2.5. Fluorite
Although fluorite is often regarded as a gangue mineral for metals production, high-grade fluorite has value in a variety of markets. The most notable use for fluorite ore is in the production of hydrofluoric acid used for the majority of fluorine compounds. It is also used in uranium production, aluminum production and as a flux.
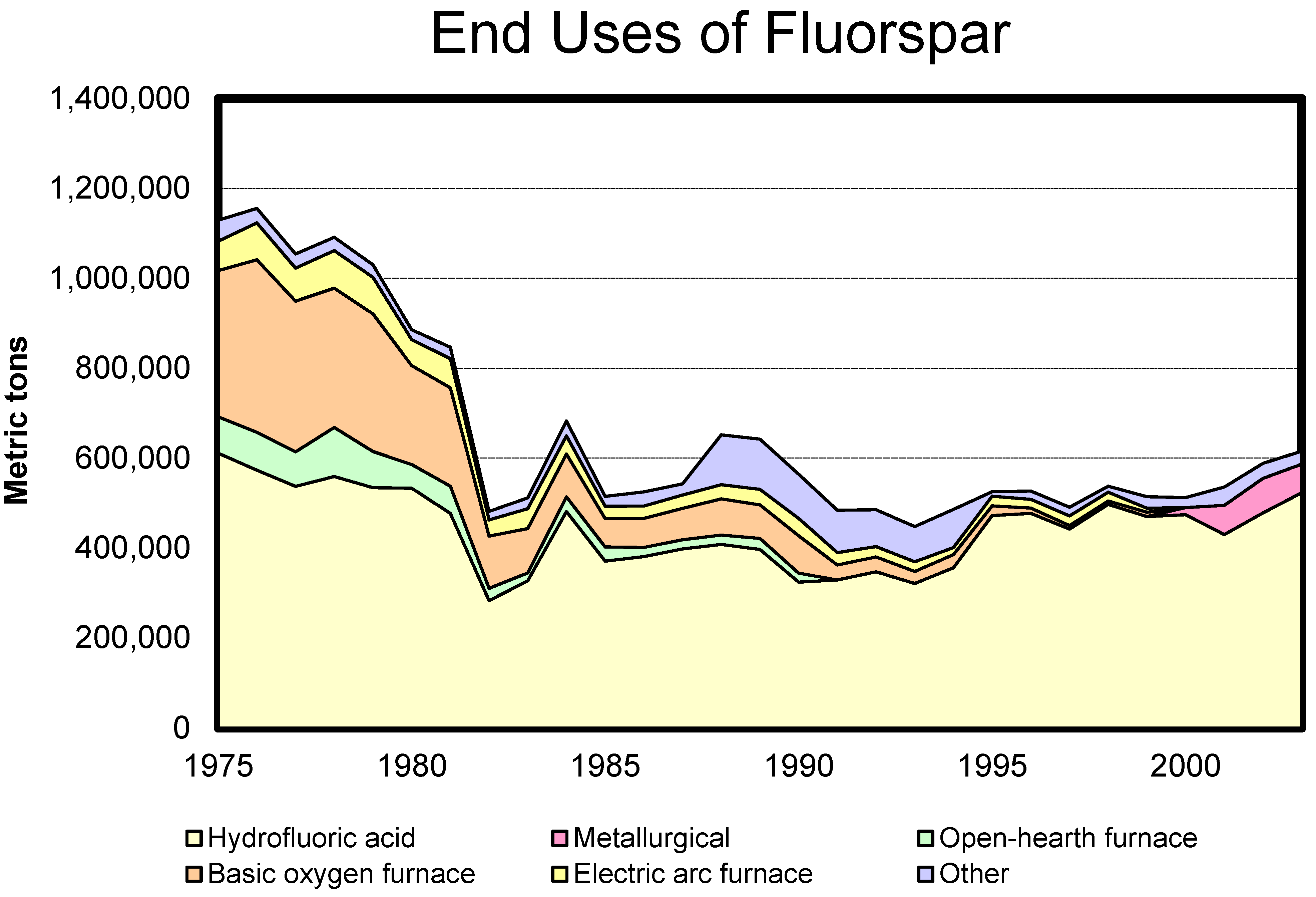
Figure 3 displays the relative amounts of fluorspar used in different applications over time.
Figure 3.
United States Geological Survey data showing the uses of fluorspar over the last few decades is illustrated [
10].
The traditional means to recover fluorspar involved utilizing ores that did not contain significant amounts calcium carbonate. Means to separate fluorite from silica were well established; however up until the 1940s, calcareous deposits were untreatable. Cullen and Lavers revealed a new method for fluorite flotation in 1936. Anderson, Stengl, and Trewartha set out to produce a high quality fluorite concentrate (no less than 97% CaF
2) from calcareous type deposits. The process involved grinding the material to around 150 microns, floating at a pH of 8 with fatty acid collectors and quebracho to depress the calcite and other gangue [
11]. Currently, quebracho and tannin extracts are the depressant of choice for generating high purity acid-grade fluorite concentrates.
The positive advantage of adding quebracho into the flotation circuit was seen in the study done by Clemmer and Anderson. The head assay of zinc and lead flotation tailings was 0.13% Pb, 0.45% Zn, 26.92% CaF
2, and 42.98% CaCO
3. The material was ground to below 100 mesh and treated with oleic acid and quebracho in a cell. The final concentrate assayed at 99% CaF
2, 0.03% Pb, and 0.05% Zn [
12]. With an enrichment ratio of 3.68, it is a very formidable process and proves to be viable for the production of fluorite in multiple applications.
The basic principle behind the success of quebracho in the fluorspar flotation circuit lies in the fact that it depresses the calcite, silicates, and metal sulfides much stronger than the fluorite. With respect to calcite, it is particularly challenging to separate from fluorite because both minerals have Ca
2+ cations in lattice. When quebracho is adsorbed, the surface becomes hydrophilic. Many attempts to describe and characterize the selective nature of quebracho have been undertaken. One of the primary insights involved the depression variance between tannin derivatives. It is clear through many studies that the condensed tannins, like that of quebracho, are far more selective than the hydrolysable tannins like that of tannic acid. The hydrolysable tannins have a greater depression affect to a disadvantage compared to the condensed tannins; the depression on the hydrolysable tannins is too strong and thus provides a less selective depression [
3].
The method of bonding also affects the behavior of tannins within a system. Plaskin and Shrader had several insights on how tannins interacted with the surfaces of minerals being floated with oleic acid. They determined the tannins to be semi-colloidal with hydrogen bonding being present. Tannins carry a negative surface charge due to the phenolic-OH groups present. Tannins thus react with the surfaces of minerals via chemisorption. This reaction isn’t exclusive to the mineral surface itself but also interacts with the collector being used. The electrokinetic potential of the mineral is slightly negative with the addition of small amounts of tannin. This negative surface charge repels anionic collectors causing depression, but attracts cationic collectors [
3].
The use of quebracho in a circuit relies heavily on the other reagents in the flotation scheme and their concentrations. In general, fatty acid collectors are used exclusively in fluorspar flotation with quebracho. Common collectors include oleic acid, sulphated soap, spermol, and oleate. The general range for collector addition is around 200–500 grams per ton while the tannins are similar at around 100–500 grams per ton. The Imperial College study determined that both the quebracho and the collector compete for adsorption on the surfaces of calcite and fluorite. Adsorption was observed for all scenarios of calcite and fluorite in the presence of quebracho and oleate. When only oleate was present in the system, fluorite adsorbed almost double the amount than the calcite. Adding only quebracho, the adsorption levels onto the calcite and the fluorite were very similar. Thus, it was shown that both the oleate and the quebracho played a significant role in the depression of calcite. With calcite holding less oleate than fluorite and quebracho adsorbing similarly onto both minerals, the fluorite floats and the calcite is depressed. In this manner, the order of conditioning is important. It was found that when the quebracho was added before the oleate, a greater depression of calcite took place and when the oleate was added prior to the quebracho, an increase in calcite flotation was observed [
3].
The effect of pH on the flotation of fluorite with quebracho has been studied quite thoroughly. In practical applications, the pH ranges from 8 to 9.5. This accounts for multiple factors of adsorption of the quebracho and the oleate, the acid consumption, and practical recovery and grade balances. The Imperial College study indicated that quebracho remains a depressant up until a pH of around 10. The optimal adsorption pH for quebracho on fluorite surface is around 7, while the dissolution of calcite requires the pH be at or above 8 [
3]. The work done by Clarence Thom suggests that pre-conditioning the ore at a pH less alkaline than that of optimal flotation levels improves the separation. Thom had optimum recovery when the quebracho conditioning was carried out at a lower pH (ideally below 8) before soda ash and oleic acid were added [
13]. While the pH of the cell has been studied extensively, temperature could be a new parameter of interest.
Another aspect that has been studied with regards to fluorite flotation is the effect of soluble ions within the system—specifically calcium ions. As both fluorite and calcite are marginally soluble, it can be concluded that a small amount of calcium ions will be present in the aqueous solution. The Imperial College work acknowledged that adding different concentrations of calcium ions into the solution, by means of calcium chloride, affected the flotation. At concentrations greater than 10
−4 M calcium ions, the fluorite was severely depressed, keeping all other factors constant. However, when the concentration of calcium is slightly lower, on the order of 10
−5 M, the calcite is heavily depressed, while the fluorite floats. In industry, it is common practice to use softened water to avoid the negative affect of dissolved calcium ions on the flotation of fluorite [
3].
In the patent from Allen and Allen, the generation of acid grade fluorspar, 97% CaF2 or greater, was investigated. The main research done involved the addition of ferrous sulfate salts and the effect of quebracho in the circuit. It was shown that a crude fluorspar ore assaying at 87.23% CaF2, 4.68% SiO2, and 4.66% CaCO3 was significantly upgraded to 97.48% CaF2 with a recovery of 91 percent. In this example, the material was crushed and fed to a wet ball mill unit running at 70 percent solids where 0.275 pounds of ferrous sulfate was added. The material was reduced to 89% passing 200 mesh, was flocculated, thickened and transferred to a conditioning tank. Reagents on the amounts of 2.2 pounds per ton of stearic fatty acid, 3.3 pounds per ton Acintol FA2 tall oil fatty acid, and 0.97 pounds of sodium carbonate were added to the conditioning cell at 207 °F for 20 min. Quebracho was added at the conditioner tank discharge at 0.18 pounds per ton of crude ore in addition to 3.67 pounds per ton of sodium silicate. This was fed to the first flotation cells, which produced a heavily mineralized fluorspar froth while maintaining the pH between 8 and 9. Quebracho was further used in the subsequent two cleaning stages at 0.37 pounds per ton. From this study, it was concluded that the addition of soluble ferrous sulfate salts (on the order of 0.2 to 0.8 pounds per ton) in combination with quebracho aided in depressing gangue minerals present including silica and calcium carbonate, whilst improving the flotation of fluorite.
Allen and Allen also delved into the effect of different types of fatty acids. Fatty acids are the most common type of collectors for fluorite flotation. This study suggested that the use of saturated fatty acids was critical in the reagent scheme. They argued that using high concentrations of the traditional unsaturated fatty acid collectors, like oleic acid and tall oil acids, was not the best practice. Instead, it was recommended having a collector composition between 30 and 60 percent saturated fatty acids. The saturated fatty acids were preferably to have between 12 and 18 carbon atoms like those of stearic acid, lauric acid, and palmitic acid. Increased recoveries of fluorite were seen with the use of saturated fatty acids.
Allen and Allen also discovered that the use of guar within the system in addition to the reagents discussed above helped to further improve the flotation of fluorite. It was shown that when guar was added to the pulp the gangue minerals including barium sulfate, pyrite, and clay slimes were significantly depressed. It was suggested that the guar be introduced into the circuit in the mill or the conditioner when the ferrous sulfate and depressants are added [
14].
While fluorite flotation is conventionally carried out in a standard bank of cells with multiple stages comprised of roughers, scavengers and cleaners, column flotation also proves to be a successful method. The Fish Creek fluorite processing plant in Eureka County Nevada proved column flotation to be superior due to increased recovery [
15]. The work done by the Gujarat Mineral Development Corporation in Kadipani proved column flotation to be more advantageous than that of the traditional route. The original processing circuit consisted of a rougher, scavenger and six stages of cleaning while the modified column flotation eliminated four of these stages. The differences between column and conventional flotation can be seen in
Table 3. The findings of this study showed that the use of tannins in a column flotation scheme as opposed to a traditional flotation circuit improved the overall separation of fluorite from the gangue consisting of CaCO
3, SiO
2, and P
2O
5 [
16].
Specific column flotation parameters were researched in fluorite flotation on an ore from the Nossa Senhora do Carmo Mining Company. The work focused on using a short column, described as the collection zone being 8 m tall, with a negative bias regime. Negative bias is obtained by having a smaller tailing output flow than the feed input flow. The cell was kept at 30% solids with a pH of 10 and sodium silicate, cornstarch, quebracho, and tall oil were added in sequence during the conditioning stage. The study concluded that increased recoveries were observed in this environment due to the negative bias allowing loaded air bubbles to more readily rise to create froth. While this scheme created a good environment for the recovery of fluorite, it should be used as a rougher as the grade of the concentrate is fairly low [
17].
Table 3.
Kadipani Flotation Data [16].
| | | Feed Assay % | | |
| | | Sample | CaF2 | CaCO3 | SiO2 | P2O5 | | |
| | | 1 | 65.84 | 5.62 | 16.68 | 3.14 | | |
| | | 2 | 74.51 | 5.10 | 10.82 | 2.16 | | |
| | | 3 | 78.8 | 4.36 | 10.60 | 1.88 | | |
| | | 4 | 84.69 | 4.67 | 4.57 | 1.78 | | |
| | | 5 | 89.42 | 4.23 | 2.56 | 0.89 | | |
| | | 6 | 90.58 | 2.43 | 3.00 | 1.20 | | |
| Concentrate Assay, % Conventional Flotation | Concentrate Assay, % Column Flotation |
| Sample | CaF2 | CaCO3 | SiO2 | P2O5 | CaF2 | CaCO3 | SiO2 | P2O5 |
| 1 | 76.80 | 5.62 | 6.73 | 2.40 | 82.80 | 5.12 | 3.70 | 3.20 |
| 2 | 80.86 | 6.60 | 6.16 | 2.13 | 88.82 | 4.86 | 1.47 | 1.41 |
| 3 | 80.01 | 2.40 | 9.80 | 2.01 | 92.56 | 1.65 | 1.53 | 1.35 |
| 4 | 88.77 | 4.64 | 4.57 | 1.78 | 91.80 | 3.40 | 1.06 | 1.07 |
| 5 | 91.75 | 3.31 | 1.64 | 0.72 | 95.21 | 1.59 | 0.60 | 0.18 |
| 6 | 92.10 | 3.61 | 1.95 | 0.68 | 94.35 | 2.12 | 1.07 | 0.58 |
A very common issue regarding flotation as a means of separation involves slime coatings. The heterocoagulation of a fluorite and gangue minerals was observed for a feed fluorite ore of the Minera de las Cuevas concentration plant in Mexico. The work focused on the flotation circuit with oleic acid as a collector and quebracho as a depressant with the addition of CMC and water glass as dispersants. SEM (scanning electron microscope) was used to observe the slime coatings of the gangue minerals, calcium carbonate and quartz, on the fluorite surface. It was found that strong heterocoagulation occurred around pH 9, which is close to general operating parameters for pH in a fluorite flotation circuit. The study suggested that this phenomenon may be due to strong electrical double layer attraction yet weak double layer repulsion between the gangue particles and the fluorite particles at these conditions. The addition of the dispersants CMC and water glass improved the flotation circuit by reducing the slime coatings; an increased recovery of fluorite from 72% to 78.5% was seen with the grade remaining continuous at 98% [
18].
The stability of the feed into a flotation circuit is a critical parameter to control, particularly as it affects the grades and recoveries of the valuable mineral in question. The work done by Schubert, Baldauf, Kramer, and Schoenherr on the development of fluorite flotation examined the effects of changing feed in high calcareous ores with the addition of quebracho, sodium hexametaphosphate, and oleoyl sarcosine. Oleoyl sarcosine with around 20% oleic acid was favored over the traditional collector containing 60%–70% oleic acid. The calcium carbonate content in the ores tested ranged from 7 percent to 45 percent while the fluorite ranged from 29 to 52 percent. As noted previously, the most important parameter to control was the concentrations of the reagents; specifically the amount of quebracho added. This study illustrated that quebracho had two major effects: when at low concentrations, depression is the primary rule and at higher concentrations the flotation of fluorite becomes activated. It was also explained that quebracho acts as a dispersant in the system particularly at high concentrations. The most favorable recoveries occurred in the pH range from 8–9 and in highly flocculated systems with a particle size from 0.04 to 0.16 mm [
19].
2.6. Sulfides: Copper-Lead-Zinc-Nickel Ores
To effectively separate polymetallic sulfide ores, depressants including lime, sulfites and cyanide in conjunction with xanthate collectors are traditionally used. Quebracho proves to be a promising alternative to these depressants and is favored for operating conditions, as the tannins are completely non-toxic and work at moderate pH levels [
3]. The order of depression by quebracho is shown below for sulfides:
Imperial College provided a comprehensive study on the effects of quebracho as a depressant for sulfides. The depressant action of quebracho was found to be a function of the –OH group content [
3]. Before this study, the quebracho form most commonly used for sulfides was Tupasol ATO; however, Tupafin ATO proved to be the most effective quebracho depressant form.
The primary aim in nearly all sulfide separation includes removing the pyrite content from the rest of the valuable sulfides. An important distinction of the form of pyrite was taken into account. As pyrite experiences high surface oxidation, both non-oxidized and oxidized pyrites were tested. In the presence of xanthate, the non-oxidized pyrite was sufficiently depressed by all three forms of quebracho. In the case of the oxidized pyrite, it is clear that quebracho is less effective for depression.
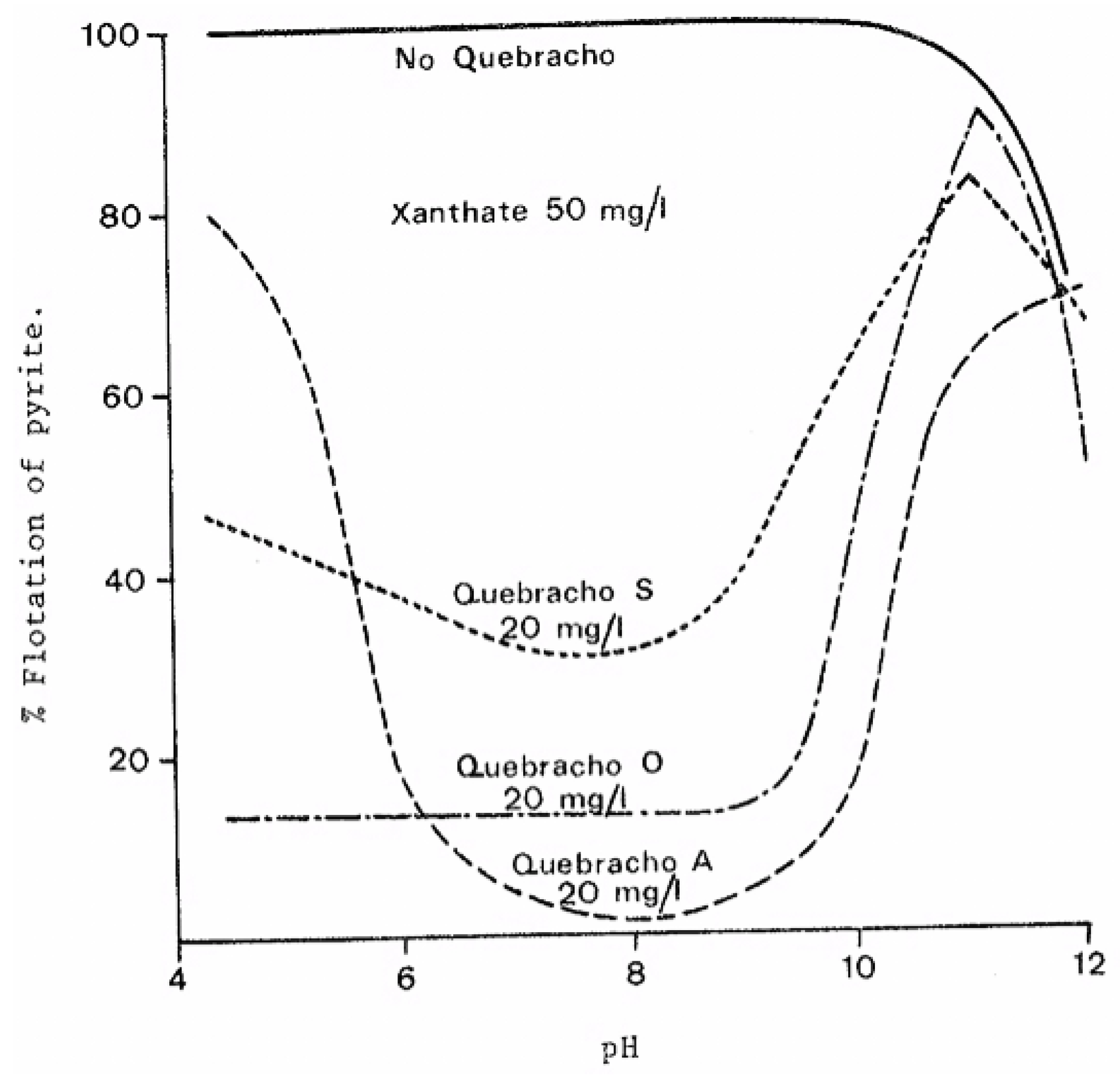
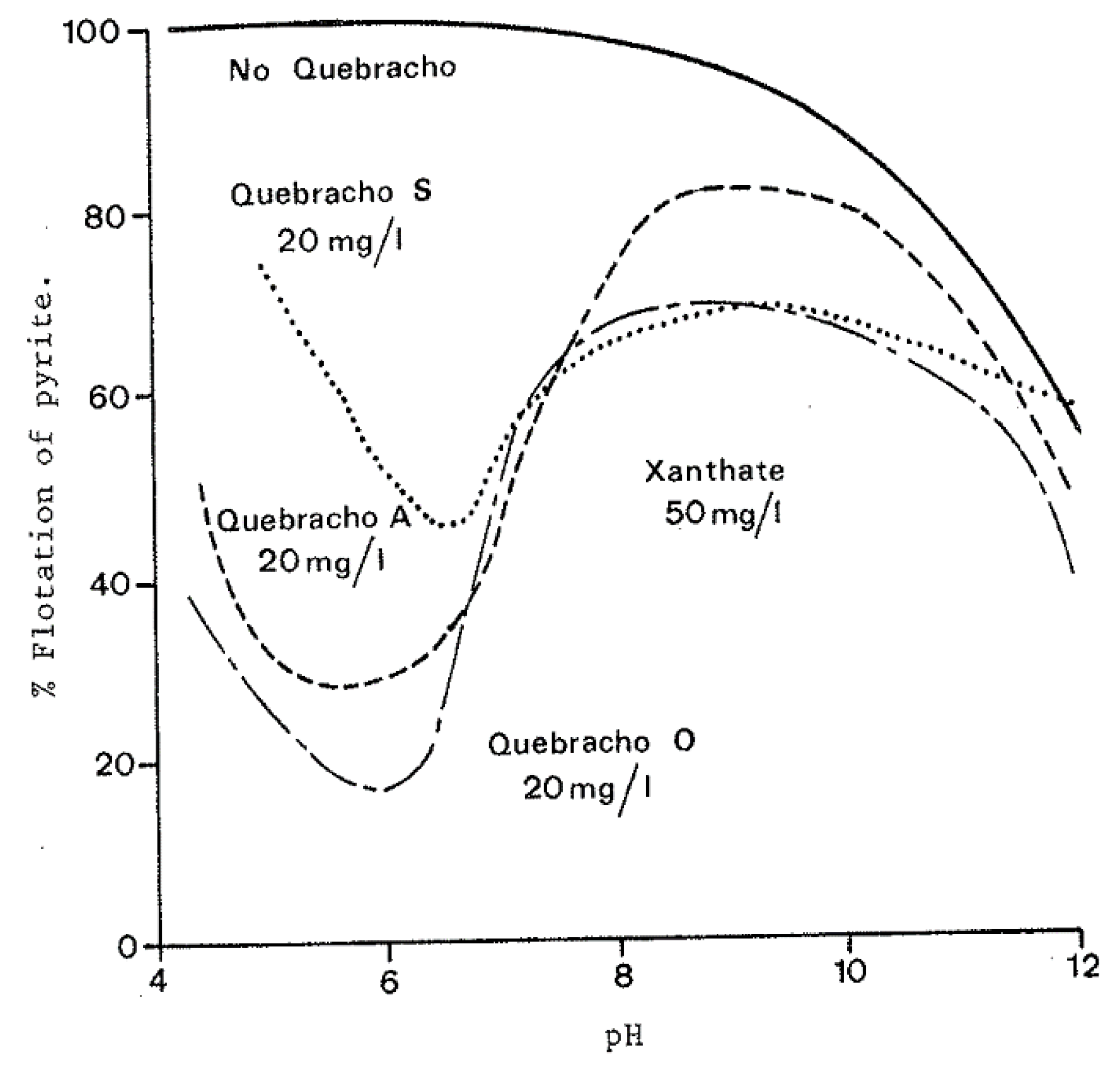
Figure 4 and
Figure 5 display the differences in depression that quebracho has on non-oxidized and oxidized pyrite. Most notably the range of pH that the pyrite is depressed is much smaller in the case of the oxidized pyrite where large fluctuations occur between pH around 6 to 9. In the case of the non-oxidized pyrite, a pH from around 4 to 9.5 was sufficient for the depression of pyrite. These figures also illustrate how the three forms of quebracho behave differently. Silvafloc (quebracho A) has the best depressing effect when the pyrite is non-oxidized, but Tupafin ATO (quebracho O) provides the best results when dealing with the oxidized pyrite. Since it is feasible that there will be a mix of both oxidized and non-oxidized pyrite in a sulfide flotation cell, the preferred quebracho type is Tupafin ATO. The reasoning behind the differences in depression effect between oxidized and non-oxidized pyrite was explained through testing with ferric salts. It was shown that when increasing amounts of Fe
3+ ions were added to the solution quebracho no longer depressed pyrite.
Figure 4.
Depression of non-oxidized pyrite with addition of xanthate and quebracho [
3].
Figure 5.
Depression of oxidized pyrite with addition of xanthate and quebracho [
3].
Quebracho is known to complex with trivalent iron especially above pH 6; thus, it was determined that as the levels of ferric iron in the pulp rose, as was the case with oxidized pyrite, the depressing effect of quebracho decreased. It should be noted that ferrous iron does not form a complex with quebracho however it readily oxidizes to ferric iron [
3]. A pH 6 was shown to provide the best conditions for depressing pyrite.
It is interesting to note that quebracho does not always behave the same way in terms of adsorption. With separation of calcite from fluorite, quebracho and the collector oleate are competitive adsorbates. Both fluorite and calcite adsorb the quebracho and oleate by the same mechanisms via pulp and lattice calcium ions. However, in the case of sulfide systems, it was shown that this was not the case. Quebracho and xanthate do not act as competitive adsorbates within the system; xanthate is adsorbed onto the mineral surfaces at very similar values with and without the addition of quebracho. This indicates that the depressing effect of quebracho is completed by rendering the surface hydrophilic [
3].
Laboratory and pilot plant testing was done on the Cayeli-Riz massive sulfide deposit in Turkey to compare two different depressant systems. The deposit has two ores; the yellow ore is mostly copper with fine-grained pyrite while the black ore contains both copper and zinc that is finely disseminated. Both ores are very high in pyrite and thus required a specific depressant system that would depress the gangue sulfides without depressing the valuable sulfides. The copper rougher flotation was taken out at a pH of 10.5–11.5, the copper cleaning at a lower pH 4.5–5.5, and the zinc flotation was at a high pH above 11.5. The first depressant system used a 1:1 mixture of cyanide and thiourea at 120 g/t in the copper rougher to depress zinc and lime was used to depress the pyrite at 8000 g/t with controlled addition of SO
2. The second system used the same additions of lime and SO
2 in addition to 2H-BC, which is a mixture of quebracho and dextrin-maleic acid. The new addition of 2H-BC was successful at depressing the ultrafine pyrite and increased both the grade and recovery of the copper and zinc concentrates by several percentage points [
20].
A recent study taken out by the National University of San Juan in Argentina set out to prove that tannins and quebracho are a cleaner alternative to the conventional flotation reagents used in copper sulfide flotation. The work specifically set out to show that the Cu/Fe ratio could be improved by depressing pyrite with the organic depressant. Hallimond tube flotation tests were taken out on both pure pyrite and pure chalcopyrite minerals. The pyrite was floated at a pH 8 and showed good depression (around 40% pyrite depressed) with increasing tannin concentration up to around 0.25 g/L where it leveled off. The effect of pH on the recovery of pyrite was shown to favor in the alkaline and acidic range where only 5%–10% pyrite was recovered with a mixture of tannins and lime. It was also illustrated that the presence of Ca
2+ ions (in the form of lime) improved the depression effect of the tannins on the pyrite system. Similar tests were carried out in the Hallimond tube for pure chalcopyrite. The recovery of pyrite and chalcopyrite with and without the addition of tannin is shown in
Figure 6 [
21].
Figure 6 clearly illustrates that the use of tannins have a marked depression effect on pyrite while having little influence on chalcopyrite recovery. Flotation work was carried out in a Denver cell where the pH was set constant at 10 with the addition of lime and a varying amount of tannins between 0 and 600 g/t. With increasing tannin addition, the pyrite was drastically reduced in the concentrate with the best results at 600 g/t. These tests concluded that the addition of tannins in the system significantly increased the grade of the copper while only sacrificing 1 percent of recovery of copper due to the depression of pyrite [
21].
Figure 6.
The effect of tannins on pure chalcopyrite and pyrite Hallimond tube flotation [
21].
A United States patent titled “Separation of Polymetallic Sulphides by Froth Flotation” by Srdjan Bulatovic and Robert S. Salter investigates the use of quebracho as a depressant in complex sulfide ores. Bulatovic and Salter’s work focuses on comparing a newly invented depressant mixture to the traditional depressant system on several polymetallic sulfides. The new depressant is made by dissolving quebracho into solution with either dextrin or guar gum. This mixture undergoes a second reaction with a lignin sulphonate to make a water-soluble polymer. The polymer is then partially monomerized by at least one of the following reagents: alkali metal cyanide, alkaline earth metal cyanide, metal sulfate, and/or a sulfite. For the experimental trials, the lignin sulphonate polymers LS7 and LS8 are used; LS7 is the compound that is reacted with sodium cyanide while LS8 is the compound that has been reacted with zinc sulfate/sodium cyanide complex.
The first set of experiments focused on the clean separation of copper and zinc from a massive sulfide deposit in Canada. The Cu–Zn ore contains valuable amounts of silver and the primary gangue minerals are quartz, pyrite and pyrrhotite. Four sets of experiments were conducted on two ore types, high and low copper, to compare the effects of the LS8 depressant versus sodium cyanide on the recovery and grade of the concentrates. The first set of experiments was laboratory scale while the second set was tested on a full-scale commercial plant. The LS8 depressant was added during the grinding process and in the copper cleaner flotation circuit, with a total of 170 g/t added. In the lab scale trials, the LS8 depressant made large improvements over the sodium cyanide on the rejection of zinc and iron sulfides from the copper concentrate by several percentage points. The sequential zinc concentrate grade and recovery was also improved with the addition of LS8. In the plant trails, similar results were obtained whereby the zinc recovery in the copper circuit was reduced, copper concentrate grade was increased, and zinc recovery in the zinc circuit was improved.
The second segment of laboratory experiments was conducted on two different massive sulfide ores containing mostly copper and nickel. The first ore, from British Columbia, contained copper and nickel as well as significant amounts of platinum and palladium. The comparison tests between the LS8 depressant and sodium cyanide proved that the LS8 reagent had a positive effect on the circuits. With the use of LS8 the copper was recovered to a higher degree in the copper flotation circuit, while the nickel was effectively depressed. The nickel recovery in the nickel circuit was increased and overall, the platinum and palladium recovery was improved. The second ore, from Northern Ontario, also showed improved results with the use of LS8. Most significantly, the nickel recovery in the nickel cleaner concentrate was increased by more than 50 percent. These examples prove that the LS8 depressant is highly effective for depressing nickel in the flotation of copper-nickel ores.
The third installment of experiments involved a massive sulfide with mostly lead and zinc with large amounts of pyrite. Lime was compared to the LS7 depressant in laboratory testing. LS7 was added during the grinding stage at 250 g/t while the lime was added in the lead circuit at 750 g/t and the zinc circuit at 3500 g/t. The recovery of lead in the lead concentrate remained the same, while the grade improved over 30 percent with the addition of LS7. Zinc grade and recovery in the zinc concentrate was also improved with the use of LS7. In plant operations, testing was done to compare the addition of 300 g/t of LS7 to no addition of LS7. By using the LS7 reagent, grade of the lead concentrate was increased with no recovery loss.
The work done by Bulatovic and Salter proved that the invention of a new series of quebracho containing depressants greatly improved the differential flotation of polymetallic sulfides in contrast to traditional reagent schemes [
22].