Nanocrystalline β-Ta Coating Enhances the Longevity and Bioactivity of Medical Titanium Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Specimen Preparation
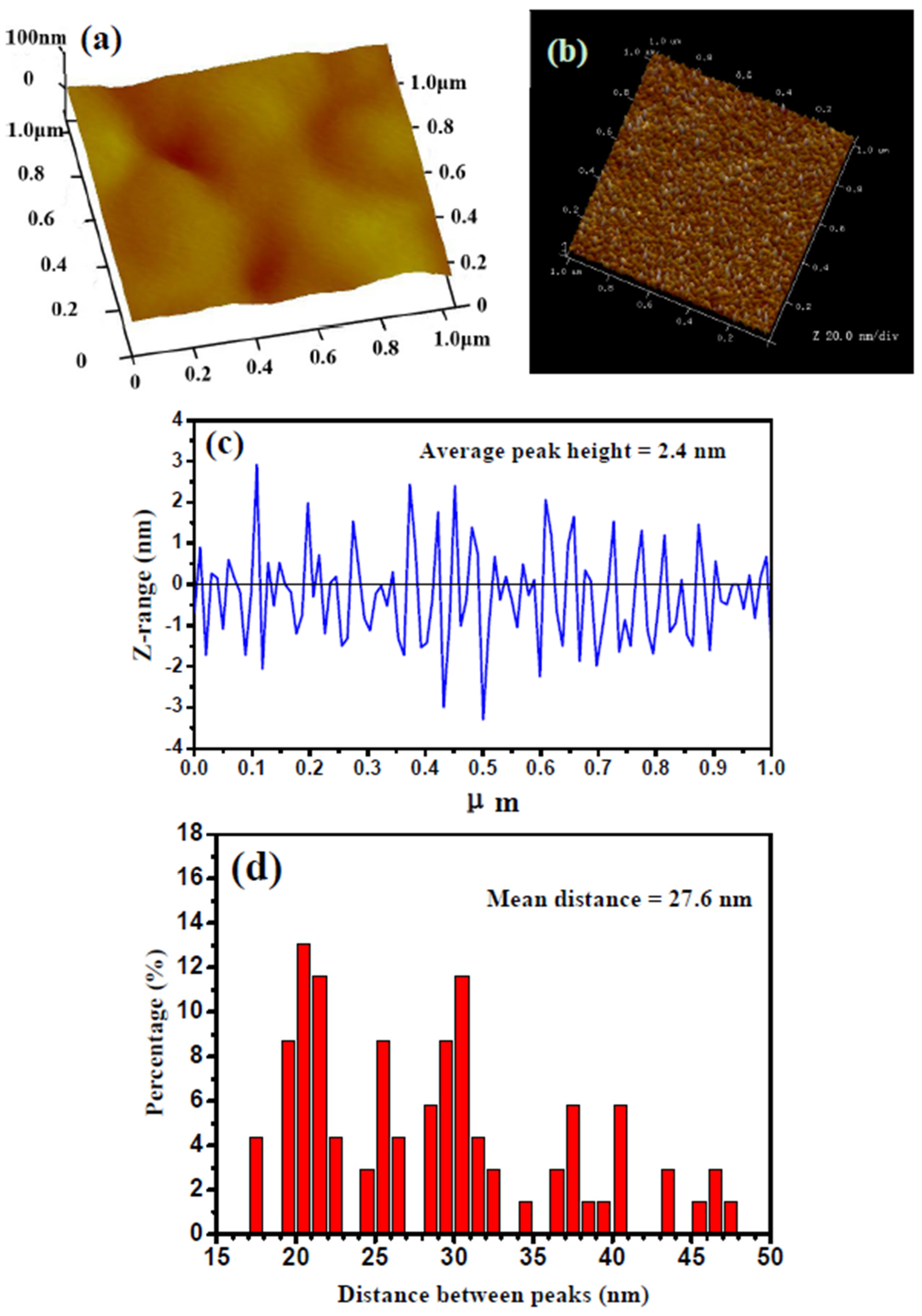
2.2. Microstructural Characterization
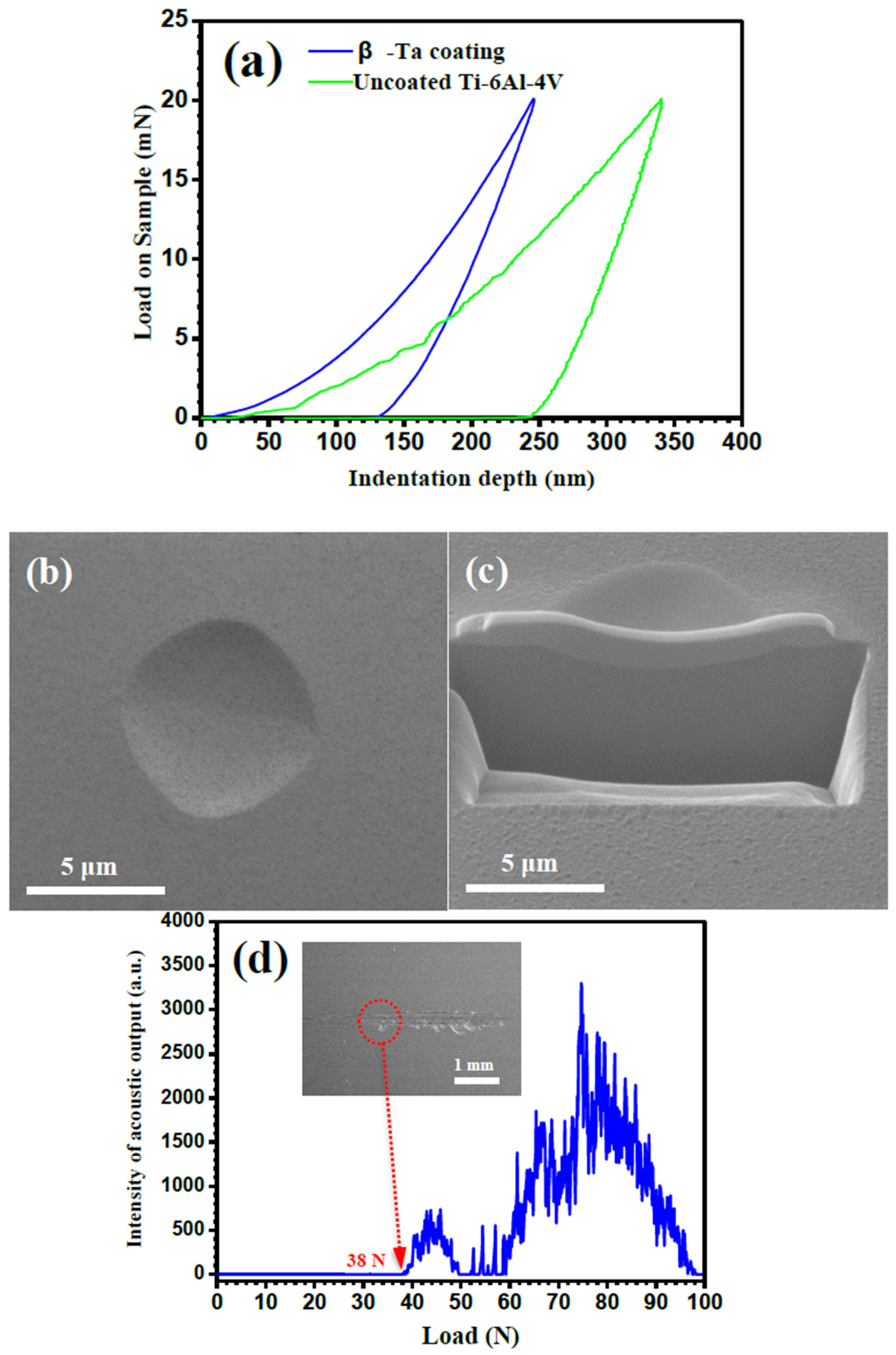
2.3. Nanoindentation and Scratch Tests
2.4. Electrochemical Measurements
2.5. In Vitro Bioactivity Evaluation
3. Results
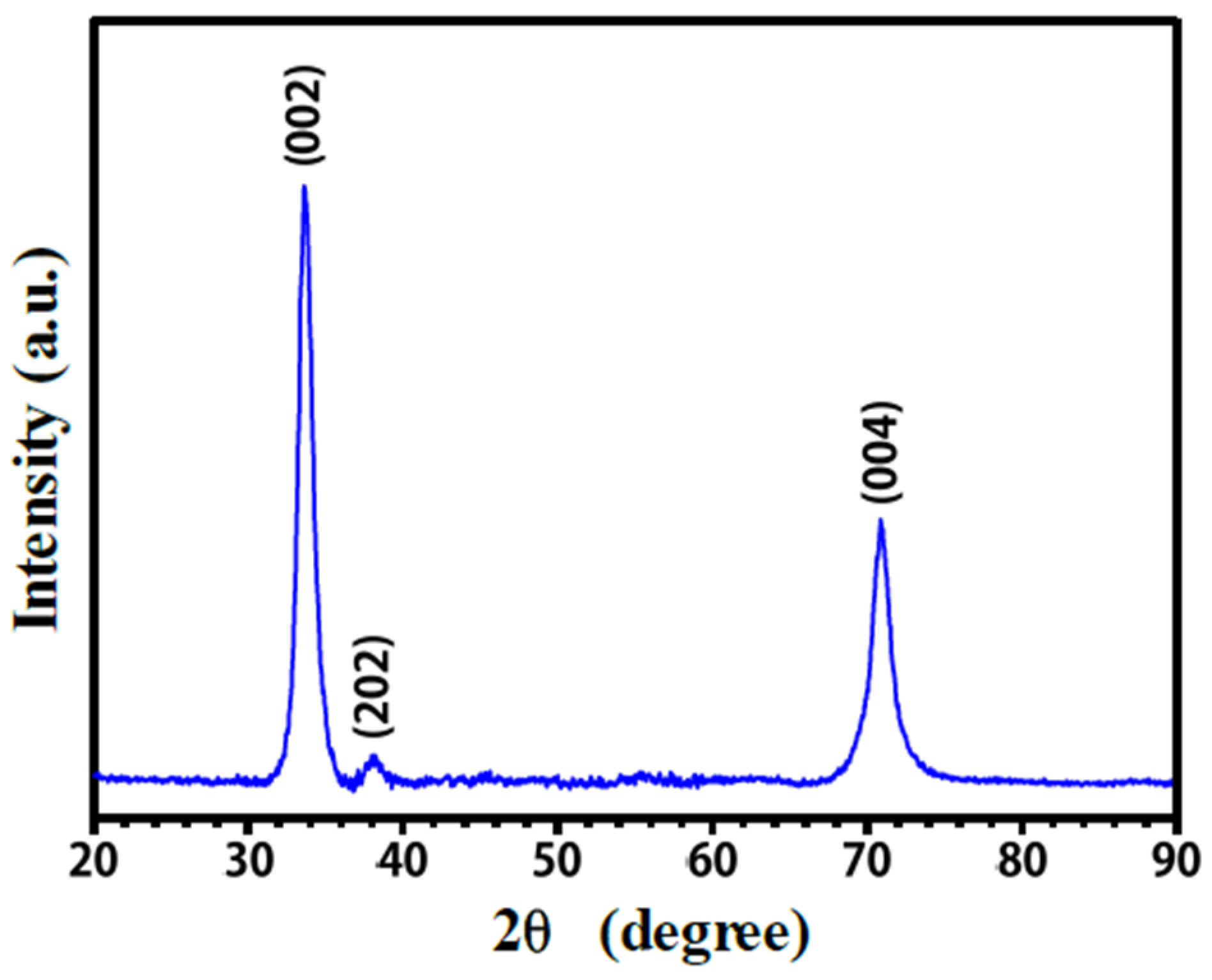
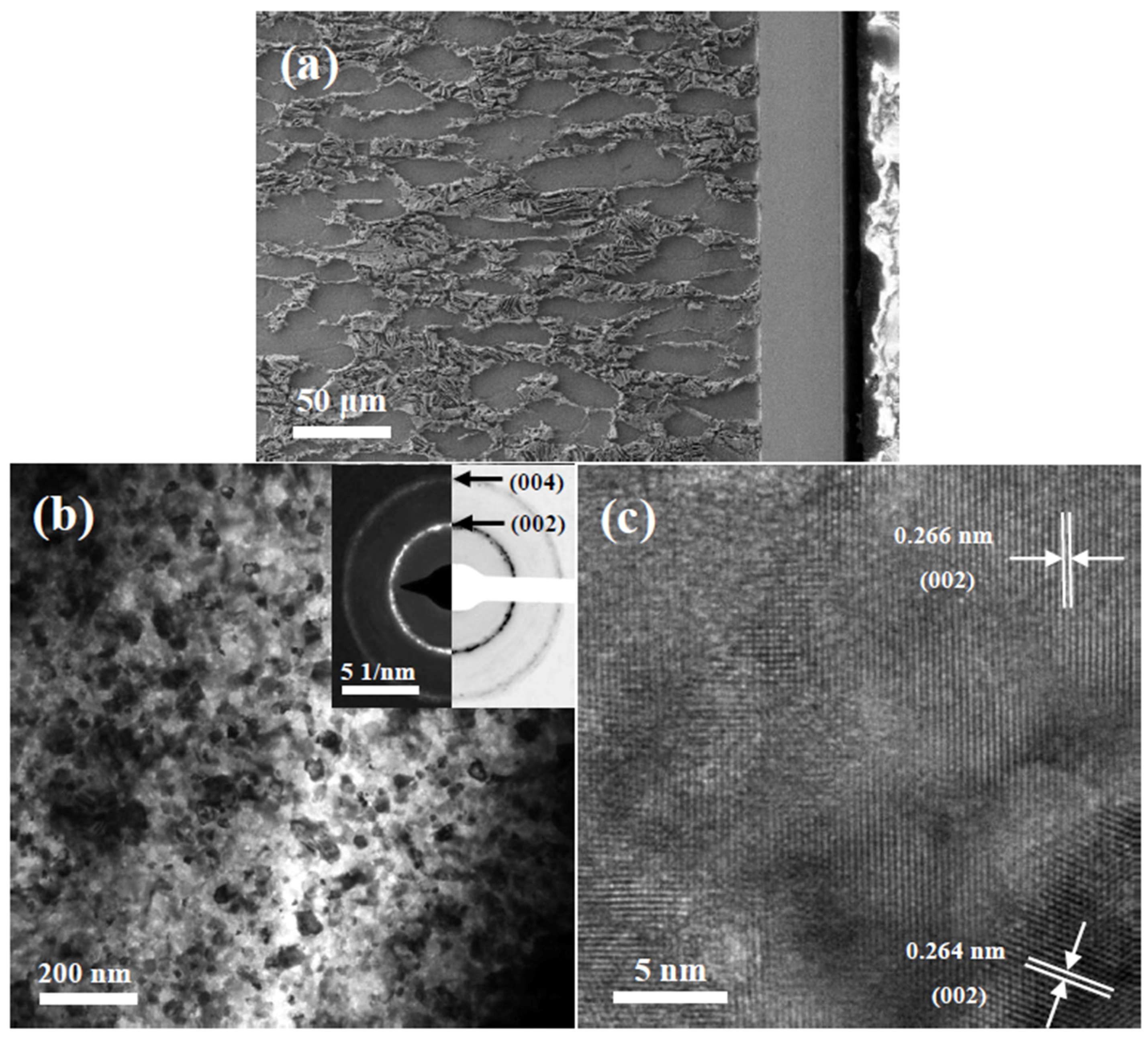
3.1. Crystalline Structure and Microstructure of Ta Coating
3.2. Nanoindentation and Scratch Tests
3.3. Electrochemical Measurements
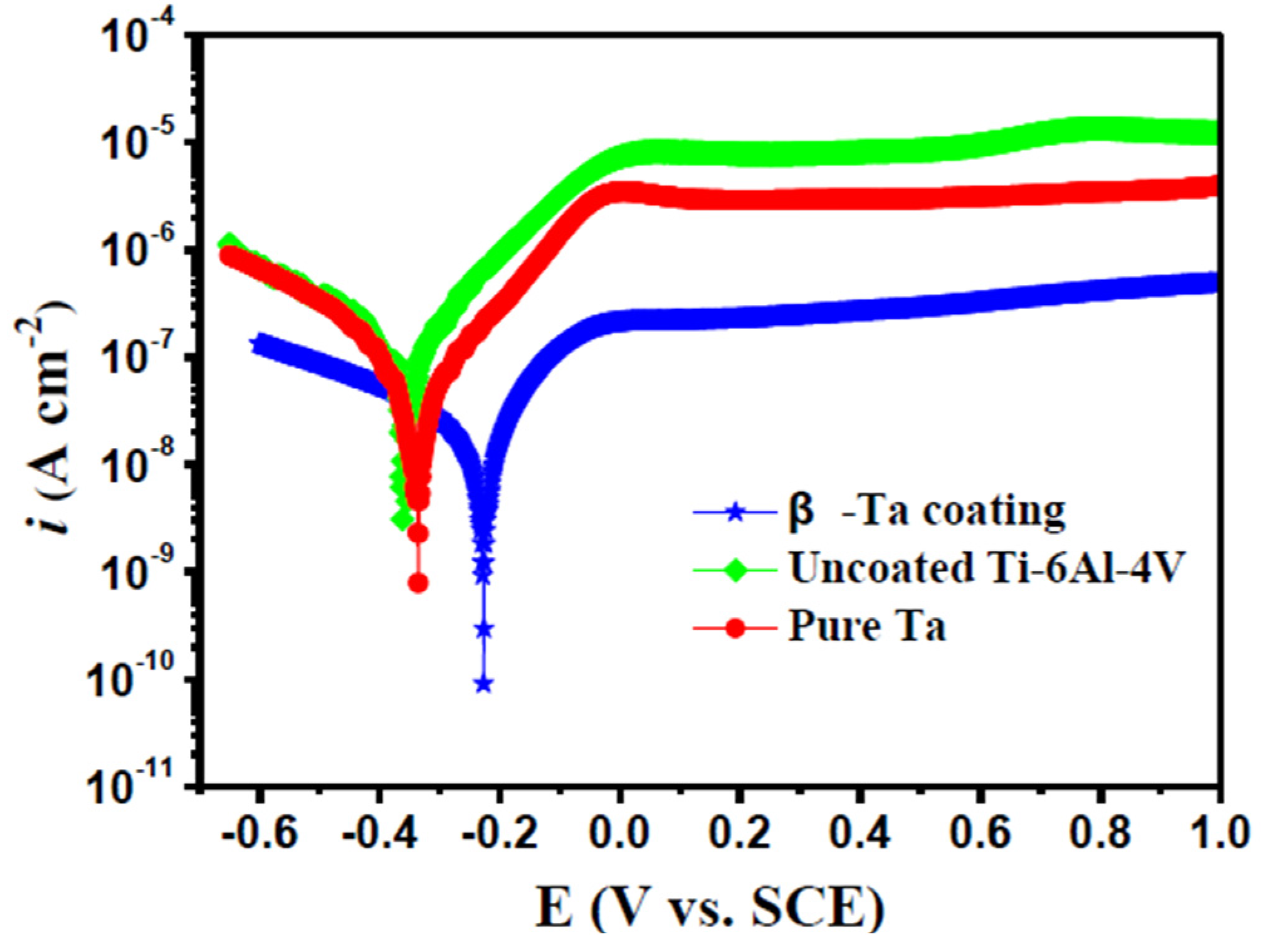
3.3.1. Potentiodynamic Polarization Tests
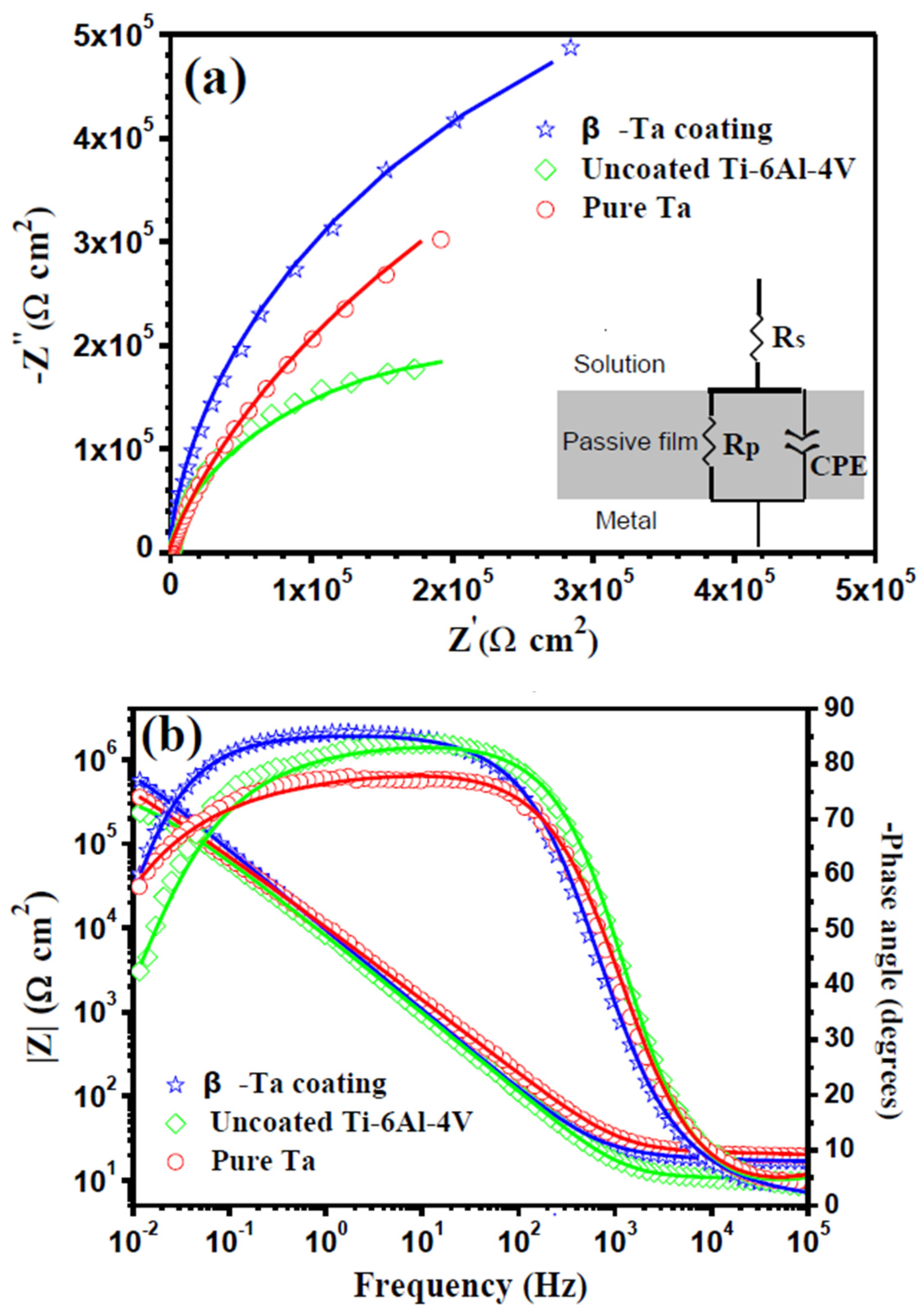
3.3.2. Electrochemical Impedance Spectroscopy (EIS)
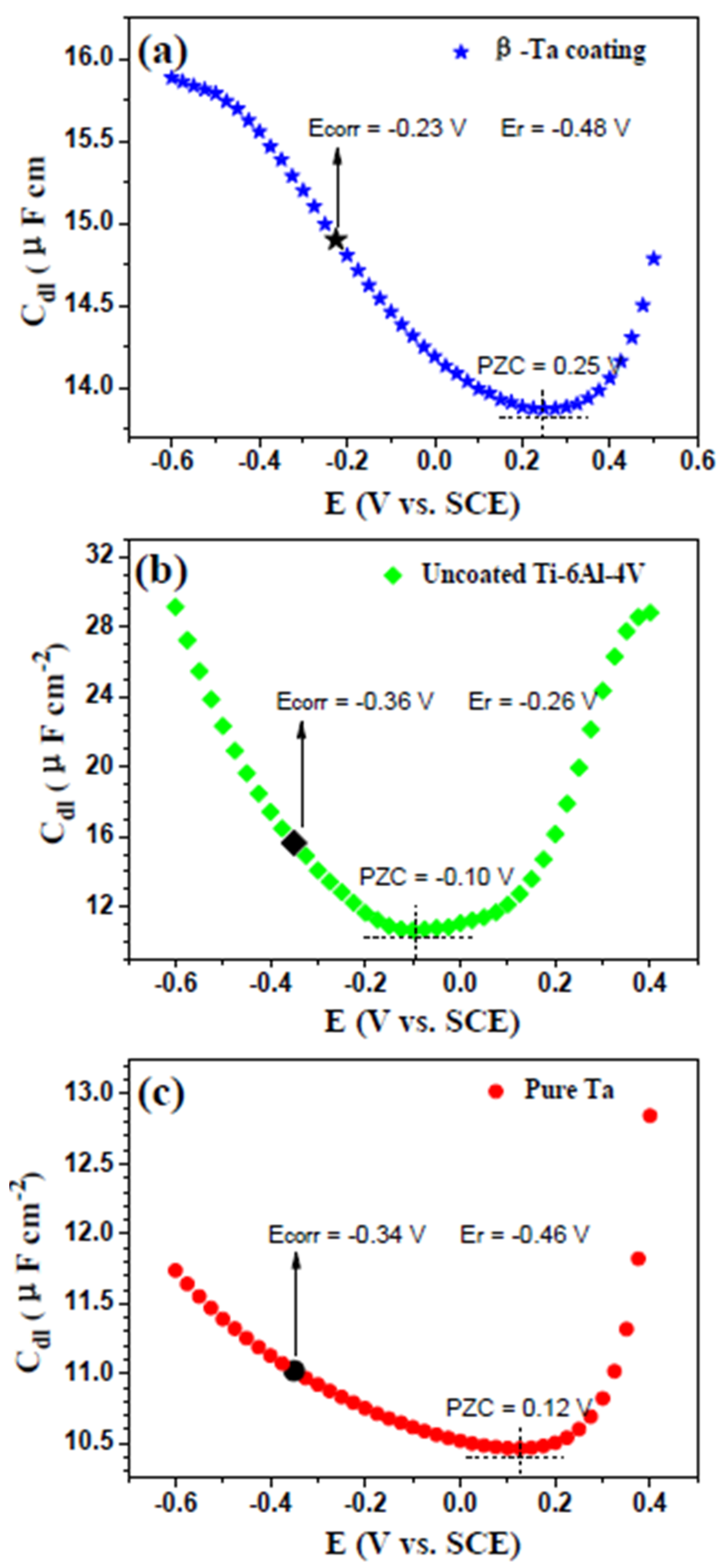
3.3.3. PZC Measurements
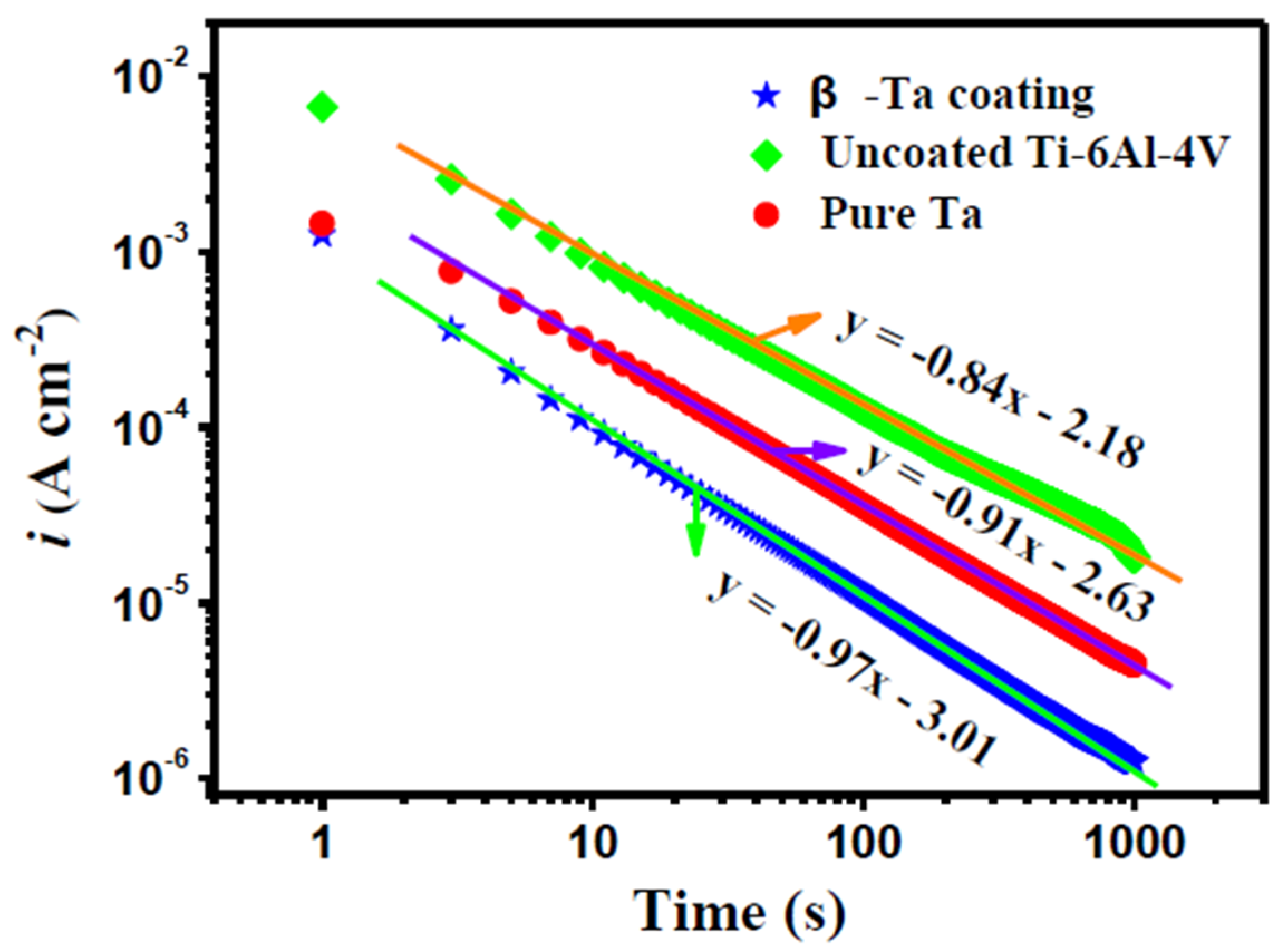
3.3.4. Potentiostatic Polarization Tests
3.3.5. Mott-Schottky Analysis
3.4. Apatite-Forming Ability
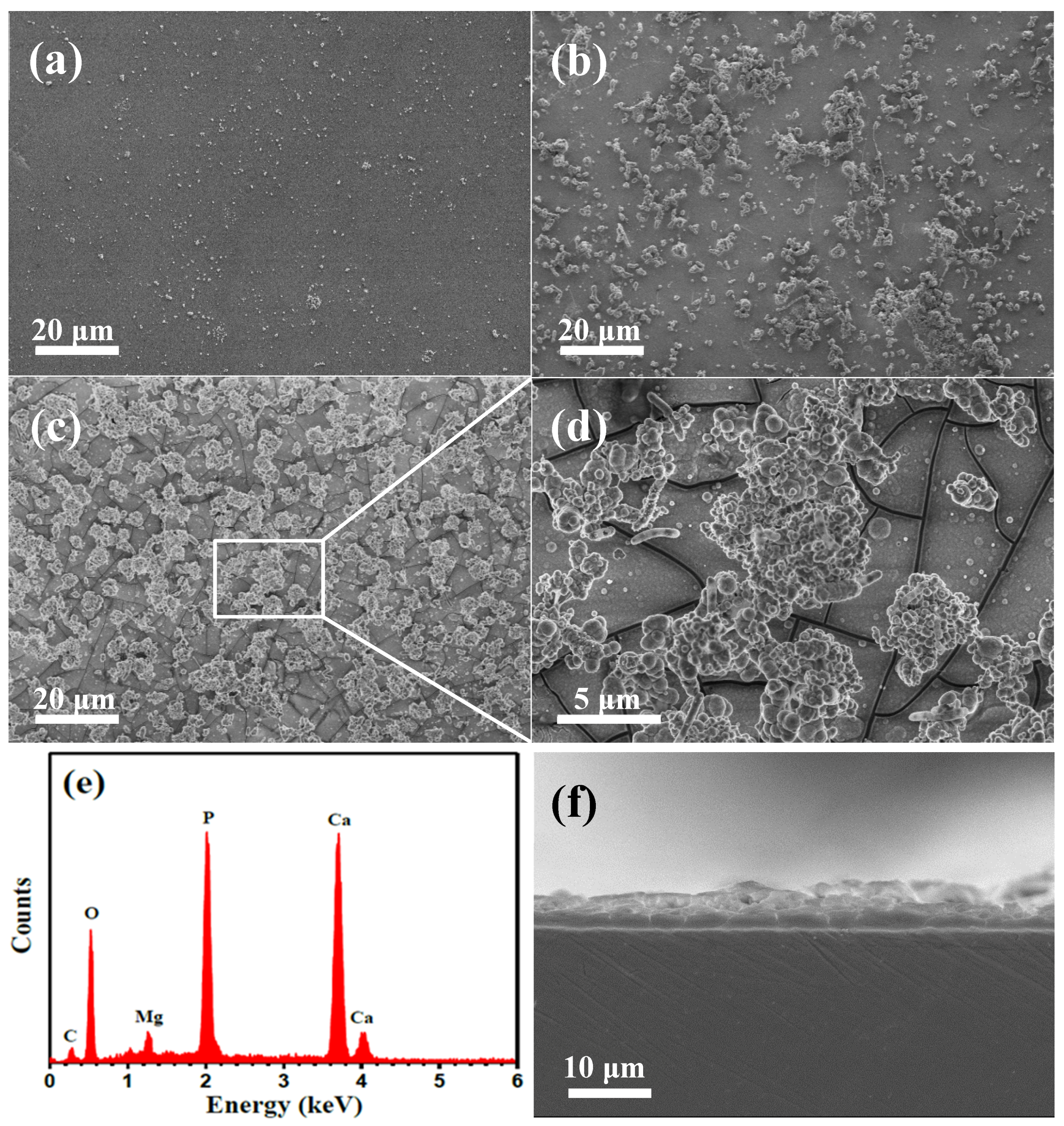
3.4.1. SEM Observation
3.4.2. XRD Analysis
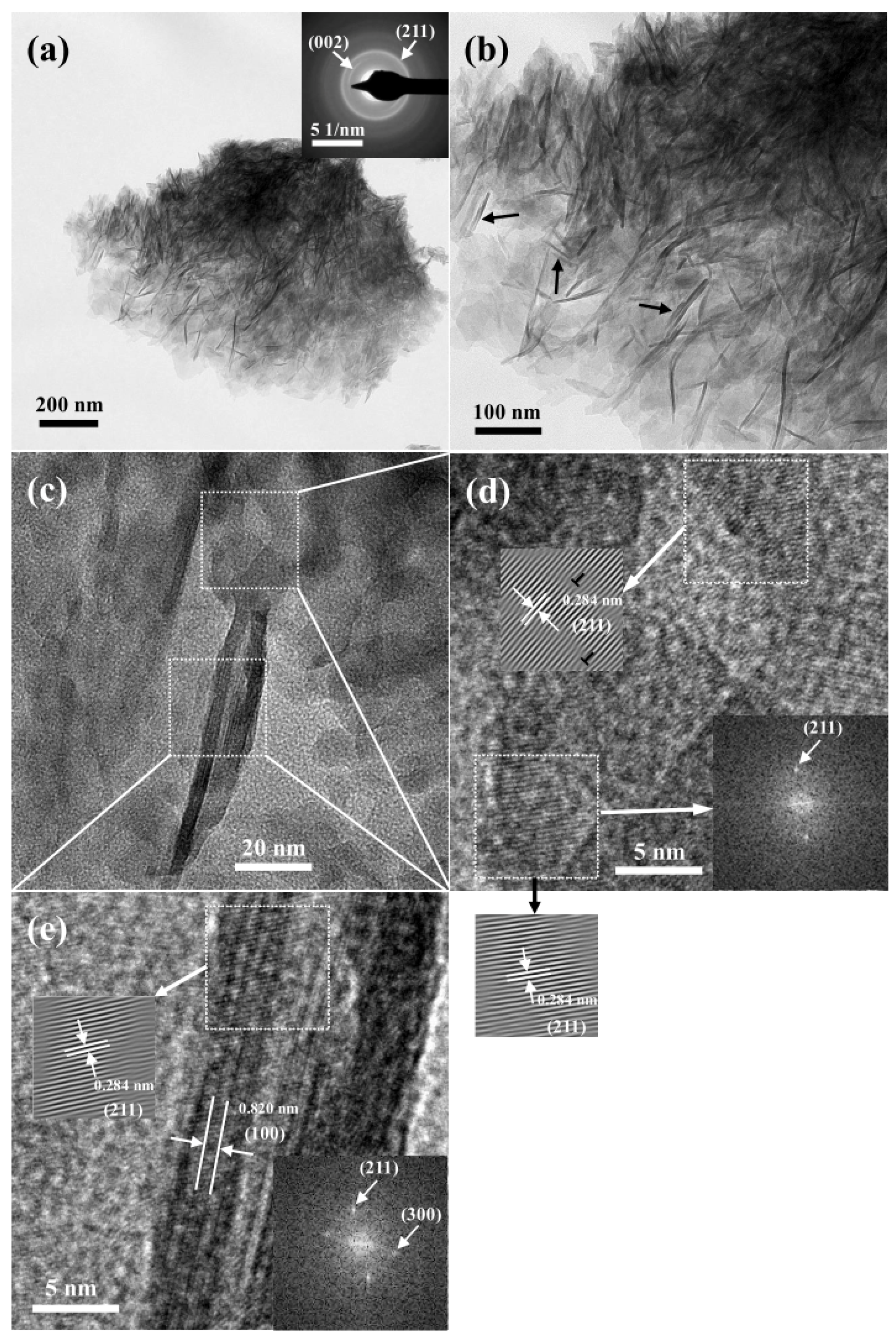
3.4.3. TEM Observation
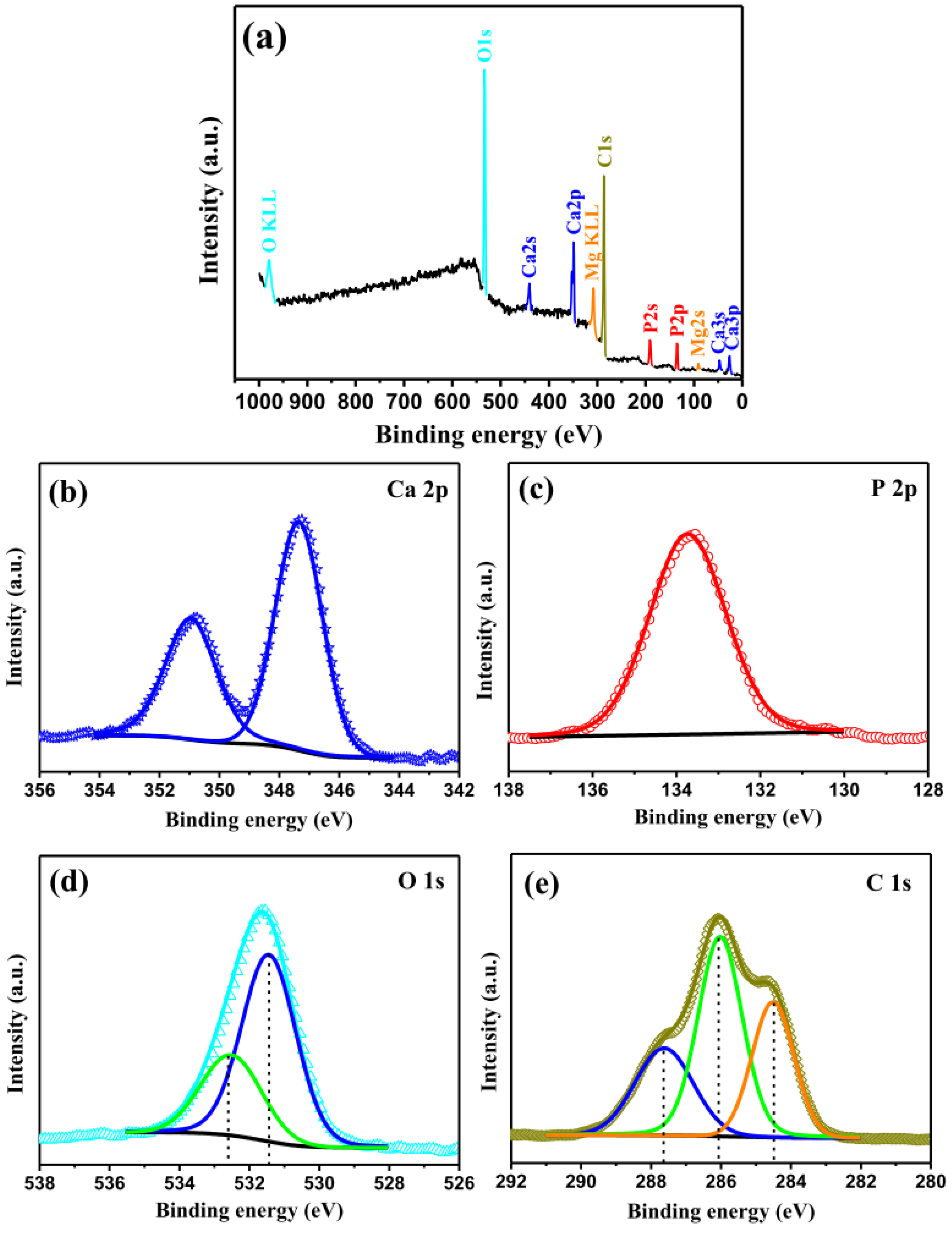
3.4.4. XPS and FT-IR Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Nishiguchi, S.; Kato, H.; Fujita, H.; Oka, M.; Kim, H.-M.; Kokubo, T.; Nakamura, T. Titanium metals form directed bonding to bone after alkali and heat treatments. Biomaterials 2001, 22, 2525–2533. [Google Scholar] [CrossRef]
- Zaffe, D.; Bertoldi, C.; Consolo, U. Element release from titanium devices used in oral and maxillofacial surgery. Biomaterials 2003, 24, 1093–1099. [Google Scholar] [CrossRef]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Sun, Z.L.; Wataha, J.C.; Hanks, C.T. Effects of metal ions on osteoblast-like cell metabolism and differentiation. J. Biomed. Mater. Res. 1997, 34, 29–37. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Jaffe, W.L.; Scott, D.F. Current concepts review—Total hip arthroplasty with hydroxyapatite-coated prostheses. J. Bone Jt. Surg. Am. 1996, 78, 1918–1934. [Google Scholar]
- Kokubo, T.; Miyaji, F.; Kim, H.-M. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Biomimetic surface modification of metallic biomaterials. In Surface Coating and Modification of Metallic Biomaterials; Wen, C., Ed.; Woodhead: Cambridge, UK, 2015; pp. 219–246. [Google Scholar]
- Liu, Y.; Layrolle, P.; de Bruijn, J.; van Blitterswijk, C.; de Groot, K. Biomimetic coprecipitation of calcium phosphate and bovine serum on titanium alloy. J. Biomed. Mater. Res. 2001, 57, 327–335. [Google Scholar] [CrossRef]
- Stigter, M.; de Groot, K.; Layrolle, P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 2002, 23, 4143–4153. [Google Scholar] [CrossRef]
- Black, J. Biological performance of tantalum. Clin. Mater. 1994, 16, 167–173. [Google Scholar] [CrossRef]
- Ching, H.A.; Choudhury, D.; Nine, M.J.; Osman, N.A.A. Effects of surface coating on reducing friction and wear of orthopaedic implants. Sci. Technol. Adv. Mater. 2014, 15, 014402. [Google Scholar] [CrossRef]
- Kato, H.; Nakamura, T.; Nishiguchi, S.; Matsusue, Y.; Kobayashi, M.; Miyazaki, T.; Kim, H.-M.; Kokubo, T. Bonding of alkali- and heat-treated tantalum implants to bone. J. Biomed. Mater. Res. 2000, 53, 28–35. [Google Scholar] [CrossRef]
- Balla, V.K.; Banerjee, S.; Bose, S.; Bandyopadhyay, A. Direct laser processing of a tantalum coating on titanium for bone replacement structures. Acta Biomater. 2010, 6, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, Z.; Tao, J.; Liu, Z.; Chen, Z.; Zhu, W. A novel synthesis method for large area metallic amorphous/nanocrystal film by the glow-discharge plasma technique. Scr. Mater. 2007, 57, 587–590. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity. Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Pajkossy, T.; Kolb, D.M. Double layer capacitance of Pt (111) single crystal electrodes. Electrochim. Acta 2001, 46, 3063–3071. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.F.; Rack, P.D.; Miller, M.K.; Nieh, T.G. Nanocrystalline tetragonal tantalum thin films. Scr. Mater. 2007, 57, 1032–1035. [Google Scholar] [CrossRef]
- Frank, S.; Gruber, P.A.; Handge, U.A.; Spolenak, R. In situ studies on the cohesive properties of α- and β-Ta layers on polyimide substrates. Acta Mater. 2011, 59, 5881–5892. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Jiang, H.G.; Rühle, M.; Lavernia, E.J. On the applicability of the X-ray diffraction line profile analysis in extracting grain size and microstrain in nanocrystalline materials. J. Mater. Res. 1999, 14, 549–559. [Google Scholar] [CrossRef]
- Lee, S.L.; Doxbeck, M.; Mueller, J.; Cipollo, M.; Cote, P. Texture, structure and phase transformation in sputter beta tantalum coating. Surf. Coat. Technol. 2004, 177–178, 44–51. [Google Scholar] [CrossRef]
- Wang, Y.M.; Hodge, A.M.; Bythrow, P.M.; Barbee, T.W., Jr.; Hamza, A.V. Negative strain rate seneitivity in ultrahigh-strength nanocrystalline tantalum. Appl. Phys. Lett. 2006, 89, 081903. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Ovid’ko, I.A. Deformation of nanostructures. Science 2002, 295, 2386. [Google Scholar] [CrossRef] [PubMed]
- Swygenhoven, H.V. Grain boundaries and dislocations. Science 2002, 296, 66–67. [Google Scholar] [CrossRef] [PubMed]
- McFadden, S.X.; Mishra, R.S.; Valiev, R.Z.; Zhilyaev, A.P.; Mukherjee, A.K. Low-temperature superplasticity in nanostructured nickel and metal alloys. Nature 1999, 398, 684–686. [Google Scholar]
- Pham, V.-H.; Lee, S.-H.; Li, Y.; Kim, H.-E.; Shin, K.-H.; Koh, Y.-H. Utility of tantalum (Ta) coating to improve surface hardness in vitro bioactivity and biocompatibility of Co-Cr. Thin Solid Films 2013, 536, 269–274. [Google Scholar] [CrossRef]
- Hogmark, S.; Jacobson, S.; Larsson, M. Design and evaluation of tribological coatings. Wear 2000, 246, 20–33. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization, I. A theoretical analysis of the shapes of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Zhu, P.; Masuda, Y.; Koumoto, K. The effect of surface charge on hydroxyapatite nucleation. Biomaterials 2004, 25, 3915–3921. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mccrate, J.M.; Lee, J.C.M.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Chen, S.; Yin, B.; Zhao, S.; Liu, X. Impedance spectroscopic study of corrosion inhibition of copper by surfactants in the acidic solutions. Corros. Sci. 2003, 45, 867–882. [Google Scholar] [CrossRef]
- El-Aziz, A.M.; Kibler, L.A.; Kolb, D.M. The potentials of zero charge of Pd(111) and thin Pd overlayers on Au(111). Electrochem. Commun. 2002, 4, 535–539. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F.H. Electrochemical corrosion behavior of nanocrystalline materials—A review. J. Mater. Sci. Technol. 2010, 26, 1–14. [Google Scholar] [CrossRef]
- Galvele, J.R.; Torresi, R.M.; Carranza, R.M. Passivity breakdown, its relation to pitting and stress-corrosion-cracking processes. Corros. Sci. 1990, 31, 563–571. [Google Scholar] [CrossRef]
- Hassan, H.H. Effect of chloride ions on the corrosion behaviour of steel in 0.1 M citrate. Electrochim. Acta 2005, 51, 526–535. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, J.L. The hydrogen-enhanced effects of chloride ions on the passivity of type 304 stainless steel. Electrochim. Acta 2000, 45, 3927–3937. [Google Scholar] [CrossRef]
- Morrison, S.R. Electrochemistry at Semiconductor and Oxidized Metal Electrodes; Plenum Press: New York, NY, USA, 1980. [Google Scholar]
- Jovic, V.D.; Barsoum, M.W. Corrosion behavior and passive film characteristics formed on Ti, Ti3SiC2, and Ti4AlN3 in H2SO4 and HCl. J. Electrochem. Soc. 2004, 151, B71–B76. [Google Scholar] [CrossRef]
- Kerrec, O.; Devilliers, D.; Groult, H.; Chemla, M. Dielectric properties of anodic oxide films on tantalum. Electrochim. Acta 1995, 40, 719–724. [Google Scholar] [CrossRef]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434–3449. [Google Scholar] [CrossRef]
- Schneider, M.; Schroth, S.; Schilm, J.; Michaelis, A. Micro-EIS of anodic thin oxide films on titanium for capacitor applications. Electrochim. Acta 2009, 54, 2663–2671. [Google Scholar] [CrossRef]
- Liu, L.L.; Xu, J.; Lu, X.L.; Munroe, P.; Xie, Z.H. Electrochemical corrosion behavior of nanocrystalline β-Ta coating for biomedical applications. ACS Biomater. Sci. Eng. 2016, 2, 579–594. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R. Influence of ytterbium on the electrochemical property of PbCaSn alloy in sulfuric acid solution. J. Power Sour. 2012, 210, 163–171. [Google Scholar] [CrossRef]
- Azumi, K.; Ohtsuka, T.; Sato, N. Mott-Schottky plot of the passive film formed on iron in neutral borate and phosphate solutions. J. Electrochem. Soc. 1987, 134, 1352–1357. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Neo, M.; Kim, H.-M.; Kokubo, T.; Nakamura, T. A comparative study between in vivo bone ingrowth and in vitro apatite formation on Na2O-CaO-SiO2 glasses. Biomaterials 2003, 24, 1349–1356. [Google Scholar] [CrossRef]
- Song, W.-H.; Jun, Y.-K.; Han, Y.; Hong, S.-H. Biomimetic apatite coatings on micro-arc oxidized titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef] [PubMed]
- Pasinli, A.; Yuksel, M.; Celik, E.; Sener, S.; Tas, A.C. A new approach in biomimetic synthesis of calcium phosphate coatings using lactic acid-Na lactate buffered body fluid solution. Acta Biomater. 2010, 6, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barrère, F.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Biomimetic hydroxyapatite coating on metal implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Darvell, B.W.; Lu, W.W. Apatite-formation ability—Predictor of “bioactivity”? Acta Biomater. 2010, 6, 4181–4188. [Google Scholar] [CrossRef] [PubMed]
- Jantou, V.; Turmaine, M.; West, G.D.; Horton, M.A.; McComb, D.W. Focused ion beam milling and ultramicrotomy of mineralized ivory dentine for analytical transmission electron microscopy. Micron 2009, 40, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Luong, L.N.; Hong, S.I.; Patel, R.J.; Outslay, M.E.; Kohn, D.H. Spatial control of protein within biomimetically nucleated mineral. Biomaterials 2006, 27, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Layani, J.D.; Cuisinier, F.J.G.; Steuer, P.; Cohen, H.; Voegel, J.C.; Mayer, I. High-resolution electron microscopy study of synthetic carbonate and aluminum containing apatites. J. Biomed. Mater. Res. 2000, 50, 199–207. [Google Scholar] [CrossRef]
- Suzuki, T.; Kumeda, I.; Teshima, K.; Qishi, S. Specific surface free energies of strontium chloraptite single crystal determined by contact angles of liquid droplets. Chem. Phys. Lett. 2006, 421, 343–347. [Google Scholar] [CrossRef]
- Kačiulis, S.; Mattogno, G.; Pandolfi, L.; Cavalli, M.; Gnappi, G.; Montenero, A. XPS study of apatite-based coatings prepared by sol-gel technique. Appl. Surf. Sci. 1999, 15, 1–5. [Google Scholar] [CrossRef]
- Kunze, J.; Müller, L.; Macak, J.M.; Greil, P.; Schmuki, P.; Müller, F.A. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochim. Acta 2008, 53, 6995–7003. [Google Scholar] [CrossRef]
- Raikar, G.N.; Gregory, J.C.; Ong, J.L.; Lucas, L.C.; Lemons, J.E.; Kawahara, D.; Nakamura, M. Surface characterization of titanium implants. J. Vac. Sci. Technol. A 1995, 13, 2633. [Google Scholar] [CrossRef]
- Gu, Y.W.; Tay, B.Y.; Lim, C.S.; Yong, M.S. Biomimetic deposition of apatite coating on surface-modified NiTi alloys. Biomaterials 2005, 26, 6916–6923. [Google Scholar] [CrossRef] [PubMed]
- Roguska, A.; Pisarek, M.; Andrezjczuk, M.; Dolata, M.; Lewandowska, M.; Janik-Czachor, M. Characterization of a calcium phosphate-TiO2 nanotube composite layer for biomedical applications. Mater. Sci. Eng. C 2011, 31, 906–914. [Google Scholar] [CrossRef]
- Gu, Y.W.; Khor, K.A.; Cheang, P. In vitro studies of plasma-sprayed hydroxyapatite/Ti-6Al-4V composite coatings in simulated body fluid (SBF). Biomaterials 2003, 24, 1603–1611. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Zhou, L.; Zhang, S. Preparation and characterization of selenite substituted hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.C.; Gibson, I.; Barbosa, M.A. The uptake of titanium ions by hydroxyapatite particles—Structural changes and possible mechanisms. Biomaterials 2006, 27, 1749–1761. [Google Scholar] [CrossRef] [PubMed]
- Kweh, S.W.K.; Khor, K.A.; Cheang, P. An in vitro investigation of plasma sprayed hydroxyapatite (HA) coatings produced with flame-spheroidized feedstock. Biomaterials 2002, 23, 775–785. [Google Scholar] [CrossRef]
- Müller, F.A.; Müller, L.; Caillard, D.; Conforto, E. Preferred growth orientation of biomimetic apatite crystals. J. Cryst. Growth 2007, 304, 464–471. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, Y.; Chen, X.; Zhu, P.; Wei, S. Biomimetic synthesis and biocompatibility evaluation of carbonated apatites template-mediated by heparin. Mater. Sci. Eng. C 2013, 33, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Fleet, M.E.; Liu, X. Coupled substitution of type A and B carbonate in sodium-bearing apatite. Biomaterials 2007, 28, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Rajzer, I.; Kwiatkowski, R.; Piekarczyk, W.; Biniaś, W.; Janicki, J. Carbon nanofibers produced from modified electrospun PAN/hydroxyapatite precursors as scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2012, 32, 2562–2569. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Y.; Zeng, Z.; Liu, W.; Xue, Q.; Hu, L.; Zhang, J. Electrochemical corrosion behavior of nanocrystalline Co coatings explained by higher grain boundary density. Electrochim. Acta 2007, 52, 4342–4350. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, F.H. Influence of nanocrystallization on passive behavior of Ni-based superalloy in acidic solutions. Electrochim. Acta 2007, 52, 2392–2400. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Fu, R.K.F.; Ho, J.P.Y.; Ding, C.; Chu, P.K. Plasma-treated nanostructured TiO2 surface supporting biomimetic growth of apatite. Biomaterials 2005, 26, 6143–6150. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Nouri, A.; Li, Y.; Lin, J.; Hodgson, P.D.; Wen, C. Effect of surface roughness of Ti, Zr, and TiZr on apatite precipitation from simulated body fluid. Biotechnol. Bioeng. 2008, 101, 278–387. [Google Scholar] [CrossRef] [PubMed]
- Peltola, T.; Jokinen, M.; Veittola, S.; Simola, J.; Yli-Urpo, A. In vitro bioactivity and structural features of mildly heat-treated sol-gel-derived silica fibers. J. Biomed. Mater. Res. 2000, 54, 579–590. [Google Scholar] [CrossRef]
- Viitala, R.; Jokinen, M.; Peltola, T.; Gunnelius, K.; Rosenholm, J.B. Surface properties of in vitro bioactive and non-bioactive sol-gel derived materials. Biomaterials 2002, 23, 3073–3086. [Google Scholar] [CrossRef]
- Kaplan, F.S.; Lee, W.C.; Keaveny, T.M.; Boskey, A.; Einhorn, T.A.; Iannotti, J.P. Form and function of bone. In Orthopedic Basic Science; Simon, S.P., Ed.; American Academy of Orthopedic Surgeons: Columbus, OH, USA, 1994; pp. 127–185. [Google Scholar]
- Lin, C.-M.; Yen, S.-K. Biomimetic growth of apatite on electrolytic TiO2 coatings in simulated body fluid. Mater. Sci. Eng. C 2006, 26, 54–64. [Google Scholar] [CrossRef]
- Chun, W.J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, L.; Yuan, S.; Zhao, Y.; Fang, J. Synthesis and photocatalytic properties of highly stable and neutral TiO2/SiO2 hydrosol. J. Colloid Interface Sci. 2009, 330, 113–118. [Google Scholar] [CrossRef] [PubMed]



| Samples | β-Ta Coating | Uncoated Ti-6Al-4V | Pure Ta |
|---|---|---|---|
| Ecorr (V vs. SCE) | −0.23 | −0.36 | −0.34 |
| βa (mV/decade) | 120.25 | 126.97 | 128.67 |
| −βc (mV/decade) | 213.76 | 224.62 | 123.04 |
| icorr (A·cm−2) | 1.75 × 10−8 | 1.25 × 10−7 | 3.54 × 10−8 |
| Rp (Ω·cm2) | 1.91 × 106 | 2.81 × 105 | 7.72 × 105 |
| Samples | β-Ta Coating | Uncoated Ti-6Al-4V | Pure Ta |
|---|---|---|---|
| Rs (Ω·cm2) | 17.52 ± 0.16 | 10.00 ± 0.13 | 19.83 ± 0.21 |
| Qp (Ω−1·cm−2·sn) | (1.89 ± 0.01) × 10−5 | (2.41 ± 0.03) × 10−5 | (2.12 ± 0.02) × 10−5 |
| n | 0.941 ± 0.002 | 0.918 ± 0.002 | 0.863 ± 0.002 |
| Rp (Ω·cm2) | (1.34 ± 0.08) × 106 | (3.77 ± 0.14) × 105 | (8.96 ± 0.54) × 105 |
| Cp (μF·cm−2) | 11.44 | 11.45 | 6.17 |
| τ (s) | 15.33 | 4.32 | 5.53 |
| χ2 | 3.34 × 10−4 | 1.56 × 10−3 | 6.22 × 10−4 |
| Samples | β-Ta Coating | Uncoated Ti-6Al-4V | Pure Ta |
|---|---|---|---|
| Nd (×1019 cm−3) | 0.87 | 2.45 | 2.46 |
| Efb (V) | −1.56 | −0.79 | −1.44 |
| δsc (nm) | 27.37 | 20.74 | 15.86 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Xu, J.; Jiang, S. Nanocrystalline β-Ta Coating Enhances the Longevity and Bioactivity of Medical Titanium Alloys. Metals 2016, 6, 221. https://doi.org/10.3390/met6090221
Liu L, Xu J, Jiang S. Nanocrystalline β-Ta Coating Enhances the Longevity and Bioactivity of Medical Titanium Alloys. Metals. 2016; 6(9):221. https://doi.org/10.3390/met6090221
Chicago/Turabian StyleLiu, Linlin, Jiang Xu, and Shuyun Jiang. 2016. "Nanocrystalline β-Ta Coating Enhances the Longevity and Bioactivity of Medical Titanium Alloys" Metals 6, no. 9: 221. https://doi.org/10.3390/met6090221





