Study on the Leaching of Mercuric Oxide with Thiosulfate Solutions
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
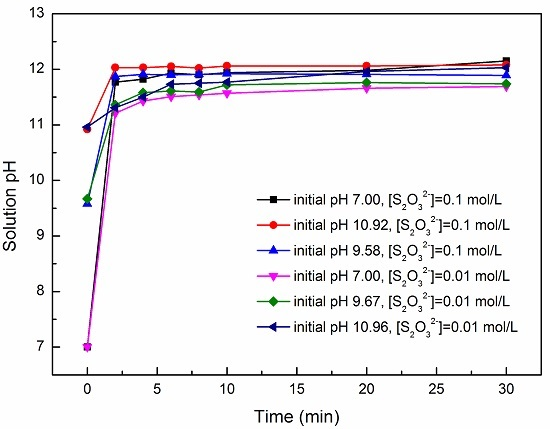
3.1. Effect of pH
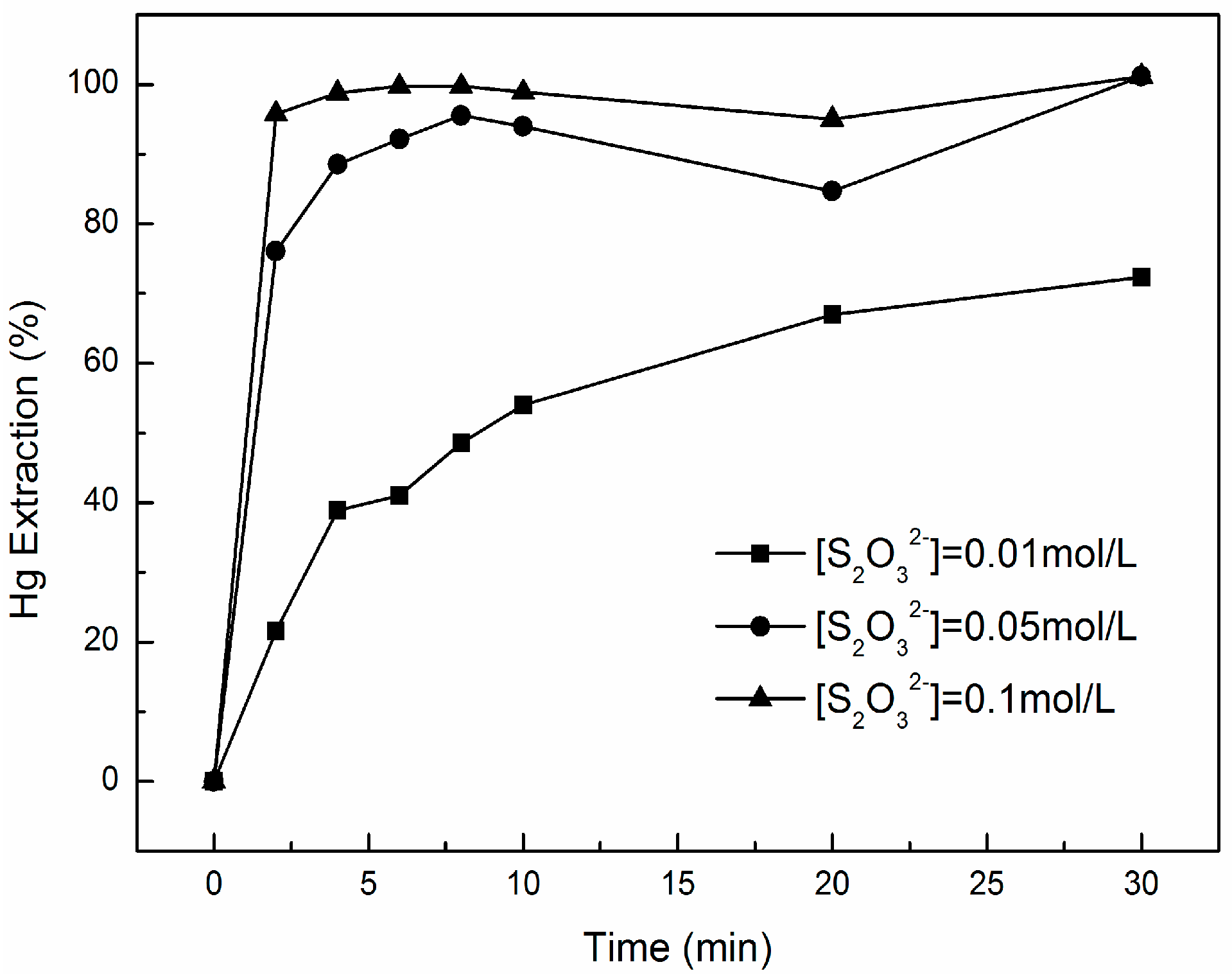
3.2. Effect of Na2S2O3 Concentration
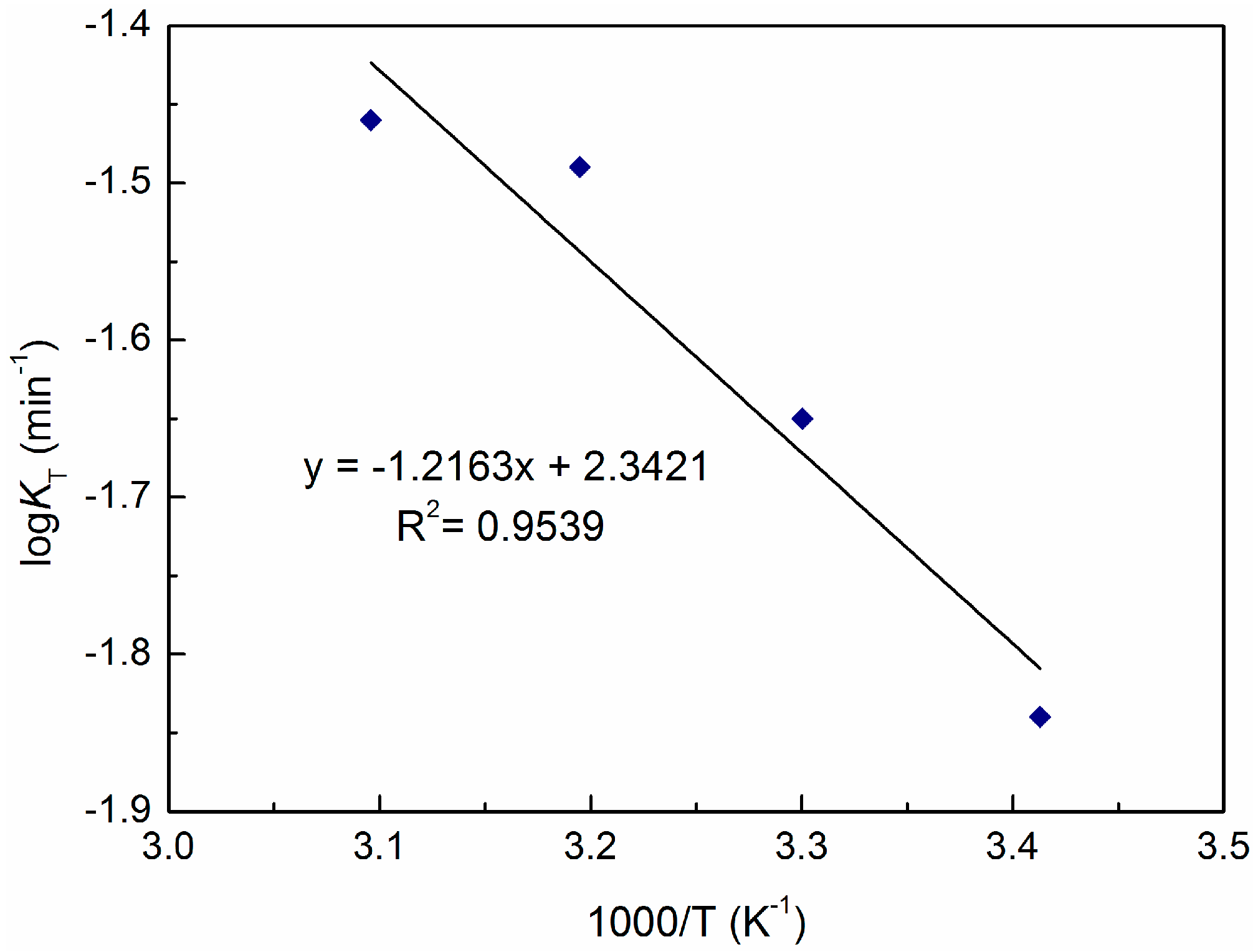
3.3. Effect of Temperature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cristina Salvador Pedroso, A.; Eusébio Reís Gomes, L.; de Carvalho Jorge, M.R. Mercury removal from process sludges via hypochlorite leaching. Environ. Technol. 1994, 15, 657–667. [Google Scholar] [CrossRef]
- Clarkson, T.W. Mercury: Major issues in environmental health. Environ. Health Prospect 1992, 100, 31–38. [Google Scholar] [CrossRef]
- Suresh Kumar Reddy, K.; Shoaibi, A.A.; Srinivasakannan, C. Elemental mercury adsorption on sulfur-impregnated porous carbon—A review. Environ. Technol. 2014, 35, 18–26. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Hg (II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Fitzgerald, W.F.; Morel, F.M.M. The biogeochemical cycling of elemental mercury: Anthropogenic influences. Geochem. Cosmochim. Acta 1994, 58, 3191–3198. [Google Scholar] [CrossRef]
- Mason, R.P.; Rolfhus, K.R.; Fitzgerald, W.F. Mercury in the North Atlantic. Mar. Chem. 1998, 61, 37–53. [Google Scholar] [CrossRef]
- Felske, A.; Vandieken, V.; Pauling, B.V. Molecular quantification of genes encoding for green-fluorescent proteins. J. Microbiol. Methods 2003, 52, 297–304. [Google Scholar] [CrossRef]
- Guentzel, J.L.; Powell, R.T.; Landing, W.M. Mercury associated with colloidal material in an estuarine and an open-ocean environment. Mar. Chem. 1996, 55, 177–188. [Google Scholar] [CrossRef]
- Kudo, A.; Miyahara, S. A case-history Minamata mercury pollution in Japan from loss of human lives to decontamination. Water Sci. Technol. 1991, 23, 283. [Google Scholar]
- Wang, Q.; Kim, D.; Dionysiou, D.D. Sources and remediation for mercury contamination in aquatic systems—A literature review. Environ. Pollut. 2004, 131, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Guo, X.; Wang, X. Removal of mercury (II), elemental mercury and arsenic from simulated flue gas by ammonium sulphide. Environ. Technol. 2015, 36, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, X.; Larssen, T. Fractionation, distribution and transport of mercury in rivers and tributaries around Wanshan Hg mining district. Guizhou province. Southwestern China: Part 1—Total mercury. Appl. Geochem. 2010, 25, 633–641. [Google Scholar] [CrossRef]
- Lima, L.; Bernardez, L.A.; Barbosa, L. Characterization and treatment of artisanal gold mine tailings. J. Hazard. Mater. 2008, 150, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.N.; Veiga, M.M.; Klein, B. Strategies for reducing the environmental impact of reprocessing mercury-contaminated tailings in the artisanal and small-scale gold mining sector: Insights from Tapajos River Basin, Brazil. J. Clean Prod. 2010, 18, 1757–1766. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 4–19. [Google Scholar]
- Gorin, A.H.; Leckey, J.H.; Nulf, L.E. Final disposal options for mercury/uranium mixed wastes from the Oak Ridge Reservation. In Office of Scientific & Technical Information Technical Reports; Oak Ridge Y-12 Plant: Oak Ridge, TN, USA, 1994; pp. 5–13. [Google Scholar]
- Piao, H.; Bishop, P.L. Stabilization of mercury-containing wastes using sulfide. Environ. Pollut. 2006, 139, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Guo, X.; Zheng, C. Removal of elemental mercury by iodine-modified rice husk ash sorbents. J. Environ. Sci. 2010, 22, 1629–1636. [Google Scholar] [CrossRef]
- Washburn, C.; Hill, E. Mercury retorts for the processing of precious metals and hazardous wastes. JOM 2003, 55, 45–50. [Google Scholar] [CrossRef]
- Busto, Y.; Cabrera, X.; Tack, F.M.G. Potential of thermal treatment for decontamination of mercury containing wastes from chlor-alkali industry. J. Hazard. Mater. 2011, 186, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Taube, F.; Pommer, L.; Larsson, T. Soil remediation-mercury speciation in soil and vapour phase during thermal treatment. Water Air Soil Pollut. 2008, 193, 155–163. [Google Scholar] [CrossRef]
- Huang, Y.T.; Hseu, Z.Y.; His, H.C. Influences of thermal decontamination on mercury removal, soil properties, and repartitioning of coexisting heavy metals. Chemosphere 2011, 84, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, M.; Melamed, D.; Kalb, P.D. Sulfur Polymer Solidification/Stabilization of elemental mercury waste. Waste Manag. 2002, 22, 327–333. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D. Immobilization of heavy metal in cement-based solidification/stabilization: A review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, Q.C.; Zhang, S.Q. Stabilization/solidification (S/S) of mercury-contaminated hazardous wastes using thiol-functionalized zeolite and Portland cement. J. Hazard. Mater. 2009, 168, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Dermont, G.; Bergeron, M.; Mercier, G. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, O.; Padilla, I.; Tayibi, H. Concerns on liquid mercury and mercury-containing wastes: A review of the treatment technologies for the safe storage. J. Environ. Manag. 2012, 101, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Issaro, N.; Besancon, S.; Bermond, A. Thermodynamic and kinetic study of the single extraction of mercury from soil using sodium-thiosulfate. Talanta 2010, 82, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huangfu, X.; Zhang, X. Strong enhancement of trace mercury removal from aqueous solution with sodium thiosulfate by in situ formed Mn-(hydr)oxides. Water Res. 2014, 65, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dyrssen, D.; Wedborg, M. The sulphur-mercury (II) system in natural waters. Water Air Soil Pollut. 1991, 56, 507–519. [Google Scholar] [CrossRef]
- Bloom, N.S.; Lasorsa, B.K. Changes in mercury speciation and the release of methyl mercury as a result of marine sediment dredging activities. Sci. Total Environ. 1999, 237–238, 379–385. [Google Scholar] [CrossRef]
- Horvat, M.; Kotnik, J.; Logar, M. Speciation of mercury in surface and deep-sea waters in the Mediterranean Sea. Atmos. Environ. 2003, 37, 93–108. [Google Scholar] [CrossRef]
- Kim, D.; Wang, Q.; Sorial, G.A. A model approach for evaluating effects of remedial actions on mercury speciation and transport in a lake system. Sci. Total Environ. 2004, 327, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Skyllberg, U.; Drott, A. Competition between disordered iron sulfide and natural organic matter associated thiols for mercury (II)—An EXAFS study. Environ. Sci. Technol. 2010, 44, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, E.K.; Dakova, I.G.; Tsalev, D.L. Mercury determination and speciation analysis in surface waters. Cent. Eur. J. Chem. 2012, 10, 1175–1182. [Google Scholar] [CrossRef]
- Wang, J.; Feng, X.; Anderson, C.W.N. Remediation of mercury contaminated sites—A review. J. Hazard. Mater. 2012, 221–222, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1995; pp. 455–464. [Google Scholar]
- Hudson, J.L.; Tsotsis, T.T. Electrochemical reaction dynamics: A review. Chem. Eng. Sci. 1994, 49, 1493–1572. [Google Scholar] [CrossRef]
- Nyman, C.J.; Salazar, T. Complex Ion Formation of Mercury (II) and Thiosulfate Ion. Anal. Chem. 1961, 33, 1467–1469. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Wang, W.; Xie, F. Study on the Leaching of Mercuric Oxide with Thiosulfate Solutions. Metals 2016, 6, 206. https://doi.org/10.3390/met6090206
Han C, Wang W, Xie F. Study on the Leaching of Mercuric Oxide with Thiosulfate Solutions. Metals. 2016; 6(9):206. https://doi.org/10.3390/met6090206
Chicago/Turabian StyleHan, Chao, Wei Wang, and Feng Xie. 2016. "Study on the Leaching of Mercuric Oxide with Thiosulfate Solutions" Metals 6, no. 9: 206. https://doi.org/10.3390/met6090206
APA StyleHan, C., Wang, W., & Xie, F. (2016). Study on the Leaching of Mercuric Oxide with Thiosulfate Solutions. Metals, 6(9), 206. https://doi.org/10.3390/met6090206