Characterization of Precipitates in a Microalloyed Steel Using Quantitative X-ray Diffraction
Abstract
:1. Introduction
2. Backgound
2.1. QXRD
2.2. Domain Size
2.3. Microstrain
2.4. Profile Functions
2.5. QXRD Programs
3. Experimental Procedure and Results
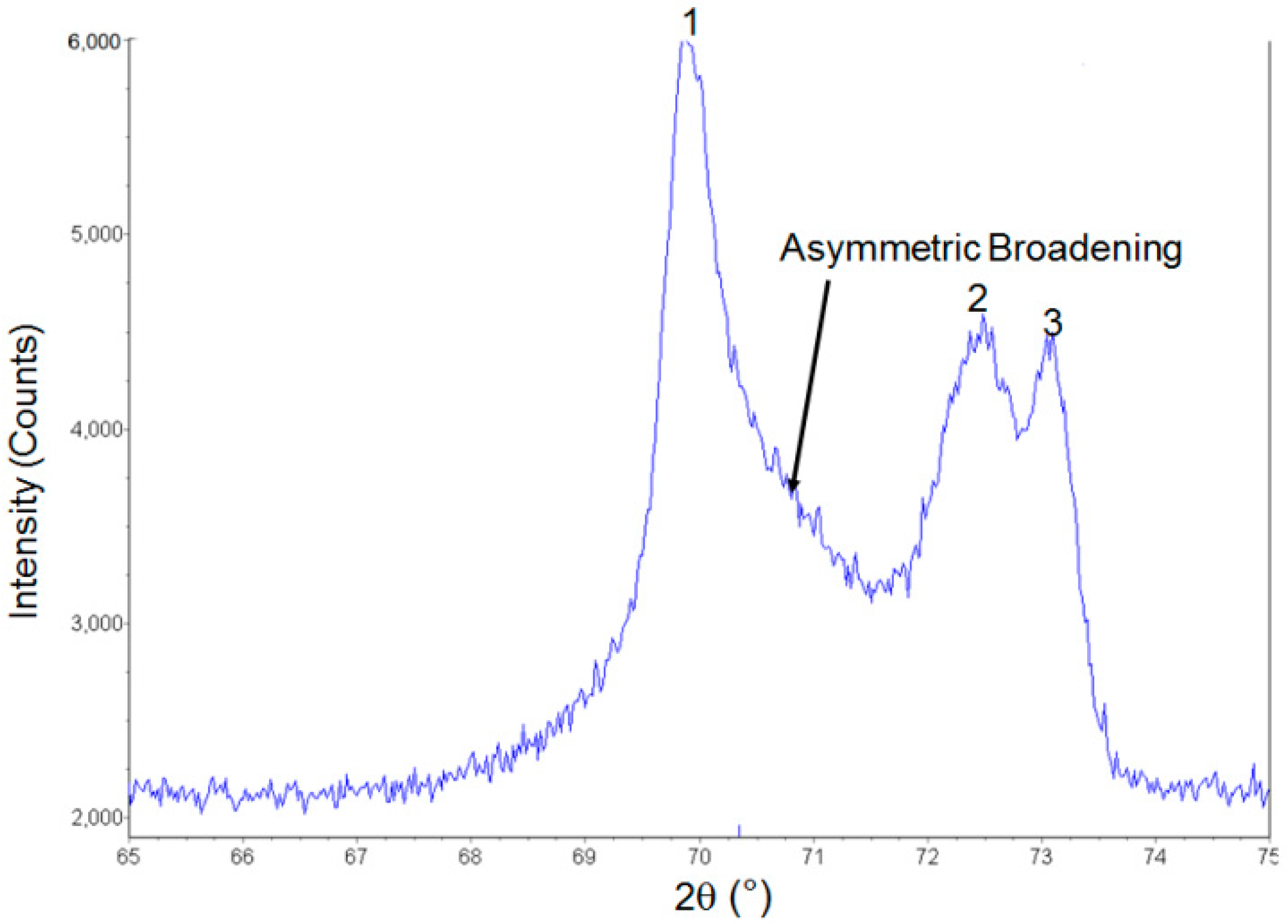
3.1. Diffraction Pattern of Grade 100 Residue
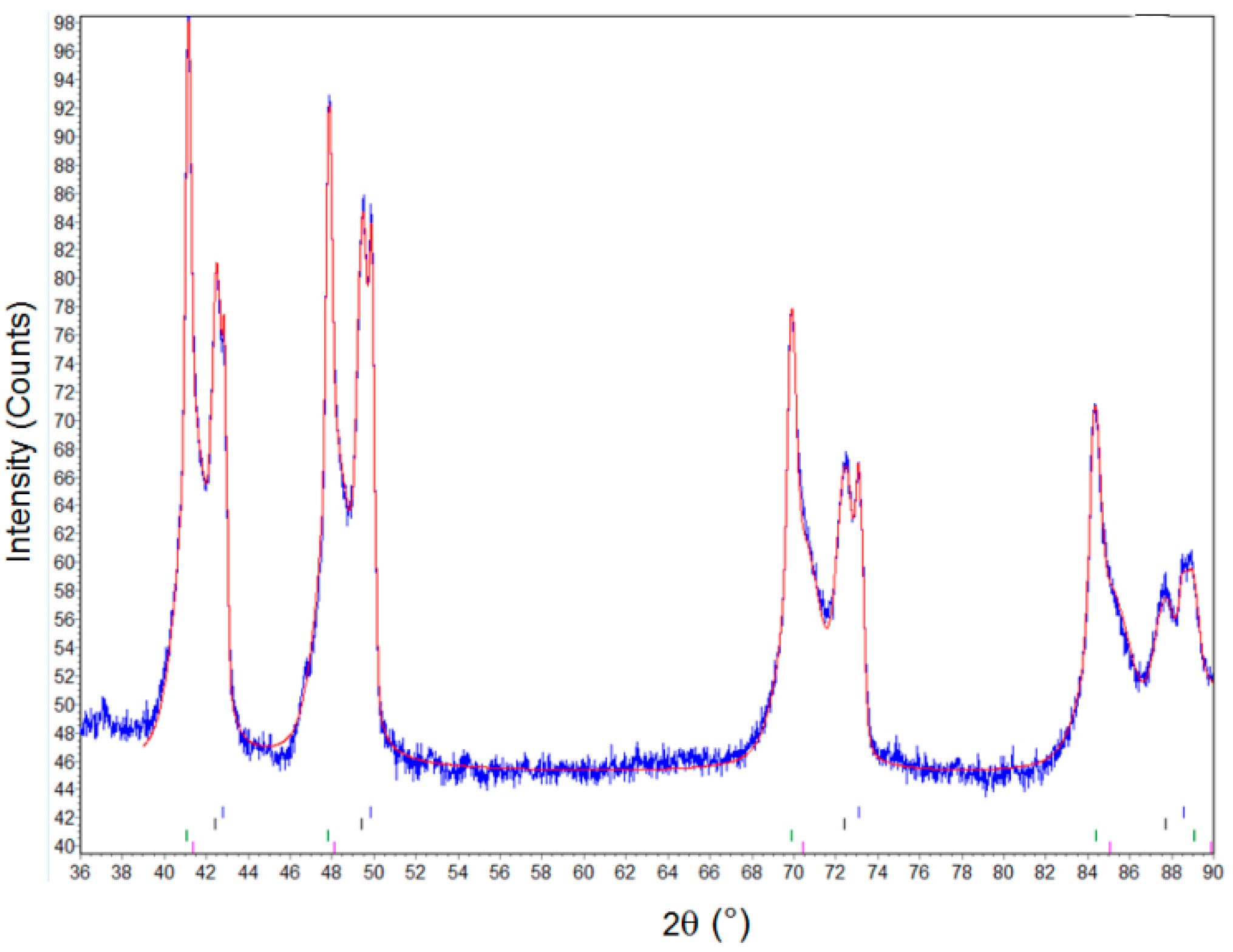
3.2. QXRD Refinement
3.3. Predicted Crystallographic and Microstructure Variables
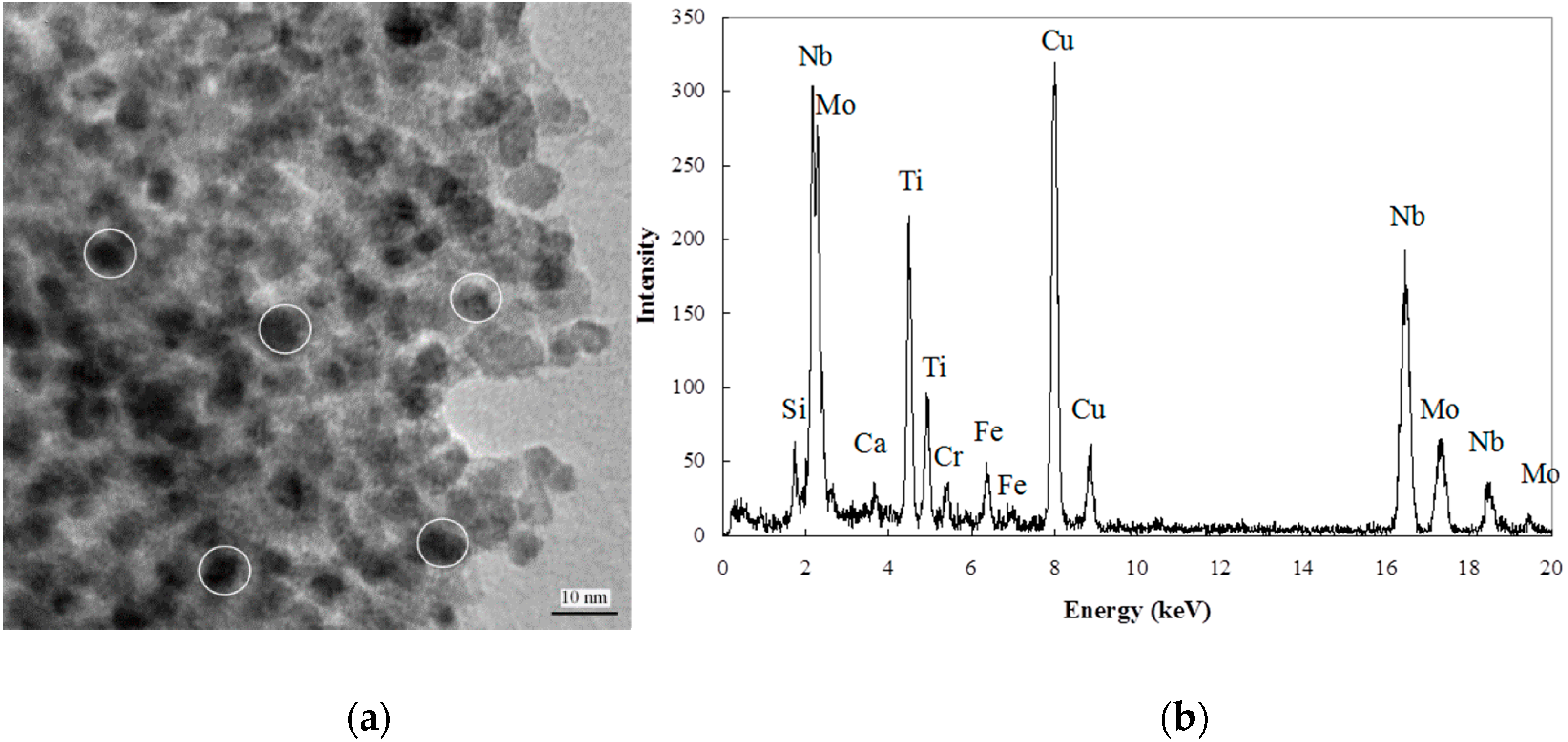
3.4. EDX/TEM Analysis
4. Discussion
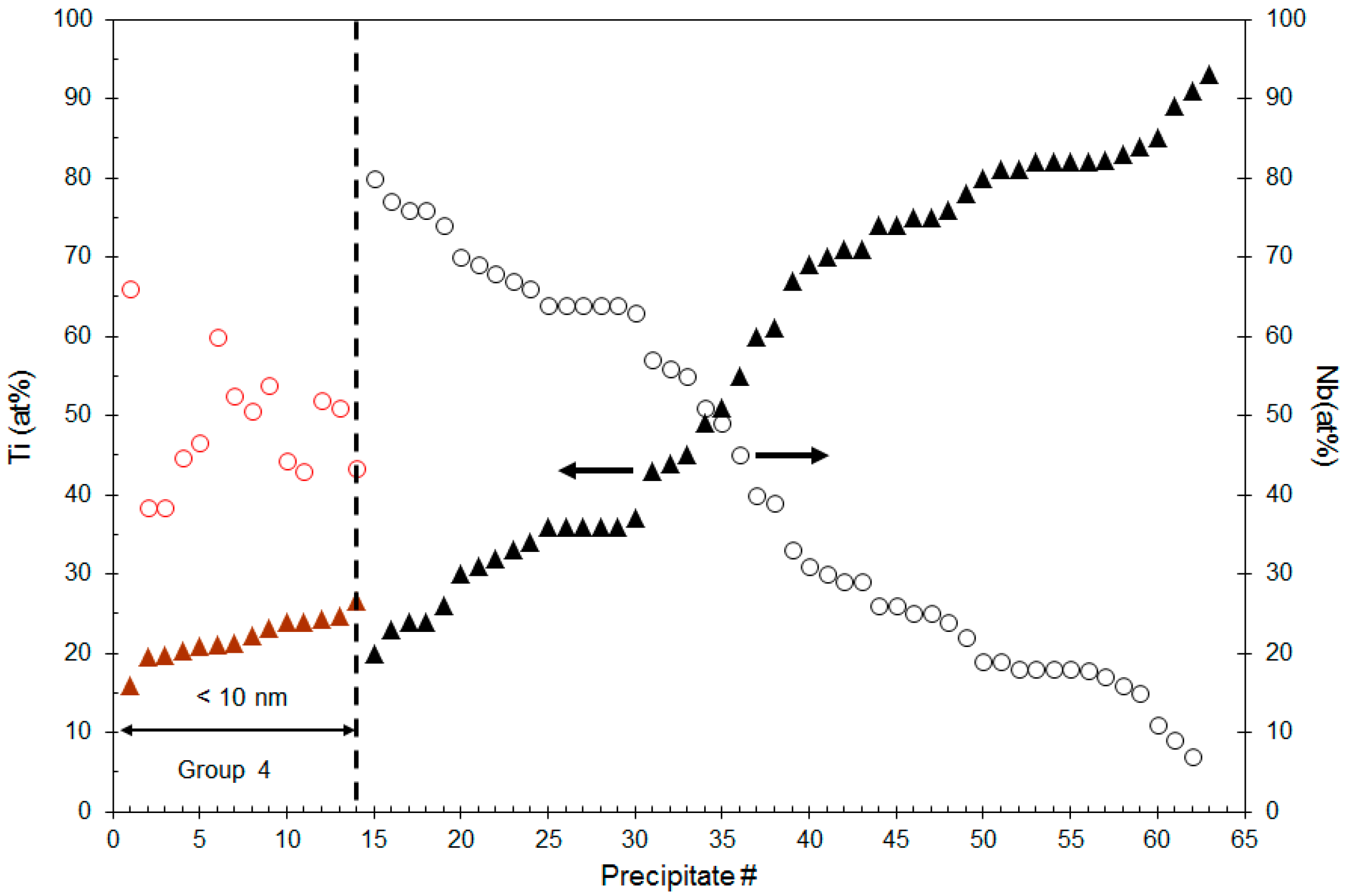
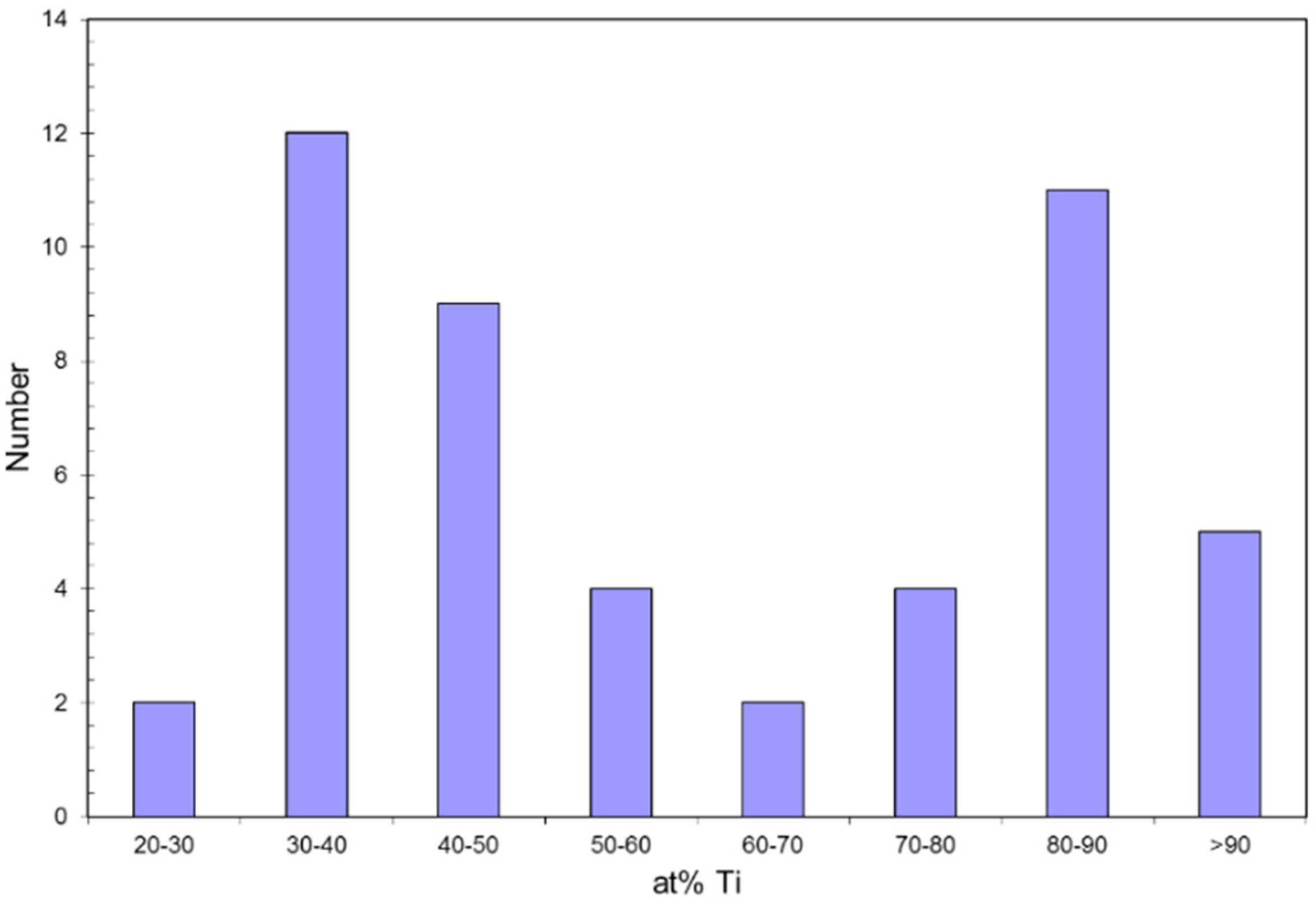
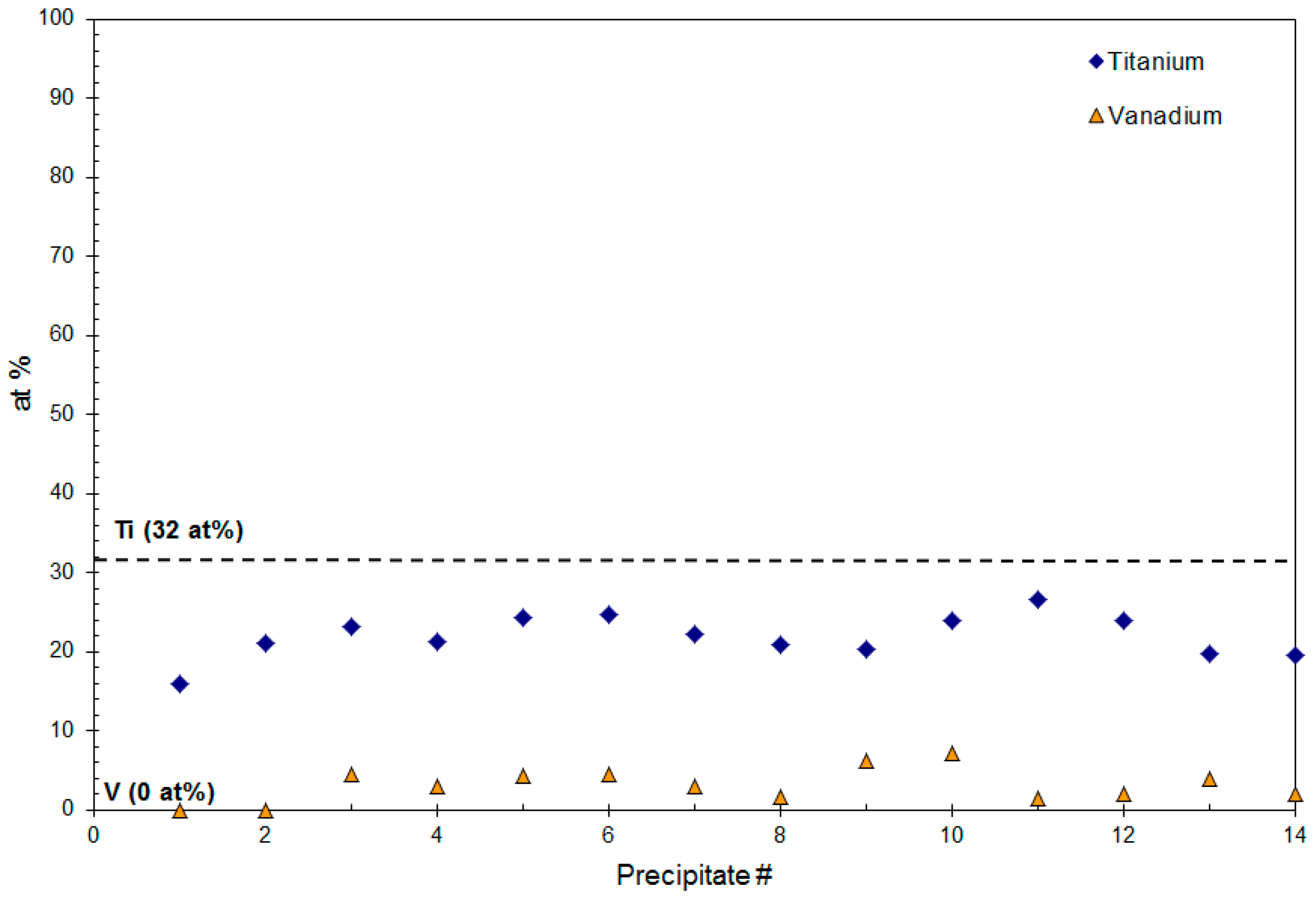
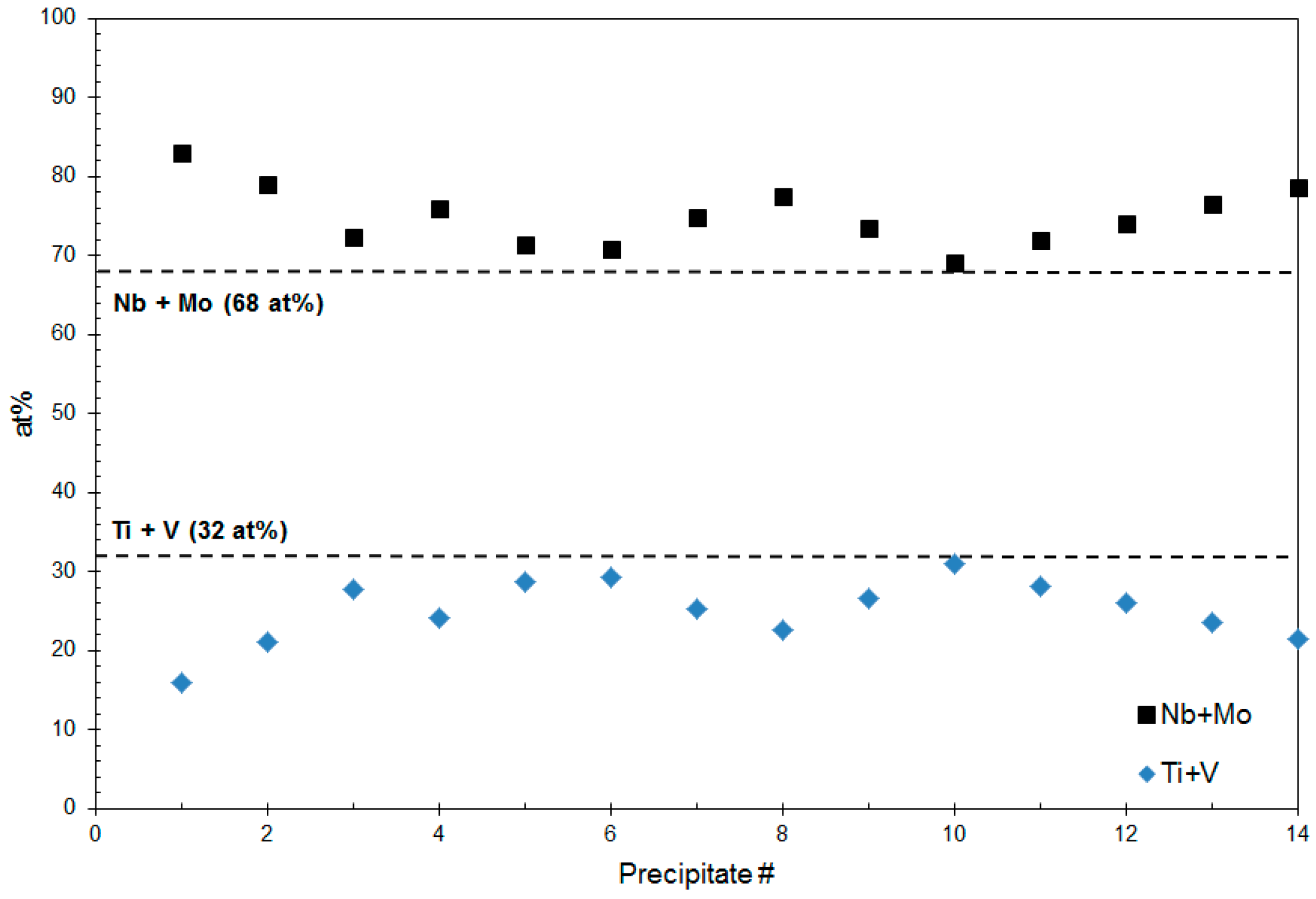
4.1. Composition Analysis
4.1.1. Composition of Precipitates >90 nm in Size
4.1.2. Composition of Precipitates <10 nm in Size
4.2. Size (Lvol) Analysis
4.2.1. Size of Precipitates >90 nm
4.2.2. Size of the Group 4 Precipitates
5. Conclusions
- Quantitative X-ray diffraction (QXRD) via the Rietveld method can be used to quantify both the average size and composition of nano-size precipitates in a microalloyed steel. The average predicted Lvol size of 4.7 nm corresponds well with precipitate size analysis conducted using transmission electron microscopy (TEM) (3.9 nm) and small angle neutron scattering (SANS) (5.5 nm). The predicted composition of the nano-size precipitates ((Nb0.50Ti0.32M0.18)(C0.59N0.41)) also compares favorably with the individual compositions measured by energy dispersive X-ray (EDX) analysis.
- The variation in the Nb and Ti composition (as measured with EDX) of the relative large (>90 nm) precipitates present in the Grade 100 steel limited the microstructure information that could be obtained from QXRD. In particular, the Lvol predicted by quantitative XRD was inconsistent with the individual precipitate sizes physically measured.
- QXRD can be used to quantify the effect of TCMP processing on precipitation in a microalloyed steel, as long as the limitations are adequately accounted for.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sharma, U. Microstructural Characterization of Microalloyed Linepipe Steels. M.Sc. Thesis, University of Alberta, Edmonton, Canada, 1999. [Google Scholar]
- Akhlaghi, S.; Ivey, D.G. Precipitation behaviour of a grade 100 structural steel. Can. Metall. Q. 2002, 41, 111–119. [Google Scholar] [CrossRef]
- Lu, J. Quantitative microstructural characterization of microalloyed steels. Ph.D. Thesis, University of Alberta, Edmonton, Canada, 2009. [Google Scholar]
- Elwazri, A.M.; Varano, R.; Siciliano, F.; Bai, D.; Yue, S. Characterization of precipitation of niobium carbide using carbon extraction replicas and thin foils by FESEM. Mater. Sci. Technol. 2006, 22, 537–541. [Google Scholar] [CrossRef]
- Nagarajan, V.; Palmiere, E.J.; Sellars, C.M. New approach for modelling strain induced precipitation of Nb(C,N) in HSLA steels during multipass hot deformation in austenite. Mater. Sci. Technol. 2009, 25, 1168–1174. [Google Scholar] [CrossRef]
- Charleux, M.; Poole, W.J.; Militzer, M.; Deschamps, A. Precipitation behaviour and its effect on strengthening of an HSLA-Nb/Ti steel. Metall. Mater. Trans. A 2001, 32A, 1635–1647. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ramisetti, N.K.; Misra, R.D.K.; Hartmann, J.; Jansto, S.G. Microstructure and high strength-toughness combination of a new 700 MPa Nb-microalloyed pipeline steel. Mater. Sci. Eng. A 2008, A478, 26–37. [Google Scholar] [CrossRef]
- Lee, W.; Hong, S.G.; Park, C.G.; Park, S.H. Carbide precipitation and high-temperature strength of hot-rolled high-strength, low-alloy steels containing Nb and Mo. Metall. Mater.Trans. A 2002, 33A, 1689–1698. [Google Scholar] [CrossRef]
- Fatehi, A.; Calvo, J.; Elwazri, A.M.; Yue, S. Strengthening of HSLA steels by cool deformation. Mater. Sci. Eng. A 2010, A27, 4233–4240. [Google Scholar] [CrossRef]
- Wiskel, J.B.; Ivey, D.G.; Henein, H. The effects of finish rolling temperature and cooling interrupt conditions on precipitation in microalloyed steels using small angle neutron scattering. Metall. Trans. A 2008, 39B, 116–124. [Google Scholar] [CrossRef]
- Hua, M.; Garcia, C.I.; Deardo, A.J. Precipitation behaviour in ultra-low-carbon steels containing titanium and niobium. Metall. Trans. A 1997, 28A, 1769–1780. [Google Scholar] [CrossRef]
- Deardo, A.J. Niobium in modern steels. Int. Met. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Cheary, R.W.; Ma-Sorrell, Y. Quantitative phase analysis by X-ray diffraction of martensite and austenite in strongly oriented orthodontic stainless steel wires. J. Mater. Sci. 2000, 35, 1105–1113. [Google Scholar] [CrossRef]
- Martiez-Blanco, D.; Gorria, P.; Blanco, J.A.; Perez, M.J.; Campo, J. Analysis of the diffraction-line broadening on nanostructured Fe: Size-strain effects induced by milling and heating. J. Phys. Cond. Mater. 2008, 2, 1–10. [Google Scholar]
- Balzar, D.; Audebrand, N.; Daymond, M.R.; Fitch, A.; Hewat, A.; Langford, J.I.; le Bail, A.; Loue, D.; Masson, O.; McCowan, C.N.; et al. Size-strain line-broadening analysis of the ceria round-robin sample. J. Appl. Crystallogr. 2004, 37, 911–924. [Google Scholar] [CrossRef]
- Pourghahramani, P.; Forssberg, E. The characterization of structural changes in hematite ground in a confined particle bed using Rietveld analysis. Int. J. Miner. Process. 2007, 83, 47–59. [Google Scholar] [CrossRef]
- Langfordy, J.I.; Louer, D. Powder diffraction. Rep. Prog. Phys. 1996, 59, 131–234. [Google Scholar] [CrossRef]
- Young, R.A. Introduction to the Rietveld Method. In The Rietveld Method; Young, R.A., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 1–30. [Google Scholar]
- Snyder, R.L. Analytical profile fitting of X-ray powder diffraction profiles. In The Rietveld Method; Young, R.A., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 112–131. [Google Scholar]
- Delhez, R.D.; de Keijser, T.H.; Langford, J.I.; Louer, D.; Mittemeijer, E.J.; Sonneveld, E.J. Crystal imperfection broadening and peak shape in the Rietveld method. In The Rietveld Method; Young, R.A., Ed.; Oxford University Press: Oxford, UK, 1993; pp. 132–166. [Google Scholar]
- Kuzel, R.; Holy, V.; Cernansky, M.; Kubena, J.; Simek, D.; Kub, J. Study of submicrocrystalline materials by diffuse scattering in transmitted waves. In Diffraction Analysis of the Microstructure of Material; Mittemeijer, E.J., Scardi, P., Eds.; Springer: New York, NY, USA, 1984; pp. 229–245. [Google Scholar]
- Balzar, D.; Popa, N.C. Crystallite size and residual strain/stress modelling in Rietveld refinement. In Diffraction Analysis of the Microstructure of Material; Mittemeijer, E.J., Scardi, P., Eds.; Springer: New York, NY, USA, 1984; pp. 125–145. [Google Scholar]
- Coelho, A. Topas Academic Version 4.1; software for analysis of powder diffraction data, Coelho software: Brisbane, Australia, 2007.
- Scardi, P; Leoni, M. Whole profile pattern modelling: theory and applications. In Diffraction Analysis of the Microstructure of Material; Mittemeijer, E.J., Scardi, P., Eds.; Springer: New York, NY, USA, 1984; pp. 51–91. [Google Scholar]
- Balzar, D. Voigt function model in diffraction-line broadening analysis. In Microstructure Analysis from Diffraction; Snyder, R.L., Bunge, H.J., Fiala, J., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 94–124. [Google Scholar]
- He, J.; Laverna, E.J.; Mateczyk, D.; Bampton, C.; Schoenung, J.M. Quantitative analysis of grain size in bimodal powders by X-ray diffraction and transmission electron microscopy. J. Mater. Sci. 2004, 39, 6957–6964. [Google Scholar] [CrossRef]
- Vives, S.; Gaffet, E.; Meunier, C. X-ray diffraction line profile analysis of iron ball milled powders. Mater. Sci. Eng. 2004, A366, 229–238. [Google Scholar] [CrossRef]
- Poorhaydari-Anaraki, K. Microstructure and Property Examination of the Weld HAZ in Grade 100 Microalloyed Steel. Ph.D. Thesis, University of Alberta, Edmonton, Canada, 2005. [Google Scholar]



| Parameter | Value | Parameter | Value |
|---|---|---|---|
| filament length | 12 mm | primary soller angle | 2.3° |
| sample length | 20 mm | axial beta | 20° |
| receiving slit length | 50 mm | axial del | 0.0053° |
| slit width | 0.15 mm | - | - |
| Group | Atomic Composition | wt. % | a (Å) | Lvol (nm) | εo (%) |
|---|---|---|---|---|---|
| 1 | TiN | 12.9 | 4.25 | 70.0 | 8.8 |
| 2 | Ti0.87Nb0.13N | 31.7 | 4.28 | 115.0 | 30.1 |
| 3 | Nb0.82Ti0.18C0.87N0.13 | 13.1 | 4.42 | 40.3 | 9.9 |
| 4 | Nb0.50Ti0.32Mo0.18C0.59N0.41 | 42.2 | 4.39 | 4.7 | 25.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiskel, J.B.; Lu, J.; Omotoso, O.; Ivey, D.G.; Henein, H. Characterization of Precipitates in a Microalloyed Steel Using Quantitative X-ray Diffraction. Metals 2016, 6, 90. https://doi.org/10.3390/met6040090
Wiskel JB, Lu J, Omotoso O, Ivey DG, Henein H. Characterization of Precipitates in a Microalloyed Steel Using Quantitative X-ray Diffraction. Metals. 2016; 6(4):90. https://doi.org/10.3390/met6040090
Chicago/Turabian StyleWiskel, J. Barry, Junfang Lu, Oladipo Omotoso, Douglas G. Ivey, and Hani Henein. 2016. "Characterization of Precipitates in a Microalloyed Steel Using Quantitative X-ray Diffraction" Metals 6, no. 4: 90. https://doi.org/10.3390/met6040090





