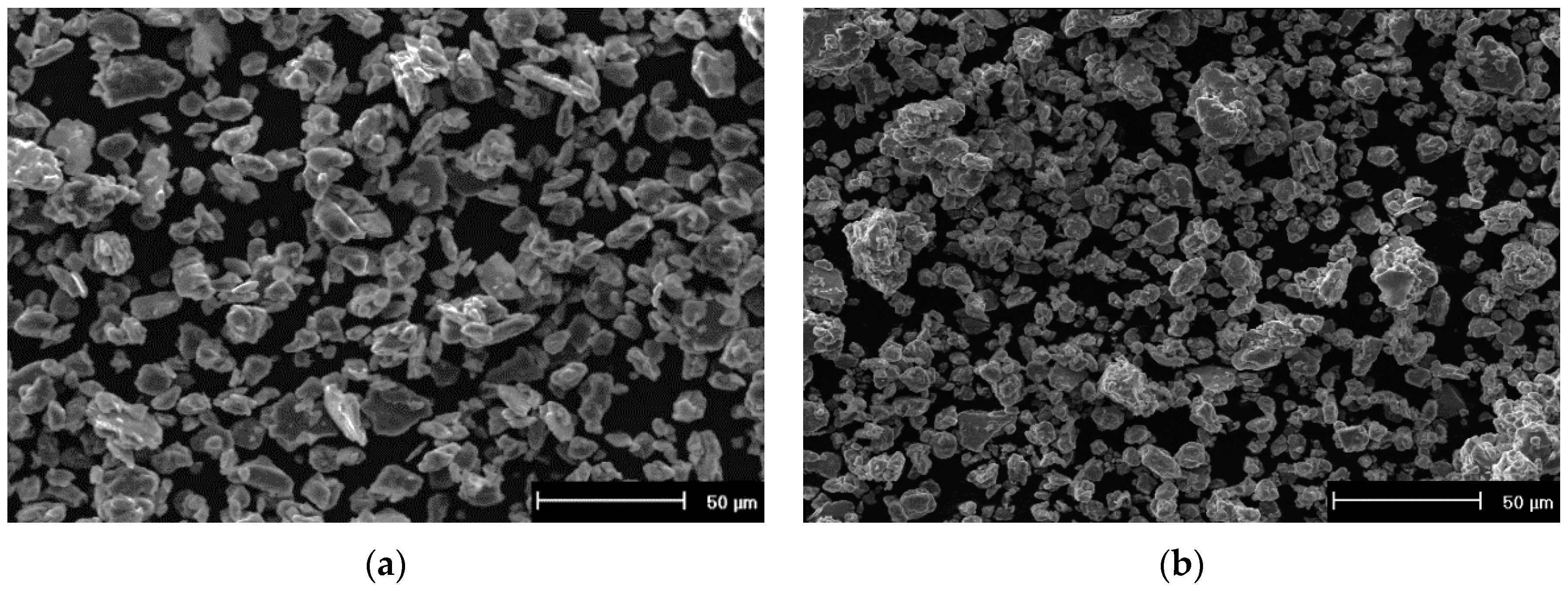
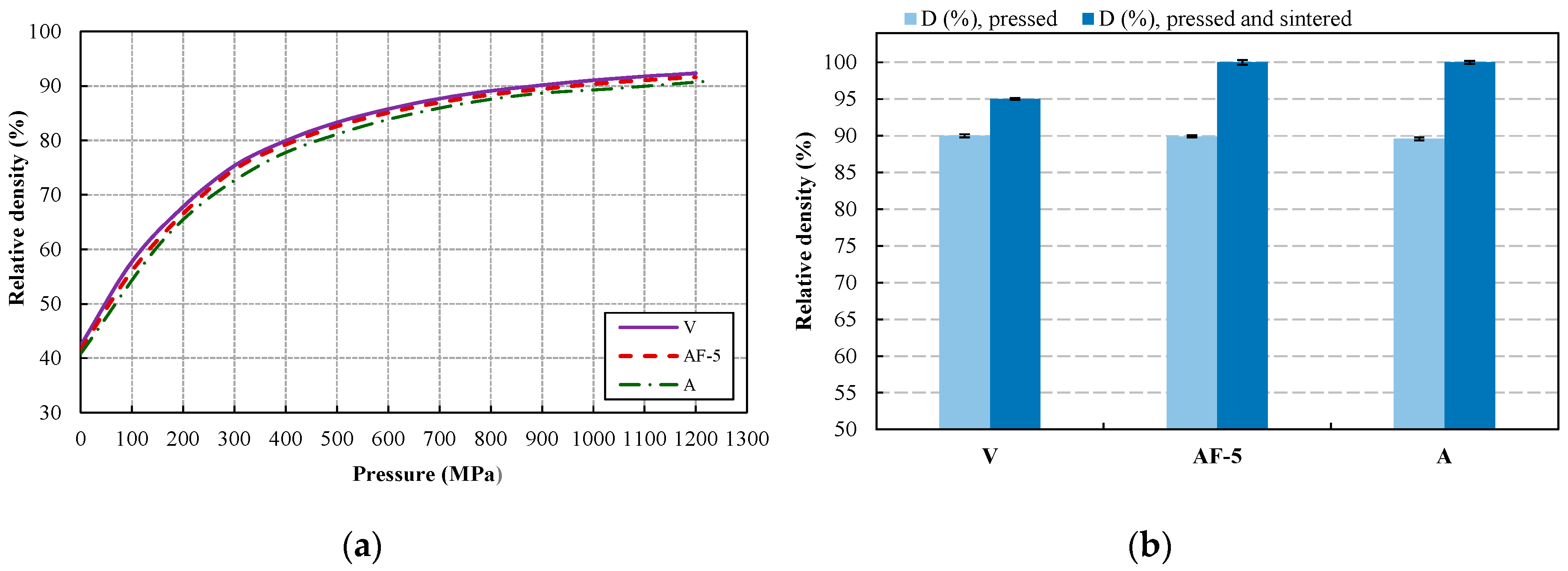
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Dragatogiannis, D.A.; Koumoulos, E.P.; Kartsonakis, I.A. Residual stress and deformation mechanism of friction stir welded aluminum alloys by nanoindentation. Mater. Sci. Eng. A 2012, 540, 226–234. [Google Scholar] [CrossRef]
- Srivastava, N.; Chaudhari, G.P. Strengthening in Al alloy nano composites fabricated by ultrasound assisted solidification technique. Mater. Sci. Eng. A 2016, 651, 241–247. [Google Scholar] [CrossRef]
- Surappa, M.K. Aluminium matrix composites: Challenges and opportunities. Sadhana 2003, 28, 319–334. [Google Scholar] [CrossRef]
- Xiang, N.; Song, R.; Zhao, J.; Li, H.; Wang, C.; Wang, Z. Microstructure and mechanical properties of ceramic coatings formed on 6063 aluminium alloy by micro-arc oxidation. Trans. Nonferr. Met. Soc. China 2015, 25, 3323–3328. [Google Scholar] [CrossRef]
- Lipińska, M.; Bazarnik, P.; Lewandowska, M. The influence of severe plastic deformation processes on electrical conductivity of commercially pure aluminium and 5483 aluminium alloy. Arch. Civ. Mech. Eng. 2016, 16, 717–723. [Google Scholar] [CrossRef]
- Ding, L.; Jia, Z.; Zhang, Z.; Sanders, R.E.; Liu, Q.; Yang, G. The natural aging and precipitation hardening behaviour of Al-Mg-Si-Cu alloys with different Mg/Si ratios and Cu additions. Mater. Sci. Eng. A 2015, 627, 119–126. [Google Scholar] [CrossRef]
- Wierzbińska, M.; Sieniawski, J. The degradation of microstructure of AlCu4Ni2Mg aluminium alloy after prolonged annealing at elevated temperature. Solid State Phenom. 2012, 186, 315–318. [Google Scholar] [CrossRef]
- Abdoli, H.; Salahi, E.; Farnoush, H.; Pourazrang, K. Evolutions during synthesis of Al–AlN-nanostructured composite powder by mechanical alloying. J. Alloy. Compd. 2008, 461, 166–172. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Qiang, W.; Wang, K. Microstructure and mechanical properties of aluminium alloy 7A52 thick plates welded by robotic double-sided coaxial GTAW process. Mater. Sci. Eng. 2016, 673, 8–15. [Google Scholar] [CrossRef]
- Mazahery, A.; Shabani, M.O. A comparative study on abrasive wear behavior of semisolid–liquid processed Al–Si matrix reinforced with coated B4C reinforcement. Trans. Indian Inst. Met. 2012, 65, 145–154. [Google Scholar] [CrossRef]
- Cöcen, U.; Önel, K. Ductility and strength of extruded SiCp/aluminium-alloy composites. Compos. Sci. Technol. 2002, 62, 275–282. [Google Scholar] [CrossRef]
- Cintas, J.; Cuevas, F.G.; Montes, J.M.; Herrera, E.J. Microstructural control of sintered mechanically alloyed Al-1%Ni material. Scr. Mater. 2005, 52, 341–345. [Google Scholar] [CrossRef]
- Cintas, J.; Montes, J.M.; Cuevas, F.G.; Gallardo, J.M. Influence of PCA content on mechanical properties of sintered MA aluminium. Mater. Sci. Forum 2006, 514–516, 1279–1283. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Yang, C.; Zhu, D.; Li, Y. (SiCp+Ti)/7075Al hybrid composites with high strength and large plasticity fabricated by squeeze casting. Mater. Sci. Eng. A 2014, 609, 250–254. [Google Scholar] [CrossRef]
- Kumar, C.; Rajadurai, J. Influence of rutile (TiO2) content on wear and microhardness characteristics of aluminium-based hybrid composites synthesized by powder metallurgy. Trans. Nonferr. Met. Soc. China 2016, 26, 63–73. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Khanduja, D. Production and some properties of Si3N4 reinforced aluminium alloy composites. J. Asian Ceram. Soc. 2015, 3, 352–359. [Google Scholar] [CrossRef]
- Ostad Shabani, M.; Mazahery, A. Fabrication of AMCs by spray forming: Setting of cognition and social parameters to accelerate the convergence in optimization of spray forming process. Ceram. Int. 2013, 39, 5271–5279. [Google Scholar] [CrossRef]
- Karabulut, S.; Gökmen, U.; Cinici, H. Study on the mechanical and drilling properties of AA7039 composites reinforced with Al2O3/B4C/SiC particles. Compos. Part B Eng. 2016, 93, 43–55. [Google Scholar] [CrossRef]
- Chen, C.-L.; Lin, C.-H. Effect of Y2O3 and TiC reinforcement particles on intermetallic formation and hardness of Al6061 composites via mechanical alloying and sintering. Metall. Mater. Trans. A 2015, 46, 3687–3695. [Google Scholar] [CrossRef]
- Mathan Kumar, N.; Senthil Kumaran, S.; Kumaraswamidhas, L.A. High temperature investigation on EDM process of Al 2618 alloy reinforced with Si3N4, AlN and ZrB2 in-situ composites. Alex. Eng. J. 2016, 663, 755–768. [Google Scholar] [CrossRef]
- Rengasamy, N.V.; Rajkumar, M.; Kumaran, S.S. Mining environment applications on Al 4032—Zrb2 and Tib2 in-situ composites. J. Alloy. Compd. 2016, 658, 757–773. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Robert, M.H.; Torralba, J.M. Mechanically alloyed AlN particle-reinforced Al-6061 matrix composites: Powder processing, consolidation and mechanical strength and hardness of the as-extruded materials. Mater. Sci. Eng. A 2006, 426, 85–94. [Google Scholar] [CrossRef]
- Gasem, Z.M.; Ali, S.S. Low-cycle fatigue behavior of powder metallurgy 6061 aluminum alloy reinforced with submicron-scale Al2O3 particles. Mater. Sci. Eng. A 2013, 562, 109–117. [Google Scholar] [CrossRef]
- Chen, Z.; Tokaji, K. Effects of particle size on fatigue crack initiation and small crack growth in SiC particulate-reinforced aluminium alloy composites. Mater. Lett. 2004, 58, 2314–2321. [Google Scholar] [CrossRef]
- Karbalaei Akbari, M.; Baharvandi, H.R.; Shirvanimoghaddam, K. Tensile and fracture behavior of nano/micro TiB2 particle reinforced casting A356 aluminum alloy composites. Mater. Des. 2015, 66, 150–161. [Google Scholar] [CrossRef]
- Yang, H.; Topping, T.D.; Wehage, K.; Jiang, L.; Lavernia, E.J.; Schoenung, J.M. Tensile behavior and strengthening mechanisms in a submicron B4C-reinforced Al trimodal composite. Mater. Sci. Eng. A 2014, 616, 35–43. [Google Scholar] [CrossRef]
- Tweed, J.H. Manufacture of 2014 aluminium reinforced with SiC particulate by vacuum hot pressing. Mater. Sci. Eng. A 1991, 135, 73–76. [Google Scholar] [CrossRef]
- Cintas, J.; Cuevas, F.G.; Montes, J.M.; Caballero, E.S.; Herrera, E.J. Strengthening of ultrafine PM aluminium using nano-sized oxycarbonitride dispersoids. Mater. Sci. Eng. A 2011, 528, 8286–8291. [Google Scholar] [CrossRef]
- Cintas, J.; Montes, J.M.; Cuevas, F.G.; Herrera, E.J. Heat-resistant bulk nanostructured P/M aluminium. J. Alloy. Compd. 2008, 458, 282–285. [Google Scholar] [CrossRef]
- Metal Powder Industries Federation. Standard Test Method 10: Method for Determination of the Tensile Properties of Powder Metallurgy (PM) Materials; Metal Powder Industries Federation: Princeton, NJ, USA, 2016. [Google Scholar]
- Cintas, J.; Caballero, E.S.; Montes, J.M.; Cuevas, F.G.; Arevalo, C. Nanocrystalline Al composites from powder milled under ammonia gas flow. Adv. Mater. Sci. Eng. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Caballero, E.S.; Cintas, J.; Herrera-García, M.; Cuevas, F.G.; Montes, J.M. Order effect of vacuum and ammonia atmospheres on aluminium nitriding by mechanical alloying. Mater. Sci. Forum 2012, 730–732, 936–941. [Google Scholar] [CrossRef]
- Young, R.A. The Rietveld Method; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Bray, J.W. ASM Handbooks Volume 2; Specific Metals and Alloys: Properties of Wrought Aluminium Alloys; ASM International (American Society of Materials): Materials Park, OH, USA, 1990. [Google Scholar]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, E.S.; Cintas, J.; Cuevas, F.G.; Montes, J.M.; Ternero, F. Influence of Milling Atmosphere on the Controlled Formation of Ultrafine Dispersoids in Al-Based MMCs. Metals 2016, 6, 224. https://doi.org/10.3390/met6090224
Caballero ES, Cintas J, Cuevas FG, Montes JM, Ternero F. Influence of Milling Atmosphere on the Controlled Formation of Ultrafine Dispersoids in Al-Based MMCs. Metals. 2016; 6(9):224. https://doi.org/10.3390/met6090224
Chicago/Turabian StyleCaballero, Eduardo S., Jesús Cintas, Francisco G. Cuevas, Juan Manuel Montes, and Fátima Ternero. 2016. "Influence of Milling Atmosphere on the Controlled Formation of Ultrafine Dispersoids in Al-Based MMCs" Metals 6, no. 9: 224. https://doi.org/10.3390/met6090224





