Composition Distribution and Electrochemical Behavior of an Ni2Al3 Coating on Q235 Steel
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Electrochemical Tests
3. Results and Discussion
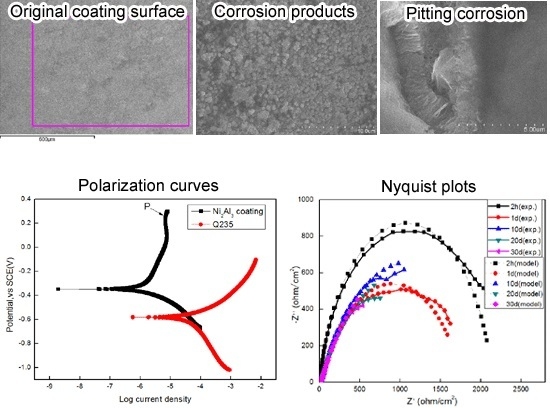
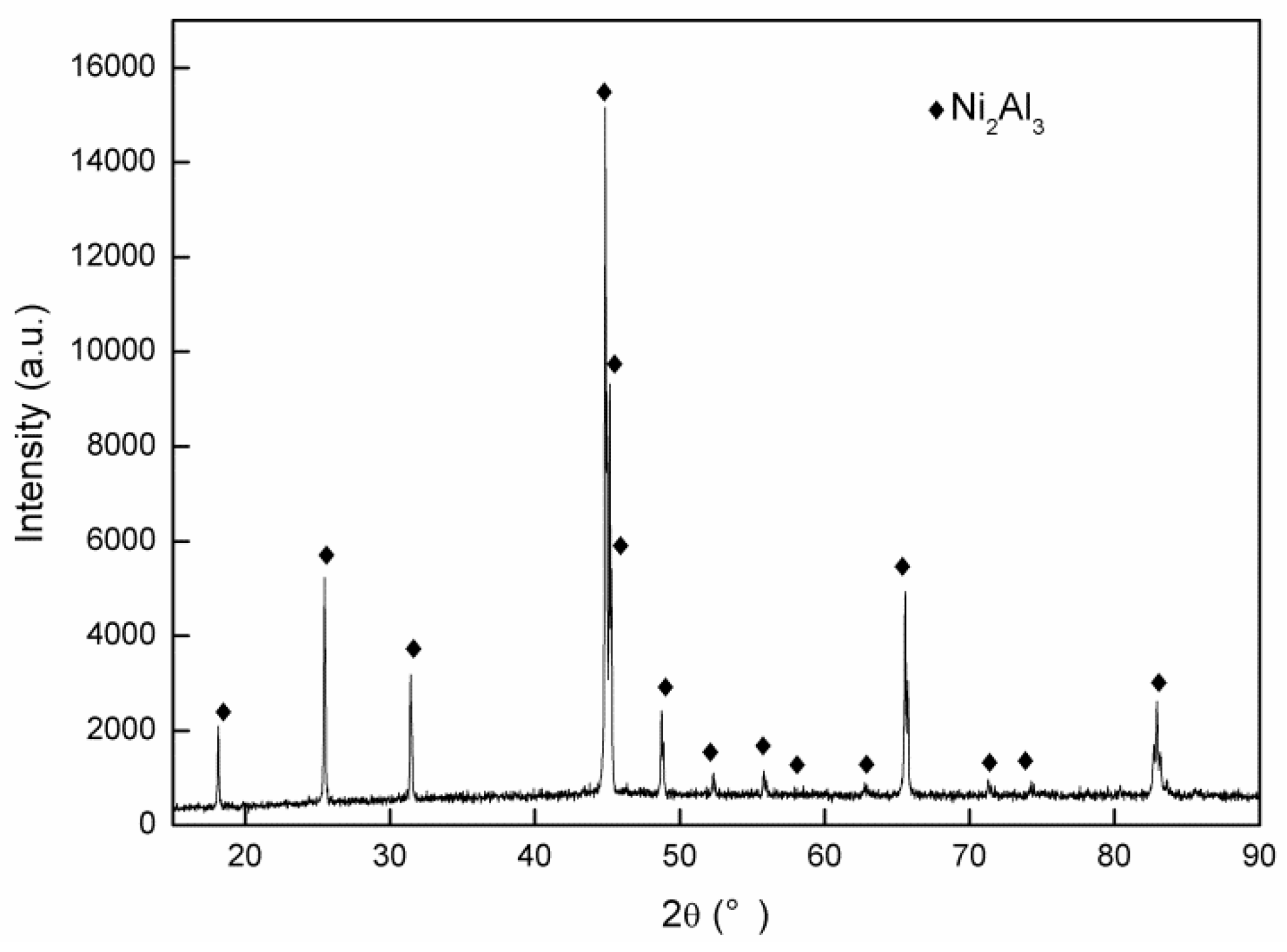
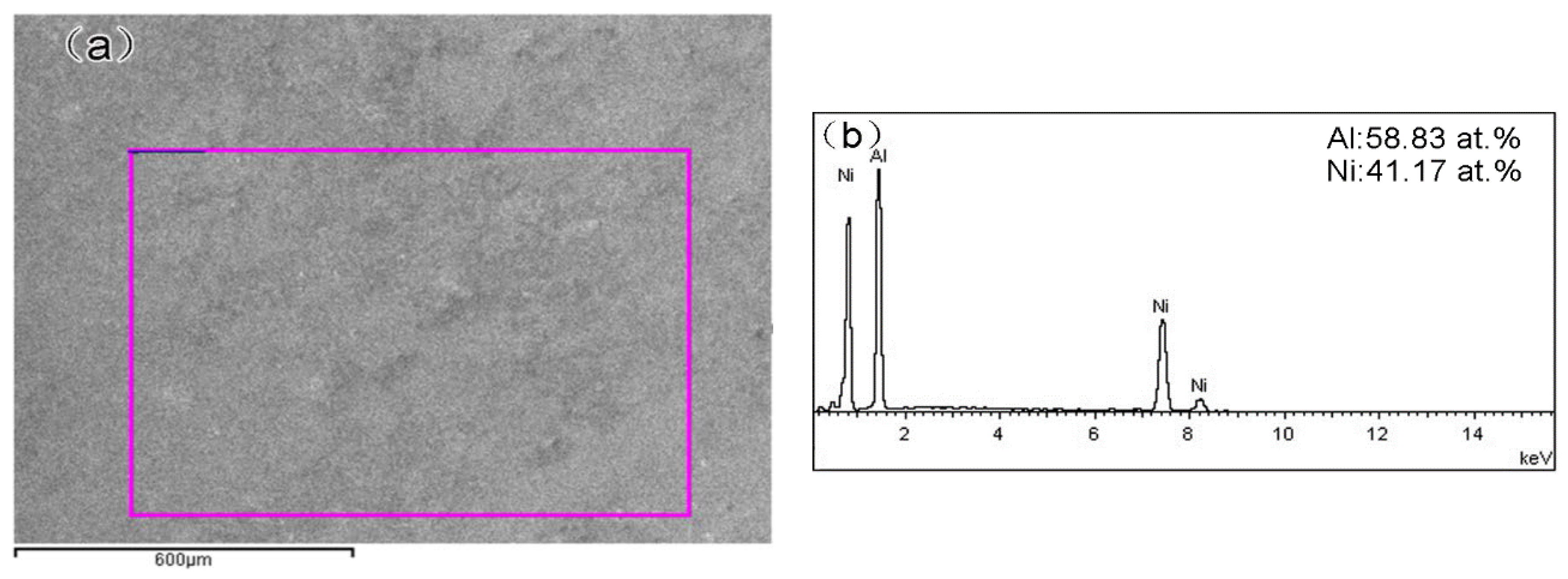
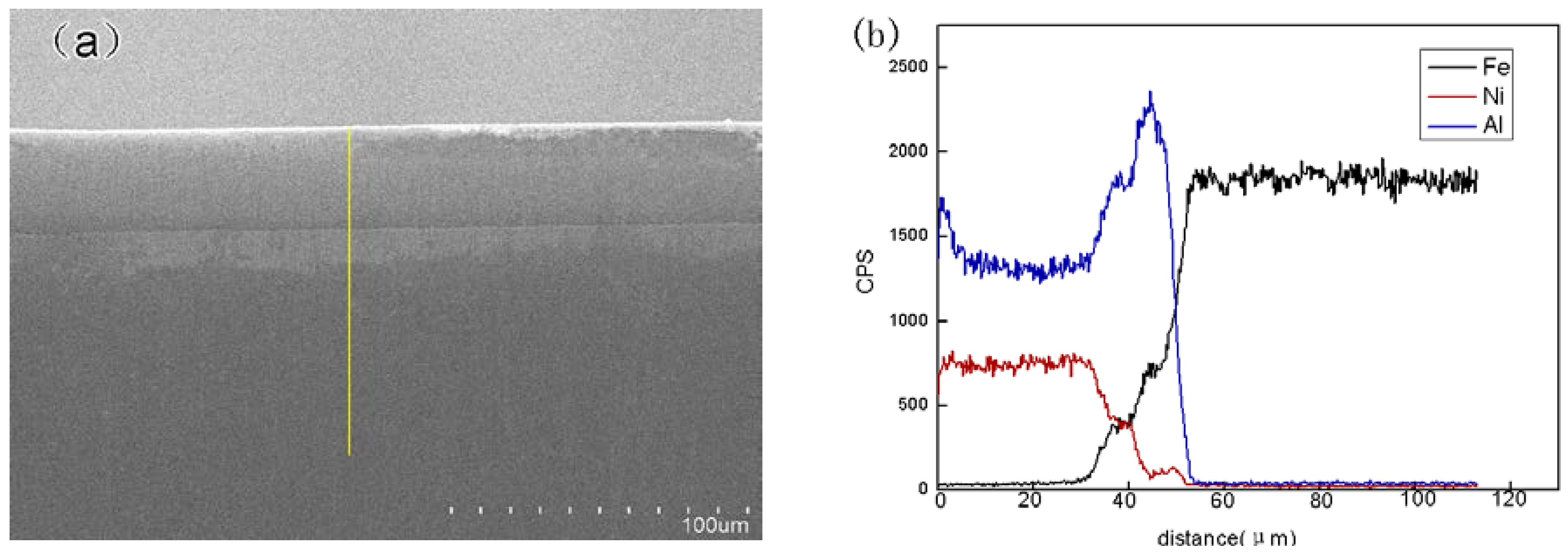
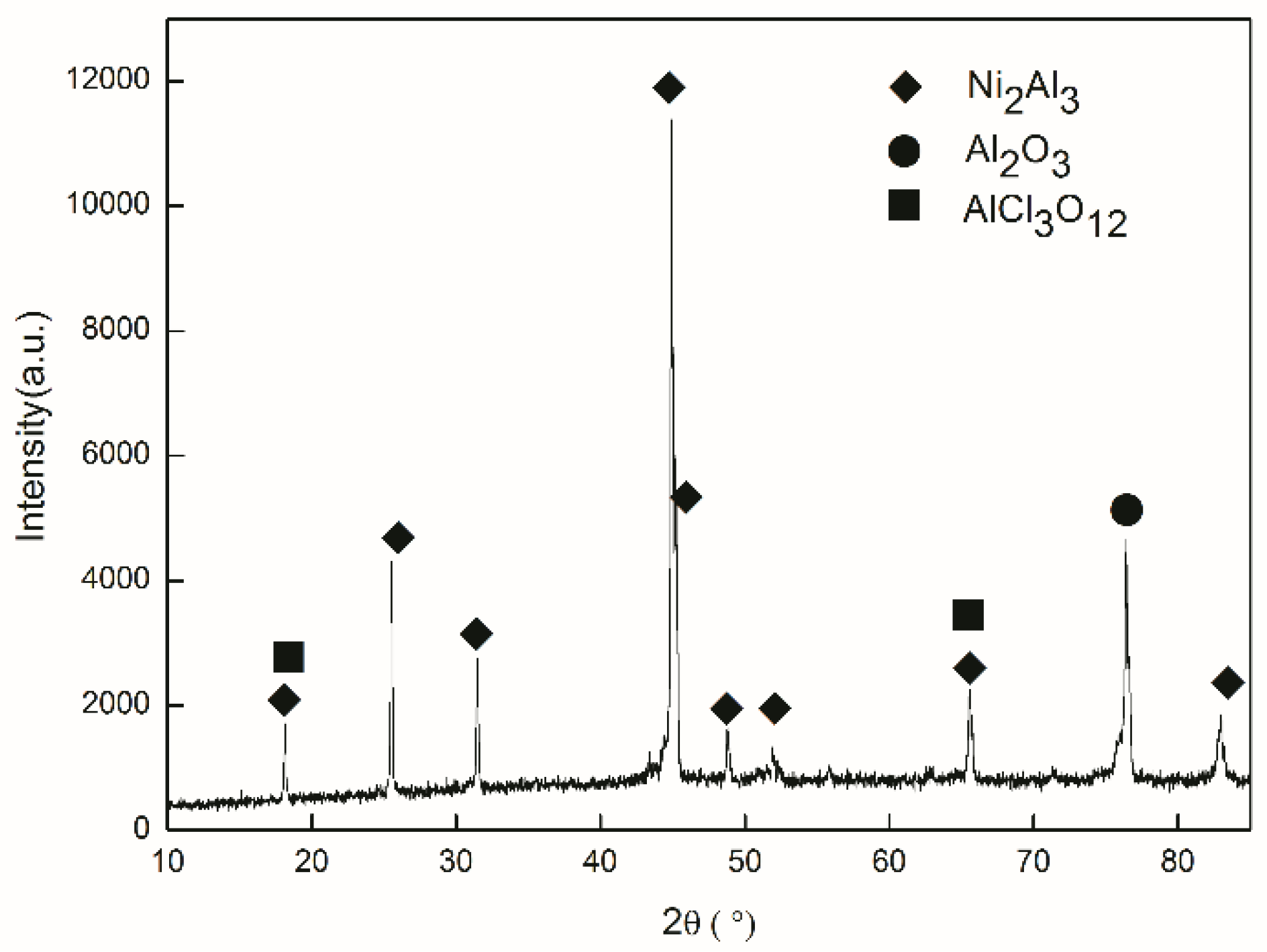
3.1. Coating Characterization
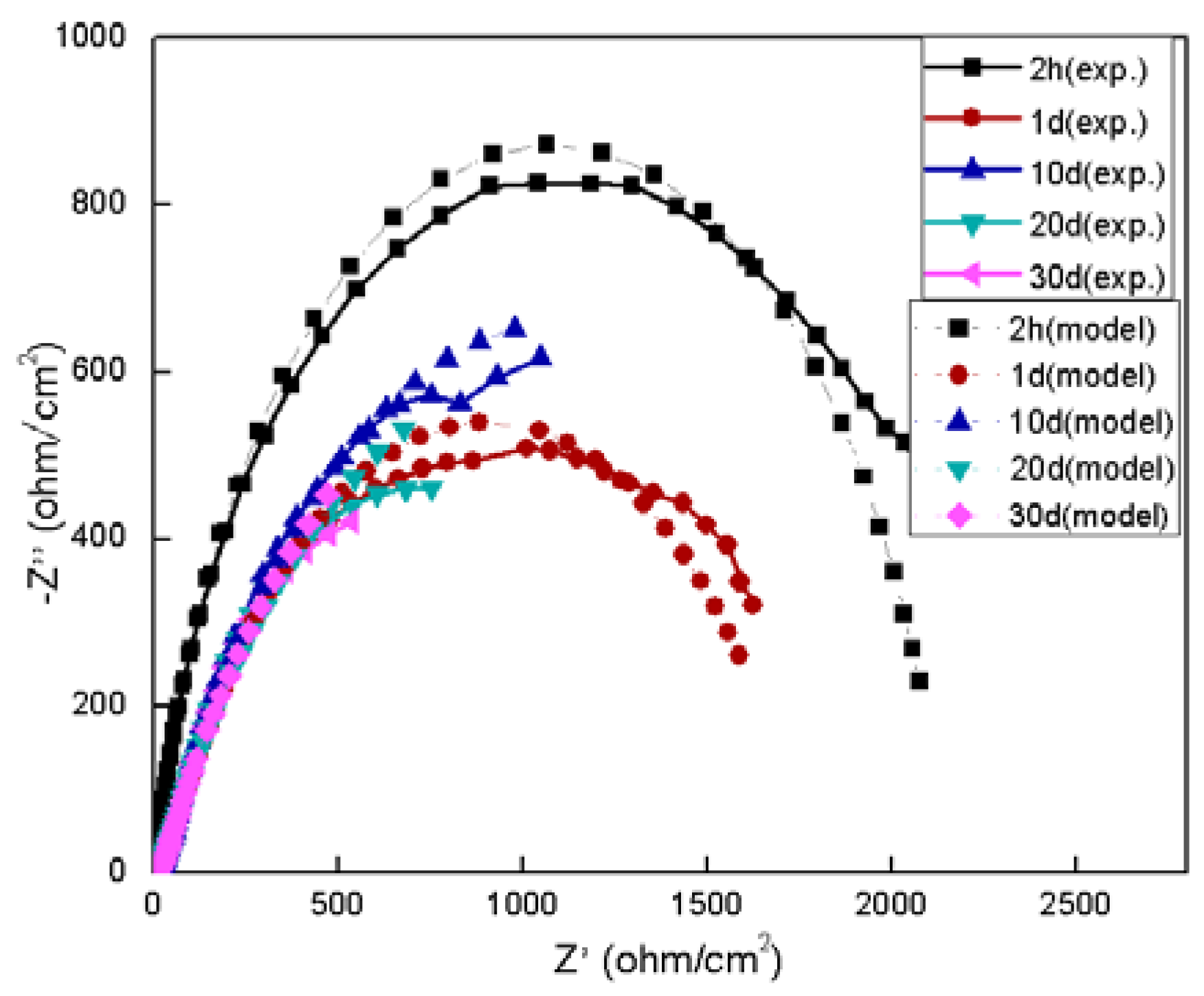
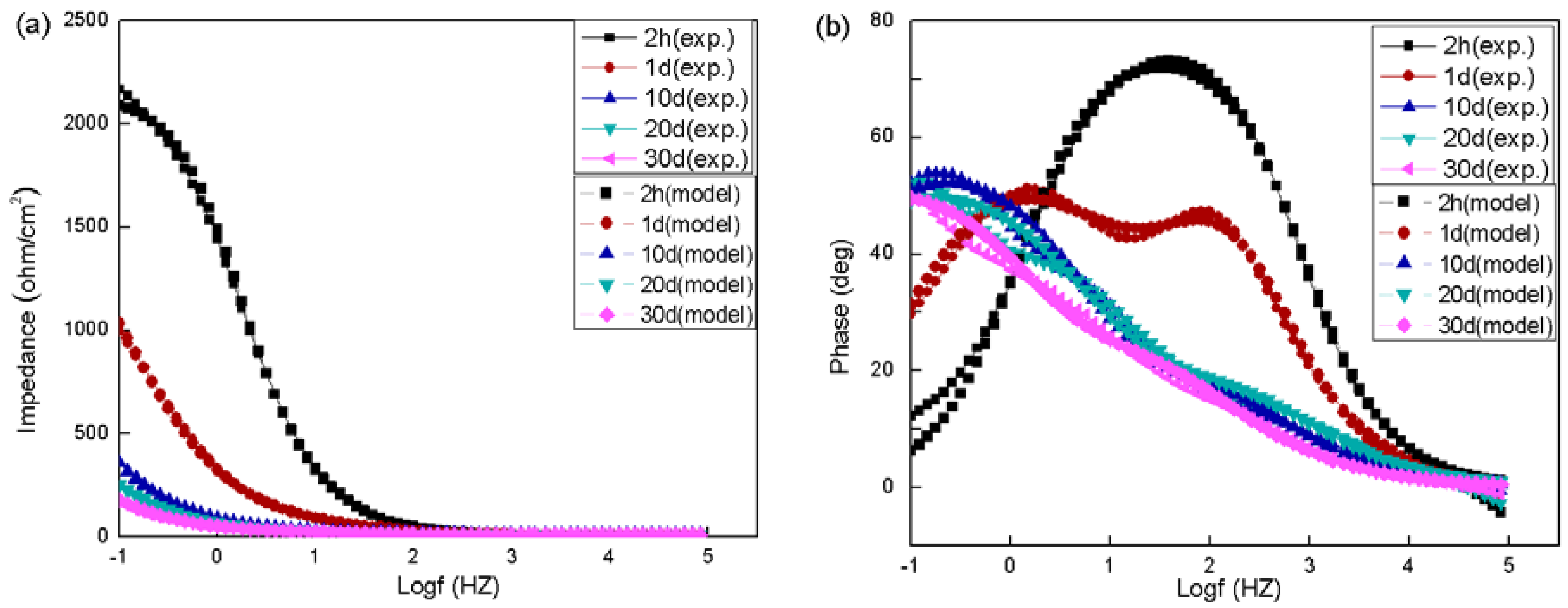
3.2. Electrochemical Behavior
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Shirvani, K.; Firouzi, S.; Rashidghamat, A. Microstructures and cyclic oxidation behavior of Pt-free and low-Pt NiAl coatings on the Ni-base superalloy Rene-80. Corros. Sci. 2012, 55, 378–384. [Google Scholar] [CrossRef]
- Hou, S.J.; Zhu, S.L.; Zhang, T.; Wang, F.H. A magnetron sputtered microcrystalline β-NiAl coating for SC superalloys. Part I. Characterization and comparison of isothermal oxidation behavior at 1100 °C with a NiCrAlY coating. Appl. Surf. Sci. 2015, 324, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W. Microstructures, properties and high-temperature carburization resistances of HVOF thermal sprayed NiAl intermetallic-based alloy coatings. Surf. Coat. Technol. 2004, 183, 18–28. [Google Scholar] [CrossRef]
- La, P.Q.; Bai, M.W.; Xue, Q.J.; Liu, W.M. A study of Ni3Al coating on carbon steel surface via the SHS casting route. Surf. Coat. Technol. 1999, 113, 44–51. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Puri, D.; Prakash, S. Characterisations of plasma sprayed and laser remelted NiCrAlY bond coats and Ni3Al coatings on boiler tube steels. Mater. Sci. Eng. A 2004, 368, 149–158. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Prakash, S. Evaluation of the corrosion behavior of plasma-sprayed Ni3Al coatings on steel in oxidation and molten salt environments at 900 °C. Surf. Coat. Technol. 2003, 166, 89–100. [Google Scholar] [CrossRef]
- Mohammadnezhad, M.; Shamanian, M.; Enayati, M. Formation of nanostructured NiAl coating on carbon steel by using mechanical alloying. Appl. Surf. Sci. 2012, 263, 730–736. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Rose, S.R.; Datta, P.K. Low-temperature formation and oxidation resistance of nickel aluminide/nickel hybrid coatings on alloy steels. Scr. Mater. 2008, 59, 99–102. [Google Scholar] [CrossRef]
- Katsman, A.; Ginzburg, A.; Werber, T.; Cohen, I.; Levin, L. Nickel-aluminide coating of TiAl by a two-stage process. Surf. Coat. Technol. 2000, 127, 220–223. [Google Scholar] [CrossRef]
- Hickl, A.J.; Heckel, R.W. Kinetics of phase layer growth during aluminide coating of nickel. Metall. Trans. A 1975, 6, 431–440. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Formation of nickel aluminide/nickel hybrid coatings on alloy steels by two step process of nickel plating and low temperature pack aluminisation. Mater. Sci. Technol. 2009, 25, 733–738. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Lin, K.L. Electrochemical corrosion study of Sn-X Ag-0.5 Cu alloys in 3.5% NaCl solution. J. Mater. Res. 2007, 22, 2573–2581. [Google Scholar] [CrossRef]
- Kaiser, M.S.; Dutta, S. Corrosion behavior of aluminium engine block in 3.5% NaCl solution. J. Mater. Sci. Chem. Eng. 2014, 2, 52–58. [Google Scholar]
- Mohanty, U.S.; Lin, K.L. Electrochemical corrosion behavior of lead-free Sn-8.5 Zn-X Ag-0.1 Al-0.5 Ga solder in 3.5% NaCl solution. Mater. Sci. Eng. A 2005, 406, 34–42. [Google Scholar] [CrossRef]
- Lv, D.M.; Ou, J.F.; Xue, M.H.; Wang, F.J. Stability and corrosion resistance of superhydrophobic surface on oxidized aluminum in NaCl aqueous solution. Appl. Surf. Sci. 2015, 333, 163–169. [Google Scholar] [CrossRef]
- Souza, V.A.D.; Neville, A. Linking electrochemical corrosion behavior and corrosion mechanisms of thermal spray cermet coatings (WC-CrNi and WC/CrC-CoCr). Mater. Sci. Eng. A 2003, 352, 202–211. [Google Scholar] [CrossRef]
- Hsu, R.W.W.; Yang, C.C.; Huang, C.A.; Chen, Y.S. Electrochemical corrosion properties of Ti-6Al-4V implant alloy in the biological environment. Mater. Sci. Eng. A 2004, 380, 100–109. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Y.F.; Zhao, L.C. Electrochemical corrosion behavior of Ti44Ni47Nb9 alloy in simulated body fluids. Mater. Sci. Eng. A 2006, 438–440, 504–508. [Google Scholar] [CrossRef]
- Javadian, S.; Yousefi, A.; Neshati, J. Synergistic effect of mixed cationic and anionic surfactants on the corrosion inhibitor behavior of mild steel in 3.5% NaCl. Appl. Surf. Sci. 2013, 285, 674–681. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, Y.; Huang, S.; Zhou, X.A. Study of the corrosion behavior and the corrosion films formed on the surfaces of Mg-x Sn alloys in 3.5 wt. % NaCl solution. Appl. Surf. Sci. 2014, 317, 1143–1150. [Google Scholar] [CrossRef]
- Madaoui, N.; Saoula, N.; Zaid, B.; Saidi, D.; Ahmed, A.S. Structural, mechanical and electrochemical comparison of TiN and TiCN coatings on XC48 steel substrates in NaCl 3.5% water solution. Appl. Surf. Sci. 2014, 312, 134–138. [Google Scholar] [CrossRef]
- Bai, Z.H.; Xia, Y.M.; Qiu, F.; Liu, Y.Y.; Hu, W.; Jiang, Q.C. Effects of RExOy addition on corrosion behavior of the Al-Cu alloys in 3.5 wt. % NaCl solution and pH = 4 acid solution. Appl. Surf. Sci. 2014, 307, 153–157. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Yin, Z.F.; Zhao, W.Z.; Lai, W.Y.; Zhao, X.H. Electrochemical behaviour of Ni-base alloys exposed under oil/gas field environments. Corros Sci. 2009, 51, 1702–1706. [Google Scholar] [CrossRef]
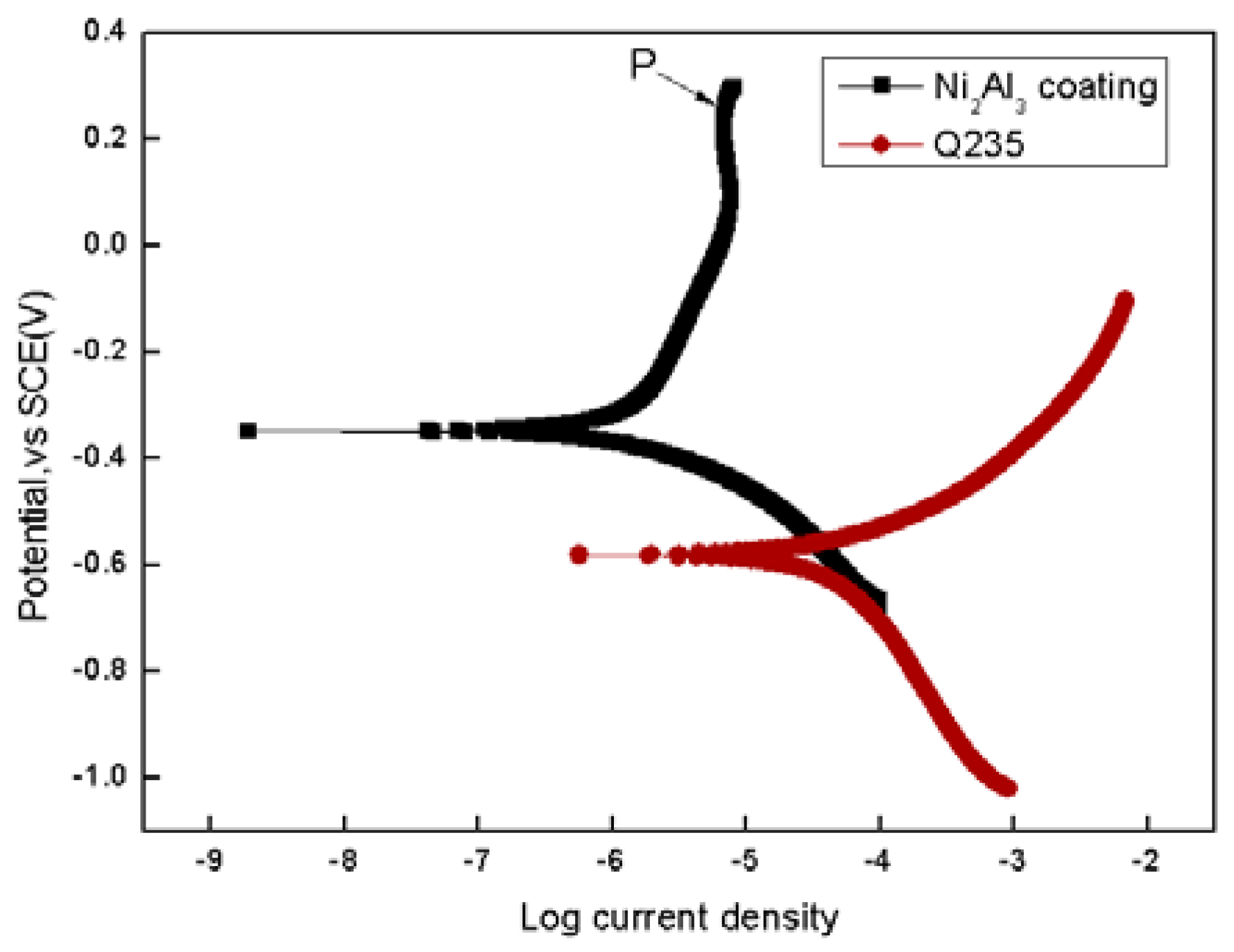
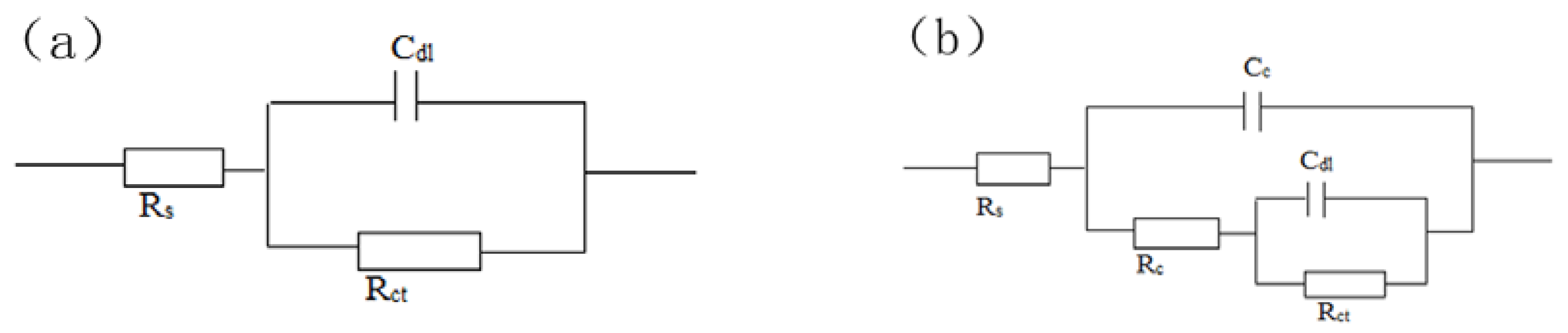

| Materials | βa (V) | βc (V) | Rp (ohm × cm−2) | Icorr (μA × cm−2) |
|---|---|---|---|---|
| Q235 | 0.822 | 0.121 | 1083 | 42.27 |
| Ni2Al3 coating | 0.116 | 0.213 | 9626 | 3.401 |
| Time | Rs | Cc | Rc | Cdl | Rct | ||
|---|---|---|---|---|---|---|---|
| (ohm·cm−2) | Y0(ohm·cm−2·sn) | n | (ohm·cm−2) | Y0(ohm·cm−2·sn) | n | (ohm·cm−2) | |
| 2 h | 6.765 | - | - | - | 8.089 × 10−5 | 0.8696 | 2144 |
| 1 d | 8.331 | 1.803 × 10−4 | 0.8232 | 78.65 | 6.963 × 10−4 | 0.6837 | 1691 |
| 10 d | 11.59 | 1.177 × 10−3 | 0.655 | 19.32 | 2.48 × 10−3 | 0.6943 | 2225 |
| 20 d | 8.072 | 1.401 × 10−3 | 0.6322 | 14.33 | 3.727 × 10−3 | 0.6395 | 2238 |
| 30 d | 9.741 | 3.491 × 10−3 | 0.5997 | 32.8 | 4.327 × 10−3 | 0.6828 | 2459 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wang, M.; Zheng, G.; Li, Y.; Chen, G. Composition Distribution and Electrochemical Behavior of an Ni2Al3 Coating on Q235 Steel. Metals 2016, 6, 58. https://doi.org/10.3390/met6030058
Li N, Wang M, Zheng G, Li Y, Chen G. Composition Distribution and Electrochemical Behavior of an Ni2Al3 Coating on Q235 Steel. Metals. 2016; 6(3):58. https://doi.org/10.3390/met6030058
Chicago/Turabian StyleLi, Ningning, Minzhi Wang, Gong Zheng, Yongsheng Li, and Guang Chen. 2016. "Composition Distribution and Electrochemical Behavior of an Ni2Al3 Coating on Q235 Steel" Metals 6, no. 3: 58. https://doi.org/10.3390/met6030058
APA StyleLi, N., Wang, M., Zheng, G., Li, Y., & Chen, G. (2016). Composition Distribution and Electrochemical Behavior of an Ni2Al3 Coating on Q235 Steel. Metals, 6(3), 58. https://doi.org/10.3390/met6030058