Use of Nanoscale Zero-Valent Iron (NZVI) Particles for Chemical Denitrification under Different Operating Conditions
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Nanosized Iron
2.2. Nitrate Reduction by NZVI
2.3. Analytical Methods and Presentation of Results
3. Results and Discussion
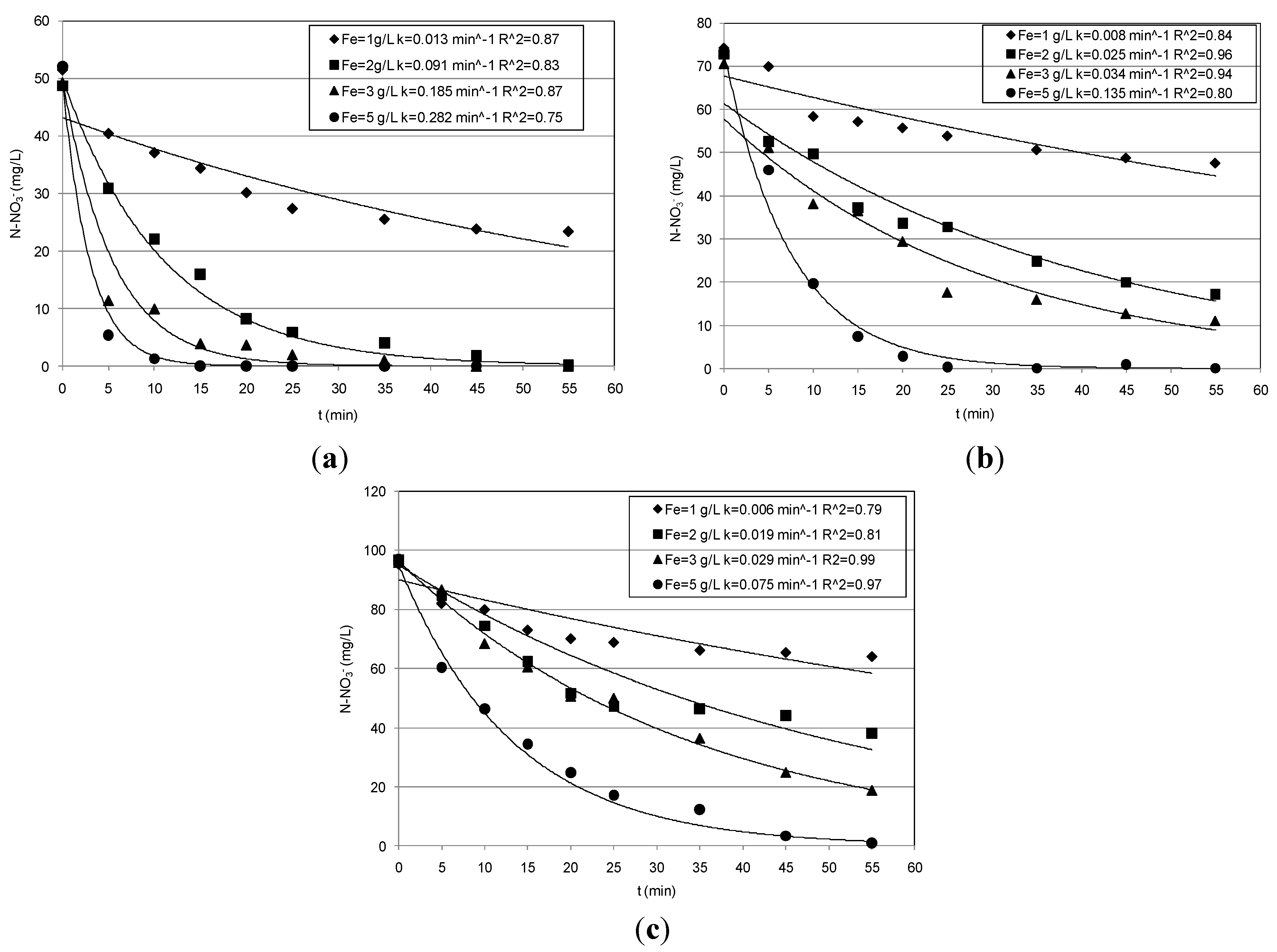
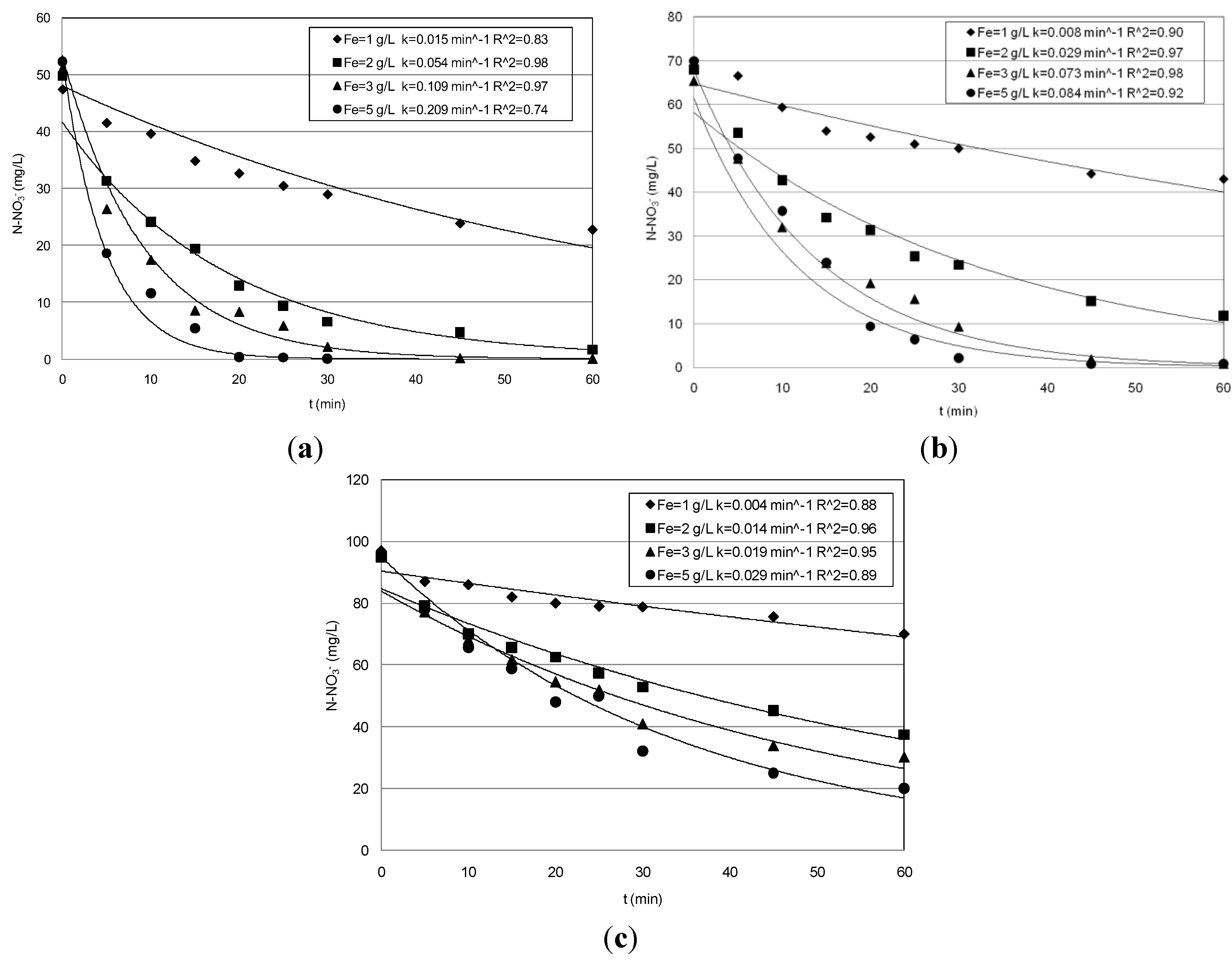
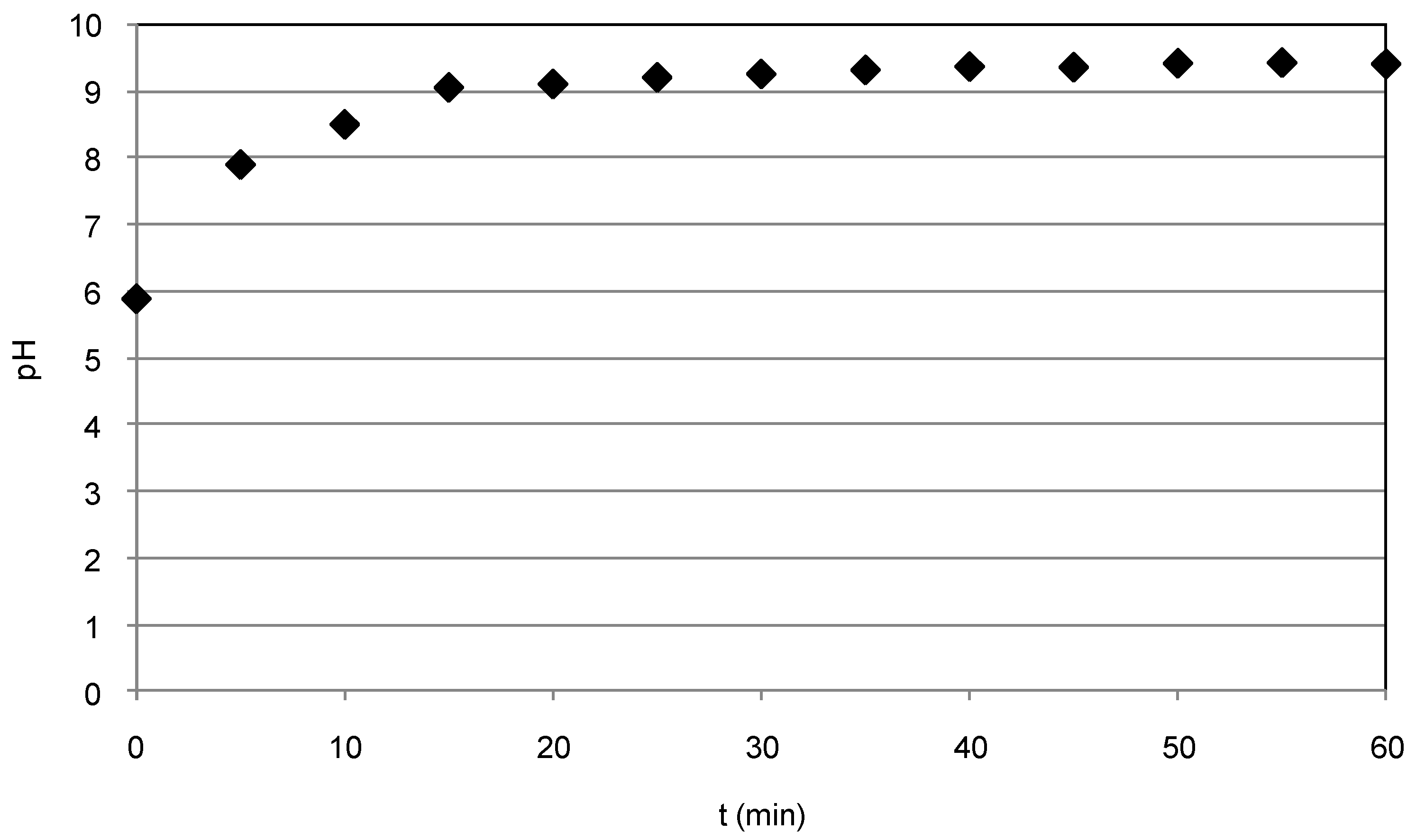
3.1. Nitrate Abatement by Fe0 Nanoparticles
| pH | Ni mg/L | Fe0 g/L | N–NO3− Abatement (%) | N–NO3− Removed (mg/L) | N–NH4+ Produced (mg/L) | Fe2+ mg/L | k min−1 |
|---|---|---|---|---|---|---|---|
| 3 | 50 | 1 | 56.1 | 28.2 | 27.6 | 198 | 0.013 |
| 2 | 99.9 | 49.2 | 47.8 | 491 | 0.091 | ||
| 3 | 99.9 | 49.6 | 46.3 | 705 | 0.185 | ||
| 5 | 99.9 | 50.8 | 48.5 | 1408 | 0.282 | ||
| 70 | 1 | 36.2 | 26.9 | 24.4 | 197 | 0.008 | |
| 2 | 74.3 | 52.9 | 49.3 | 605 | 0.025 | ||
| 3 | 84.4 | 59.2 | 58.5 | 803 | 0.034 | ||
| 5 | 99.9 | 71.7 | 70.2 | 1530 | 0.135 | ||
| 95 | 1 | 32.1 | 31.2 | 31.0 | 198 | 0.006 | |
| 2 | 60.2 | 58.6 | 57.6 | 491 | 0.019 | ||
| 3 | 92.4 | 89.6 | 88.3 | 705 | 0.029 | ||
| 5 | 99.9 | 98.5 | 97.3 | 1408 | 0.075 | ||
| 5 | 50 | 1 | 52.0 | 24.6 | 23.0 | 48 | 0.015 |
| 2 | 97.1 | 48.3 | 47.6 | 230 | 0.054 | ||
| 3 | 99.9 | 51.0 | 48.1 | 350 | 0.109 | ||
| 5 | 99.9 | 52.2 | 48.3 | 1200 | 0.209 | ||
| 70 | 1 | 38.3 | 26.3 | 23.0 | 96 | 0.008 | |
| 2 | 83.0 | 56.4 | 55.1 | 232 | 0.029 | ||
| 3 | 99.9 | 65.3 | 62.4 | 235 | 0.073 | ||
| 5 | 99.9 | 69.4 | 69.1 | 530 | 0.084 | ||
| 95 | 1 | 28.4 | 27.5 | 26.7 | 32 | 0.004 | |
| 2 | 61.0 | 57.9 | 56.5 | 126 | 0.014 | ||
| 3 | 69.2 | 66.4 | 64.6 | 168 | 0.019 | ||
| 5 | 73.1 | 71.2 | 70.1 | 370 | 0.029 | ||
| uncontrolled | 50 | 1 | 42.3 | 20.3 | 16.0 | 4.0 | 0.010 |
| 2 | 77.1 | 37.3 | 33.1 | 4.2 | 0.021 | ||
| 3 | 80.0 | 39.2 | 34.1 | 4.6 | 0.022 | ||
| 5 | 98.0 | 44.6 | 40.5 | 6.0 | 0.060 | ||
| 70 | 1 | 32.4 | 22.0 | 16.4 | 5.0 | 0.007 | |
| 2 | 59.2 | 42.9 | 30.1 | 6.3 | 0.015 | ||
| 3 | 60.5 | 44.1 | 34.6 | 7.0 | 0.016 | ||
| 5 | 87.1 | 59.2 | 41.5 | 6.8 | 0.034 | ||
| 95 | 1 | 29.5 | 28.7 | 20.7 | 4.0 | 0.005 | |
| 2 | 45.2 | 44.0 | 30.8 | 5.4 | 0.010 | ||
| 3 | 47.4 | 46.3 | 40.3 | 5.7 | 0.010 | ||
| 5 | 63.0 | 61.7 | 43.0 | 5.7 | 0.015 |
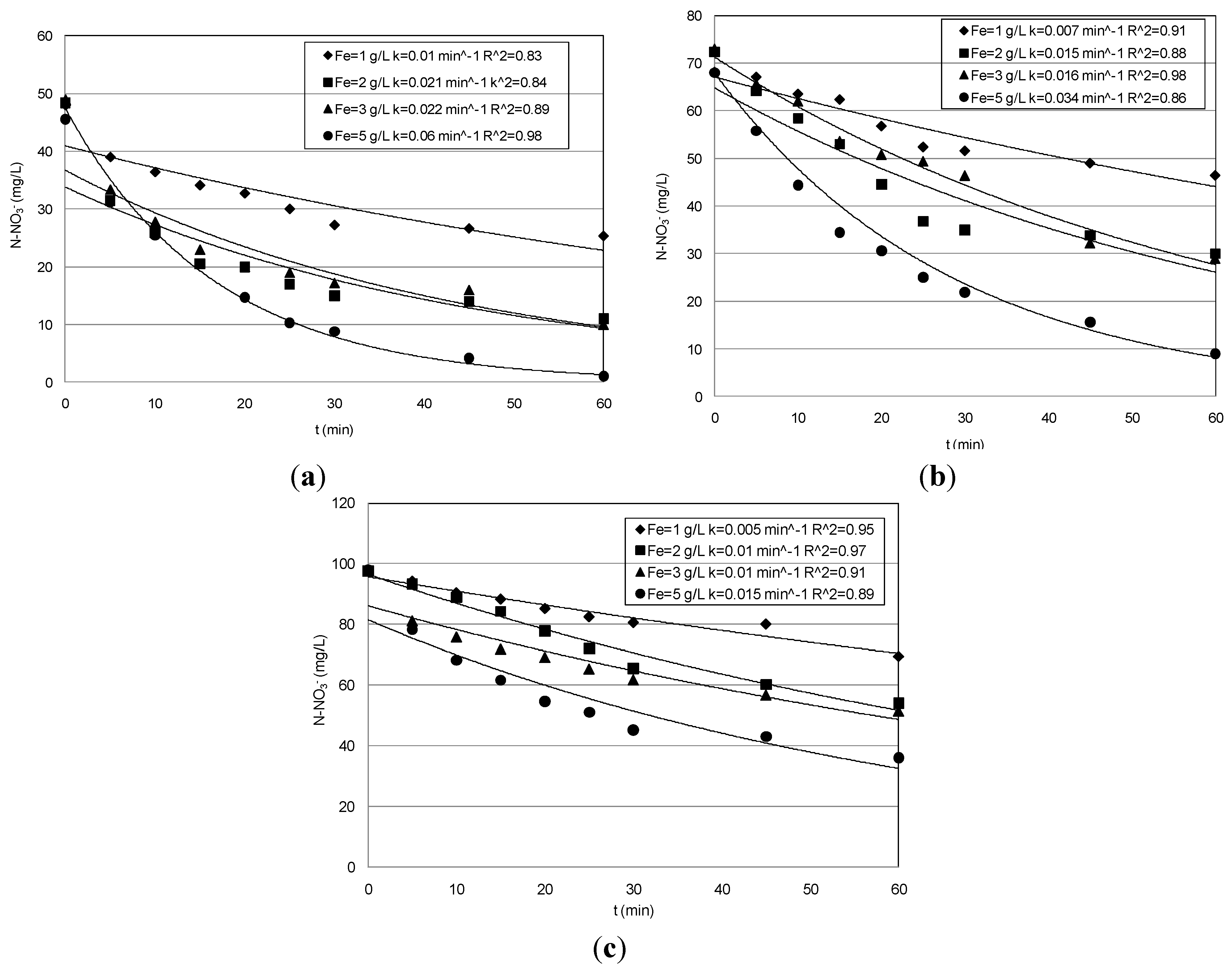
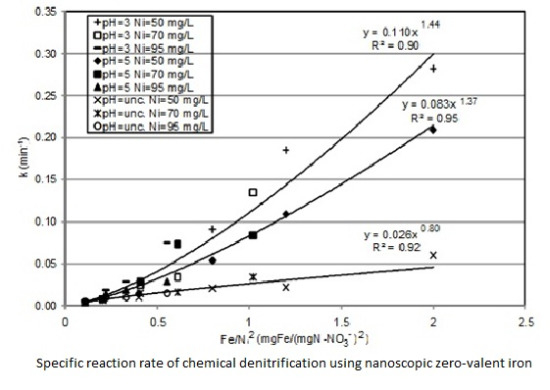
3.2. Kinetic Analysis
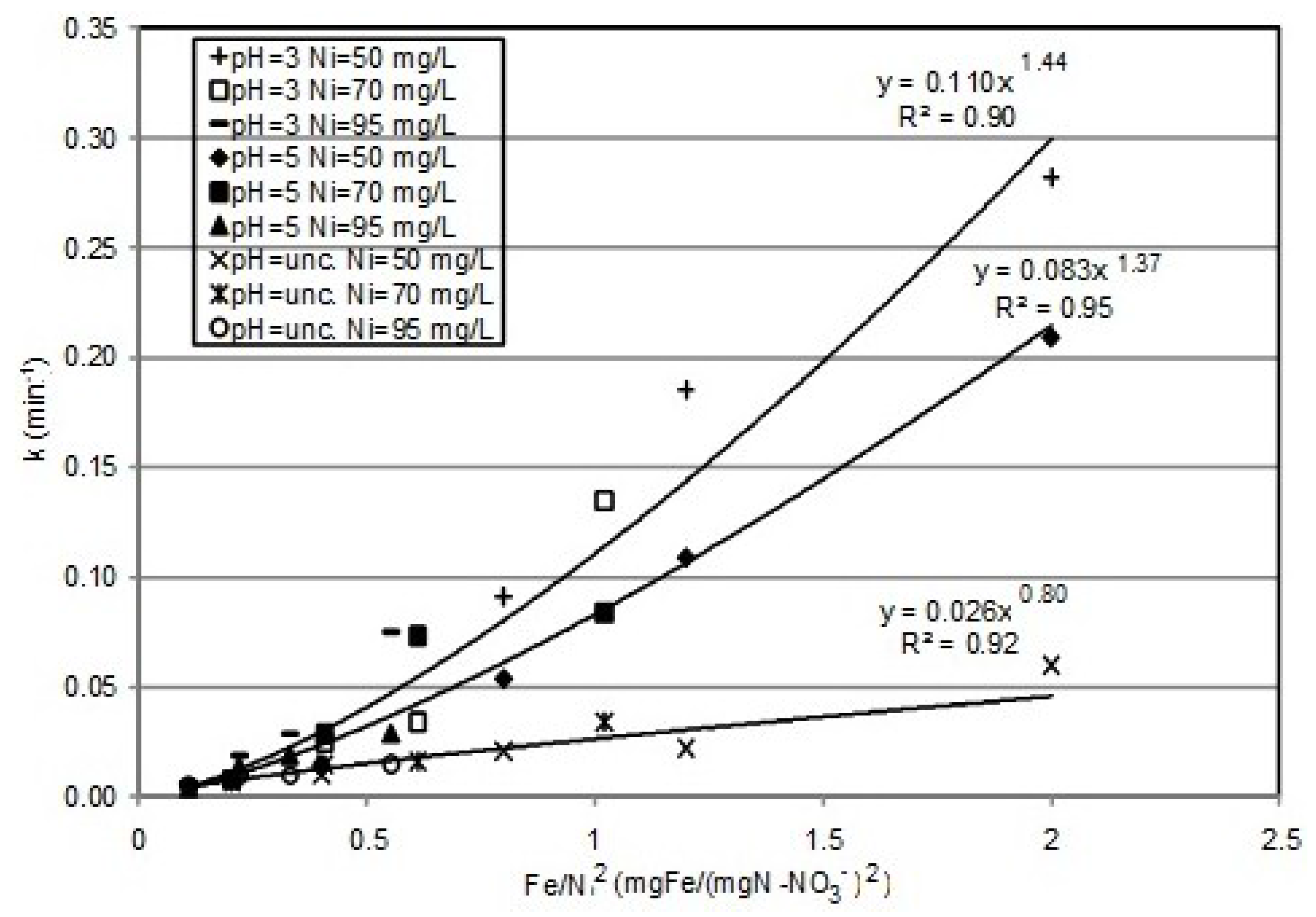
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hwang, Y.H.; Kim, D.G.; Shin, H.S. Mechanism study of nitrate reduction by nano zero valent iron. J. Hazard. Mater. 2011, 185, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Noubactep, C.; Caré, S. On nanoscale metallic iron for groundwater remediation. J. Hazard. Mater. 2010, 182, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Ruangchainikom, C.; Liao, C.H.; Anotai, J.; Lee, M.T. Effects of water characteristics on nitrate reduction by the Fe0/CO2 process. Chemosphere 2006, 63, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zhang, T.C. Effects of dissolved oxygen on formation of corrosion products and concomitant oxygen and nitrate reduction in zero-valent iron systems with or without aqueous Fe2+. Water Res. 2005, 39, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.C.C.; Lee, H.L. Chemical reduction of nitrate by nanosized iron: Kinetics and Pathways. Water Res. 2005, 39, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Guldberg, S.; Erbs, M.; Koch, C.B. Kinetics of nitrate reduction by green rusts—Effects of interlayer anion and Fe(II):Fe(III) ratio. Appl. Clay Sci. 2001, 18, 81–91. [Google Scholar] [CrossRef]
- Huang, C.P.; Wang, H.W.; Chiu, P.C. Nitrate reduction by metallic iron. Water Res. 1998, 32, 2257–2264. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, T.C. Effects of low pH on nitrate reduction by iron powder. Water Res. 2004, 38, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maroto, J.M.; García-Herruzo, F.; García-Rubio, A.; Gómez-Lahoz, C.; Vereda-Alonso, C. Kinetics of the chemical reduction of nitrate by zero-valent iron. Chemosphere 2009, 74, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jin, S.; Fallgren, P.H.; Colberg, P.J.S.; Johnson, P.A. Prevention of iron passivation and enhancement of nitrate reduction by electron supplementation. Chem. Eng. J. 2010, 160, 185–189. [Google Scholar] [CrossRef]
- Tsai, Y.J.; Chou, F.C.; Cheng, T.C. Coupled acidification and ultrasound with iron enhances nitrate reduction. J. Hazard. Mater. 2009, 163, 743–747. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington DC, USA, 1998. [Google Scholar]
- Kassaee, M.Z.; Motamedi, E.; Mikhak, A.; Rahnemaie, R. Nitrate removal from water using iron nanoparticles produced by arc discharge vs. Reduction. Chem. Eng. J. 2011, 166, 490–495. [Google Scholar] [CrossRef]
- Liou, Y.H.; Lo, S.L.; Lin, C.J.; Kuan, W.H.; Weng, S.C. Chemical reduction of an unbuffered nitrate solution using catalyzed and uncatalyzed nanoscale iron particles. J. Hazard. Mater. 2005, 127, 102–110. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Siciliano, A. A catalytic oxidation process of olive oil mill wastewaters using hydrogen peroxide and copper. Desalination Water Treat. 2010, 23, 187–193. [Google Scholar] [CrossRef]
- Cheng, F.; Muftikian, R.; Fernando, Q.; Korte, N. Reduction of nitrate to ammonia by zero-valent iron. Chemosphere 1997, 35, 2689–2695. [Google Scholar] [CrossRef]
- Siciliano, A.; de Rosa, S. Recovery of ammonia in digestates of calf manure through a struvite precipitation process using unconventional reagents. Environ. Technol. 2014, 35, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Ruggiero, C.; de Rosa, S. A new integrated treatment for the reduction of organic and nitrogen loads in methanogenic landfill leachates. Process Saf. Environ. Prot. 2013, 91, 311–320. [Google Scholar] [CrossRef]
- Choe, S.; Liljestrand, H.M.; Khim, J. Nitrate reduction by zero-valent iron under different pH regimes. Appl. Geochem. 2004, 19, 335–342. [Google Scholar] [CrossRef]
- Stucki, J.W.; Su, K.; Pentrakova, L.; Pentrak, M. Methods for handling redox-sensitive smectite dispersions. Clay Miner. 2014, 49, 359–377. [Google Scholar] [CrossRef]
- Pentrak, M.; Pentrakova, L.; Stucki, J.W. Iron and manganese reduction-oxidation. In Methods in Biogeochemistry of Wetlands; DeLaune, R.D., Reddy, K.R., Richardson, C.J., Megonigal, J.P., Eds.; Soil Science Society of America: Madison, WI, USA, 2013; pp. 701–721. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siciliano, A. Use of Nanoscale Zero-Valent Iron (NZVI) Particles for Chemical Denitrification under Different Operating Conditions. Metals 2015, 5, 1507-1519. https://doi.org/10.3390/met5031507
Siciliano A. Use of Nanoscale Zero-Valent Iron (NZVI) Particles for Chemical Denitrification under Different Operating Conditions. Metals. 2015; 5(3):1507-1519. https://doi.org/10.3390/met5031507
Chicago/Turabian StyleSiciliano, Alessio. 2015. "Use of Nanoscale Zero-Valent Iron (NZVI) Particles for Chemical Denitrification under Different Operating Conditions" Metals 5, no. 3: 1507-1519. https://doi.org/10.3390/met5031507
APA StyleSiciliano, A. (2015). Use of Nanoscale Zero-Valent Iron (NZVI) Particles for Chemical Denitrification under Different Operating Conditions. Metals, 5(3), 1507-1519. https://doi.org/10.3390/met5031507