Separation and Recycling for Rare Earth Elements by Homogeneous Liquid-Liquid Extraction (HoLLE) Using a pH-Responsive Fluorine-Based Surfactant
Abstract
:1. Introduction

2. Experimental Section
2.1. Reagents
2.2. Apparatuses
2.3. Experimental Procedure
2.3.1. Extraction Characteristics of Elements by HoLLE with Zonyl FSA®
2.3.2. The Recovery of Elements from the Zonyl FSA® Phase and the Redissolution of the Zonyl FSA® Phase
3. Results and Discussion
3.1. The Extraction of THF within the Phase Separation Phenomenon

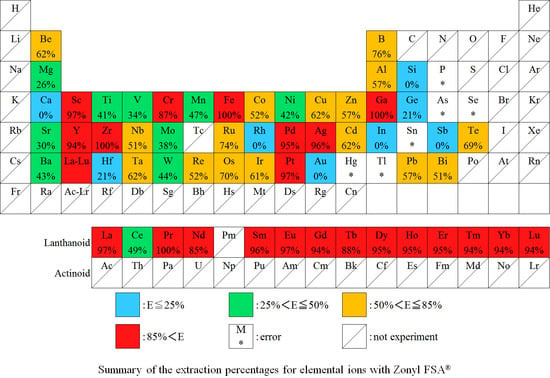
3.2. Screening of Elements
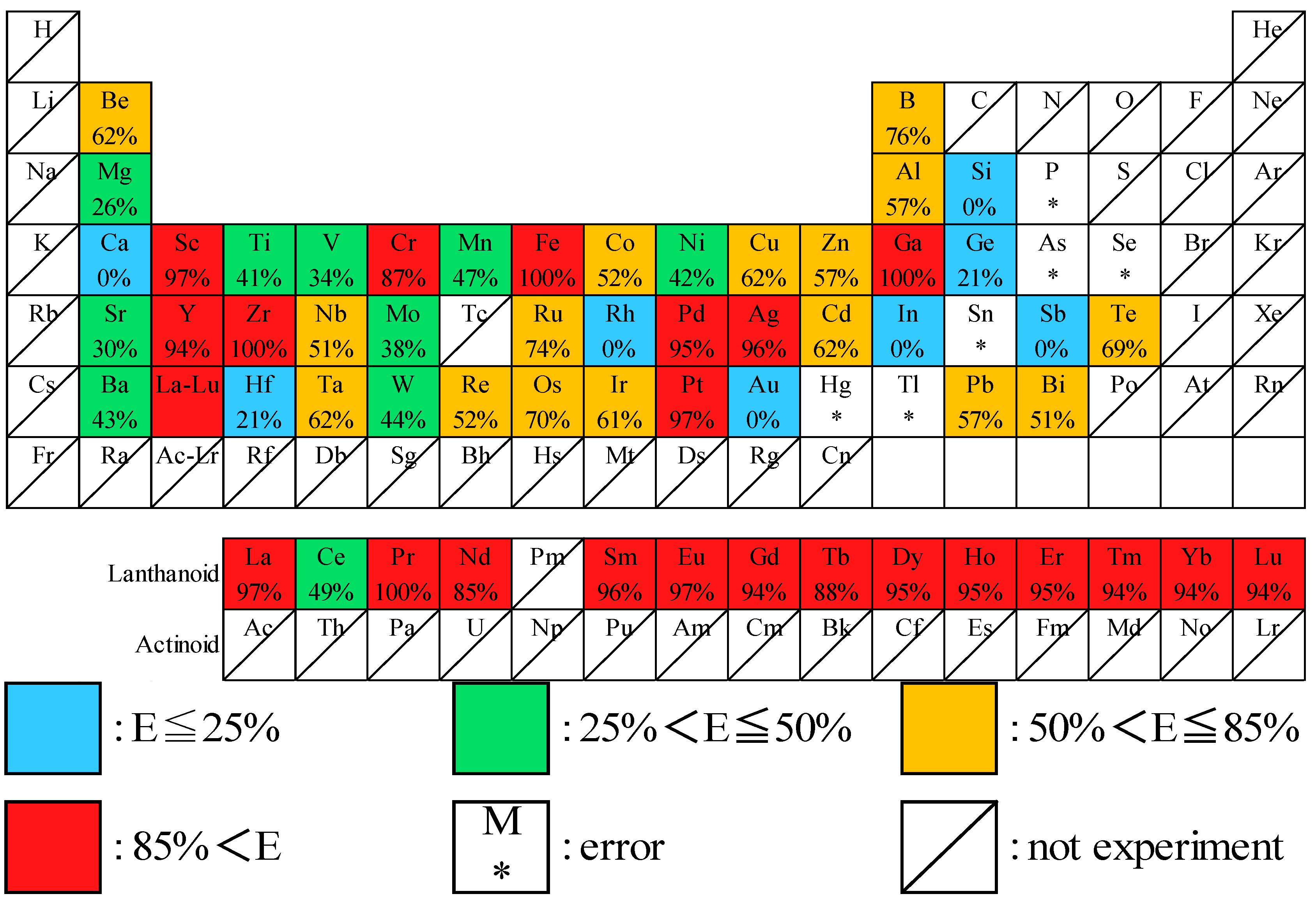
3.3. Stripping of Rare Earth Elements
| Metal | Sc | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery % | 81 | 92 | 88 | 90 | 91 | 88 | 91 | 92 | 92 | 91 | 92 | 92 | 84 | 92 | 92 | 92 |
3.4. Reuse of Spent Zonyl FSA®
| Metal | Sc | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Extraction % | 97 | 94 | 97 | 49 | 100 | 85 | 96 | 97 | 94 | 88 | 95 | 95 | 95 | 94 | 94 | 94 |
| 2nd Extraction % | 80 | 100 | 93 | 53 | 100 | 84 | 100 | 100 | 100 | 94 | 100 | 100 | 94 | 100 | 100 | 100 |
3.5. Overview of Separation and Recycling System of Rare Earth Elements Using Zonyl FSA®

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, J.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. One step effective separation of platinum and palladium in an acidic chloride solution by using undiluted ionic liquids. Solvent Extr. Res. Dev. Jpn. 2014, 21, 129–135. [Google Scholar] [CrossRef]
- Yang, J.; Kubota, F.; Baba, Y.; Kamiya, N.; Goto, M. Separation of precious metals by using undiluted ionic liquids. Solvent Extr. Res. Dev. Jpn. 2014, 21, 89–94. [Google Scholar] [CrossRef]
- Saitoh, T.; Sugiura, Y.; Asano, K.; Hiraide, M. Chitosan-conjugated thermos-responsive polymer for the rapid removal of phenol in water. React. Funct. Polym. 2009, 69, 792–796. [Google Scholar] [CrossRef]
- Kato, T.; Igarashi, S.; Ohno, O.; Watanabe, Y.; Murakami, K.; Takemori, T.; Yamaguchi, H.; Ando, R. Separation and recovery properties of rare earth elements using a pH-sensitive polymer having benzoic acid substituent group. Bunseki Kagaku 2012, 61, 235–242. [Google Scholar] [CrossRef]
- Igarashi, S.; Saito, S.; Kato, T.; Okano, G.; Yamaguchi, H. Development of separation and recovery system of gold and rare earth elements using stimuli-responsive polymers—G-MOVE system and La-VEBA system. J. Surf. Finish. Soc. Jpn. 2012, 63, 630–632. [Google Scholar] [CrossRef]
- Saito, S.; Igarashi, S.; Yamaguchi, H. Selective collection characteristics and separation/recovery method of gold(III), silver(I) and platinum(II) with pH-sensitive polymer under addition of L-ascorbic acid. Bunseki Kagaku 2014, 63, 791–795. [Google Scholar] [CrossRef]
- Inoue, K.; Ohto, K.; Yoshizuka, K.; Shinbaru, R.; Baba, Y.; Kina, K. Adsorption behavior of metal ions on some carboxymethylated chitosans. Bunseki Kagaku 1993, 42, 725–731. [Google Scholar] [CrossRef]
- Parajuli, D.; Kawakita, H.; Inoue, K.; Ohto, K.; Kajiyam, K. Persimmon peel gel for the selective recovery of gold. Hydrometallurgy 2007, 87, 133–139. [Google Scholar] [CrossRef]
- Gurung, M.; Adhikari, B.B.; Kawakita, H.; Ohto, K.; Inoue, K. Recovery of Au(III) by using low cost adsorbent prepared from persimmon tannin extract. Chem. Eng. J. 2011, 174, 556–563. [Google Scholar] [CrossRef]
- Igarashi, S.; Yotsuyanagi, T. A novel homogeneous liquid-liquid Extraction by pH dependent phase separation with fluorocarbon ionic surfactant. In Proceedings of the Symposium on Solvent Extraction 1988, Tokyo, Japan, 5–7 December 1988; pp. 175–180.
- Igarashi, S.; Yotsuyanagi, T. Temperature dependent phase transformation in the homogeneous liquid-liquid extraction with fluorocarbon surfactant. In Proceedings of the Symposium on Solvent Extraction 1989, Sendai, Japan, 9–11 November 1989; pp. 51–54.
- Igarashi, S.; Yotsuyanagi, T. New homogeneous liquid-liquid extraction by phase separation and transformation with fluorocarbon surfactant and quaternary ammonium salt. In Solvent extraction 1990, Proceedings of the International Solvent Extraction Conference (ISEC ’90), Kyoto, Japan, 16–21 July 1992; Sekine, T., Ed.; Elsevier: New York, NY, USA, 1992; pp. 1725–1730. [Google Scholar]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Yamaguchi, H. Did the study of ionic liquids begin from analytical chemistry? Bunseki 2007, 11, 608–609. [Google Scholar]
- Igarashi, S.; Yotsuyanagi, T. Homogeneous liquid-liquid extraction by pH dependent phase separation with a fluorocarbon ionic surfactant and its application to the preconcentration of porphyrin compounds. Mikrochimica Acta 1992, 106, 37–44. [Google Scholar] [CrossRef]
- Oshite, S.; Igarashi, S. Homogeneous liquid-liquid extraction using perfluorooctanesulfonic acid and calcium and its application to the separation and recovery vitamin B12. J. Chem. Technol. Biotechnol. 1999, 74, 1183–1187. [Google Scholar] [CrossRef]
- Oshite, S.; Furukawa, M.; Igarashi, S. Homogeneous liquid-liquid extraction method for the selective spectrofluorimetric determination of trace amoutns of tryptophan. Analyst 2001, 126, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Ueki, Y.; Igarashi, S. Highly efficient homogeneous liquid-liquid extraction of rare earth metal ions from perfluorocarboxylate surfactant solutions using ion-pair phase separation systems. Solvent Extr. Res. Dev. Jpn. 2001, 8, 235–240. [Google Scholar]
- Hoogerstraete, T.V.; Ohghena, B.; Binnemans, K. Homogeneous liquid-liquid extraction of rare earths with the betaine-batainium bis(trifluoromethylsulfonyl)imide ionic liquid system. Int. J. Mol. Sci. 2013, 14, 21353–21377. [Google Scholar] [CrossRef] [PubMed]
- Takagai, Y.; Igarashi, S. Homogeneous liquid-liquid extraction method as simple and powerful preconcentration for capillary gas chromatography and capillary electrophoresis. Am. Lab. 2002, 34, 29–30. [Google Scholar]
- Fuchimukai, J.; Yamaguchi, H.; Meguro, Y.; Kubota, T.; Igarashi, S. Highly efficient homogeneous liquid-liquid extraction of lanthanoid ions in a strong acidic solution. Solvent Extr. Res. Dev. Jpn. 2006, 13, 139–146. [Google Scholar]
- Sudo, T.; Igarashi, S. Homogeneous liquid-liquid extraction method for spectrofluorimetric determination of chlorophyll a. Talanta 1996, 43, 233–237. [Google Scholar] [CrossRef]
- Ghiasvand, A.R.; Moradi, F.; Sharghi, H.; Hasaninejad, A.R. Determination of silver(I) by electrothermal-AAS in a microdroplet formed from a homogeneous liquid-liquid extraction system using tetraspirocyclohexylcalix[4]pyrroles. Anal. Sci. 2005, 21, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, A.R.; Shadabi, S.; Kakanejadifard, A.; Khajehkoolaki, A. Synthesis of a new α-dioxime derivative and its application for selective homogeneous liquid-liquid extraction of Cu(II) into a microdroplet followed by direct GFAAS determination. Bull. Korean Chem. Soc. 2005, 26, 781–785. [Google Scholar]
- Kato, T.; Igarashi, S.; Ishiwatari, Y.; Furukawa, M.; Yamaguchi, H. Separation and concentration of indium from a liquid crystal display via homogeneous liquid-liquid extraction. Hydrometallurgy 2013, 137, 148–155. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Itoh, S.; Igarashi, S.; Kobayashi, T. Homogeneous liquid-liquid extraction of metal-1,10-phenanthoroline chelates in a weak acidic solution. Bunseki Kagaku 2005, 54, 227–230. [Google Scholar] [CrossRef]
- Sinha, S.P. Complexes of the Rare Earths; Pergamon Press Ltd.: Oxford, UK, 1966; p. 26. [Google Scholar]
- Suzuki, Y. Kidorui no Hanashi [The Story of Rare Earth Elements]; Shokabo Publishing Co., Ltd.: Chiyoda, Japan, 1998; pp. 66–72. [Google Scholar]
- Burgess, J. Metal Ions in Solution; Ellis Horwood Ltd.: New York, NY, USA, 1978; pp. 266–267. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Ohno, O.; Igarashi, S.; Kato, T.; Yamaguchi, H. Separation and Recycling for Rare Earth Elements by Homogeneous Liquid-Liquid Extraction (HoLLE) Using a pH-Responsive Fluorine-Based Surfactant. Metals 2015, 5, 1543-1552. https://doi.org/10.3390/met5031543
Saito S, Ohno O, Igarashi S, Kato T, Yamaguchi H. Separation and Recycling for Rare Earth Elements by Homogeneous Liquid-Liquid Extraction (HoLLE) Using a pH-Responsive Fluorine-Based Surfactant. Metals. 2015; 5(3):1543-1552. https://doi.org/10.3390/met5031543
Chicago/Turabian StyleSaito, Shotaro, Osamu Ohno, Shukuro Igarashi, Takeshi Kato, and Hitoshi Yamaguchi. 2015. "Separation and Recycling for Rare Earth Elements by Homogeneous Liquid-Liquid Extraction (HoLLE) Using a pH-Responsive Fluorine-Based Surfactant" Metals 5, no. 3: 1543-1552. https://doi.org/10.3390/met5031543