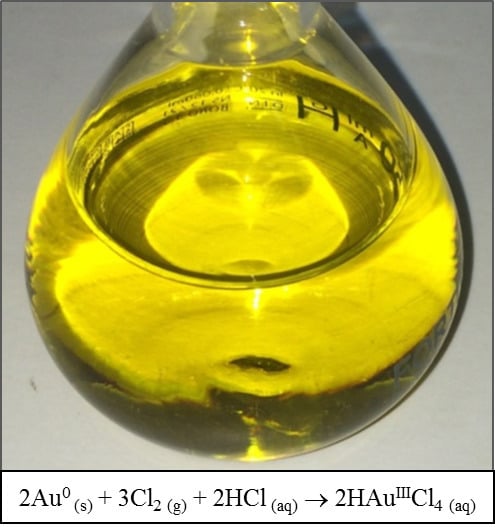
A Straightforward Route to Tetrachloroauric Acid from Gold Metal and Molecular Chlorine for Nanoparticle Synthesis
Abstract
:1. Introduction
2. Experimental Section
2.1. General
2.2. Synthesis of Tetrachloroauric Acid (HAuCl4)
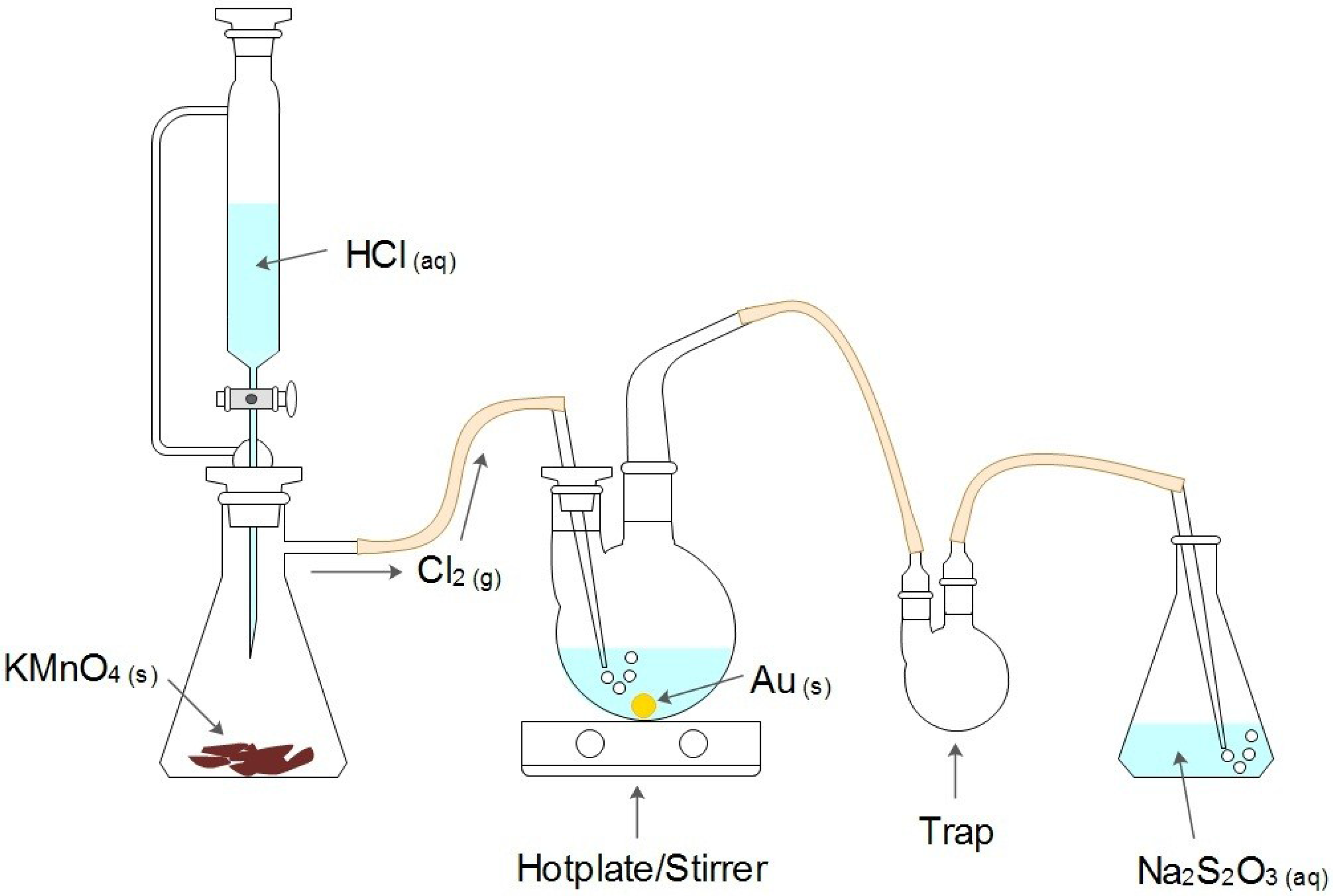
2.3. Measurement of Reaction Times
2.4. Synthesis of Citrate-Stabilised Gold Nanoparticles
3. Results and Discussion

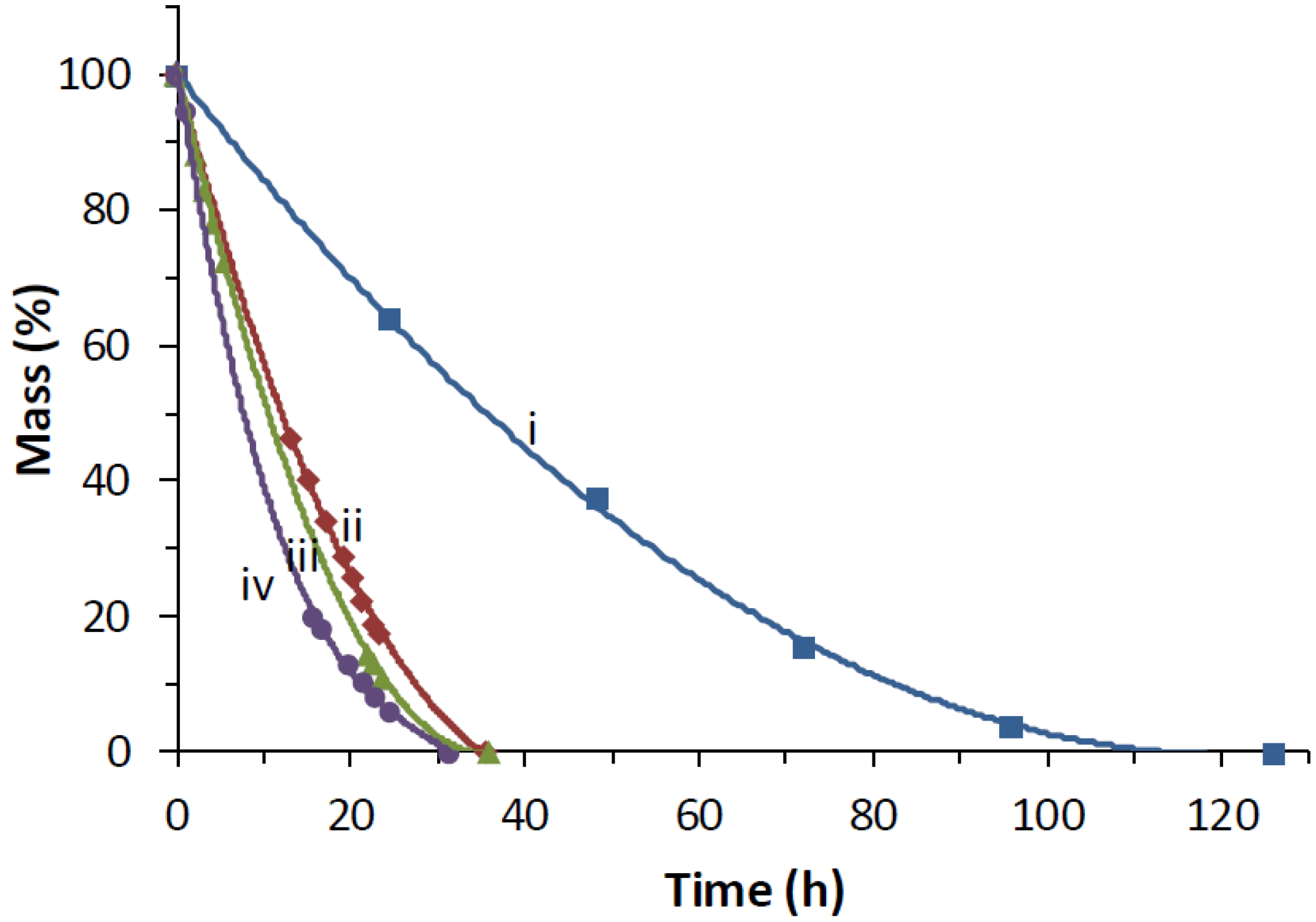
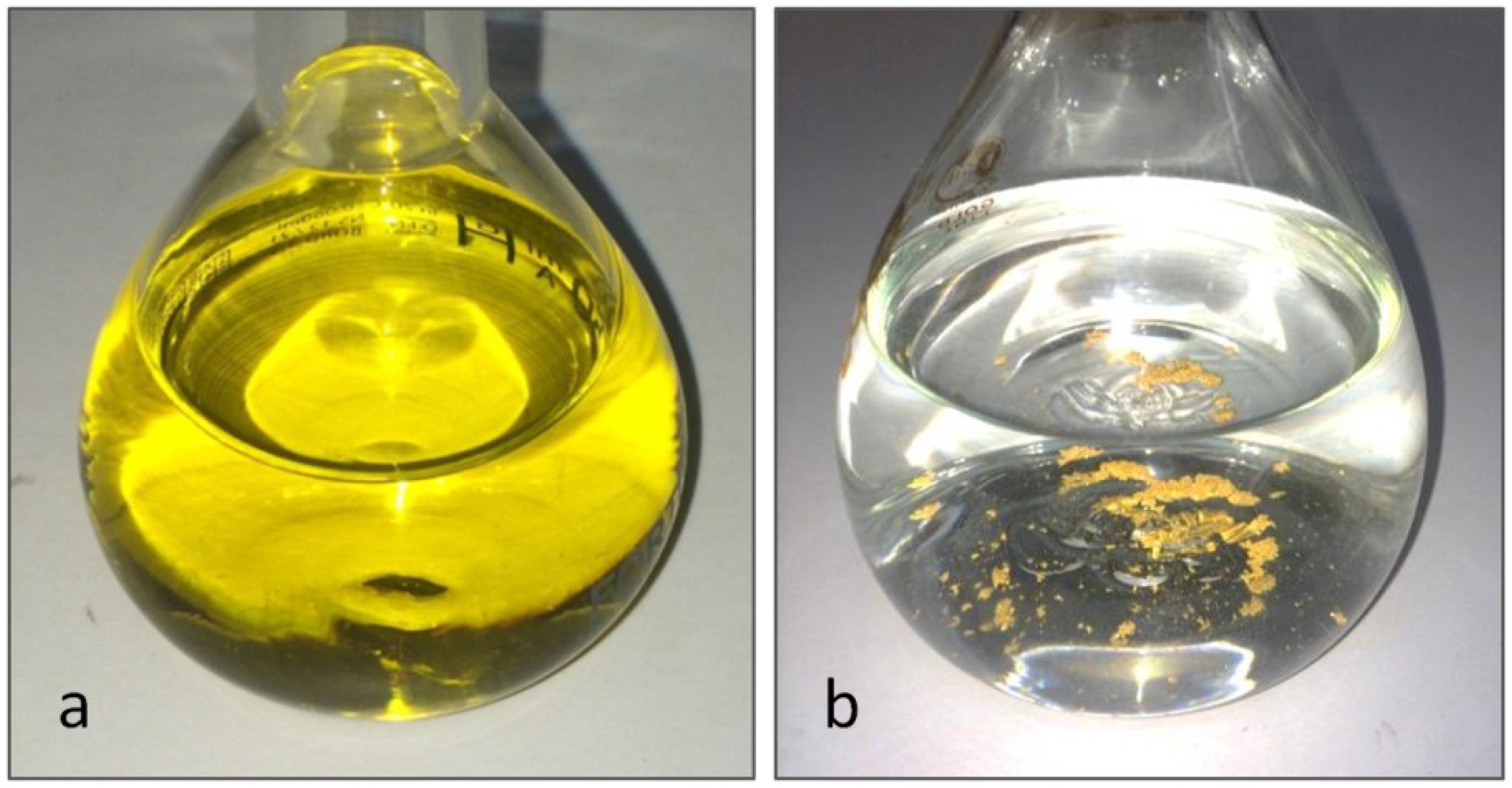
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nam, K.S.; Jung, B.H.; An, J.W.; Ha, T.J.; Tran, T.; Kim, M.J. Use of Chloride-Hypochlorite Leachants to Recover Gold from Tailing. Int. J. Miner. Process. 2008, 86, 131–140. [Google Scholar] [CrossRef]
- Mellor, J.W. A Comprehensive Treatise on Inorganic and Theoretical Chemistry, 2nd ed.; Longmans, Green and Co. Ltd.: London, UK, 1928; Volume 3, pp. 491–618. [Google Scholar]
- Konyratbekova, S.S.; Baikonurova, A.; Akcil, A. Non-cyanide Leaching Processes in Gold Hydrometallurgy and Iodine-Iodide Applications: A Review. Miner. Process. Extr. Metall.: Int. J. 2014, 36, 198–212. [Google Scholar] [CrossRef]
- French, A. Process of Obtaining Gold, Silver and Copper from Ores. U.S. Pat. Off. US490193, 17 January 1893. [Google Scholar]
- La Brooy, S.R.; Linge, H.G.; Walker, G.S. Review of Gold Extraction from Ores. Miner. Eng. 1994, 7, 1213–1241. [Google Scholar] [CrossRef]
- Syed, S. Recovery of Gold from Secondary Sources—A Review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Kallmes, J. Improvement in Treating Gold and Silver Ores. U.S. Pat. Off. US138500, 6 May 1873. [Google Scholar]
- Puvvada, G.V.K.; Murthy, D.S.R. Selective Precious Metals Leaching from a Chalcopyrite Concentrate Using Chloride/Hypochlorite Media. Hydrometallurgy 2000, 58, 185–191. [Google Scholar] [CrossRef]
- Simpson, C.H. Chlorination Process for Removing Precious Metals from Ore. U.S. Pat. Off. US4439235, 27 March 1984. [Google Scholar]
- Choi, H.; Chen, W.T.; Kamat, P.V. Know Thy Nano Neighbor. Plasmonic versus Electron Charging Effects of Metal Nanoparticles in Dye-Sensitized Solar Cells. ACS Nano 2012, 6, 4418–4427. [Google Scholar] [CrossRef] [PubMed]
- Skoog, D.A.; West, D.M.; Holler, F.J. Fundamentals of Analytical Chemistry, 6th ed.; Saunders College Publishing: Sydney, Australia, 1992; pp. 373–374. [Google Scholar]
- Carlin, R.L. Transition Metal Chemistry; Marcel Dekker, Inc.: New York, NY, USA, 1965; Volume 1, pp. 245–253. [Google Scholar]
- Gangopadhayay, A.K.; Chakravorty, A. Charge Transfer Spectra of some Gold(III) Complexes. J. Chem. Phys. 1961, 35, 2206–2209. [Google Scholar] [CrossRef]
- Peck, J.A.; Tait, C.D.; Swanson, B.I.; Brown, G.E., Jr. Speciation of Aqueous Gold(III) Chlorides from Ultraviolet/Visible Absorption and Raman/Resonance Raman Spectroscopies. Geochim. Cosmochim. Acta 1991, 55, 671–676. [Google Scholar] [CrossRef]
- Alkan, M.; Oktay, M.; Kocakerim, M.M.; Copur, M. Solubility of Chlorine in Aqueous Hydrochloric Acid Solutions. J. Hazard. Mater. 2005, 119, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J. Colloidal Gold. Part II Colour, Coagulation, Adhesion, Alloying and Catalytic Properties. Gold Bull. 1985, 18, 125–131. [Google Scholar] [CrossRef]
- Vinals, J.; Nunez, C.; Herroros, O. Kinetics of the Aqueous Chlorination of Gold in Suspended Particles. Hydrometallurgy 1995, 38, 125–147. [Google Scholar] [CrossRef]
- Diaz, M.A.; Kelsall, G.H.; Welham, N.J. Electrowinning Coupled to Gold Leaching by Electrogenerated Chlorine: I. Au(III)–Au(I)/Au Kinetics in Aqueous Cl2/Cl− Electrolytes. J. Electroanal. Chem. 1993, 361, 25–38. [Google Scholar] [CrossRef]
- Sun, T.M.; Yen, W.T. Kinetics of Gold Chloride Adsorption onto Activated Carbon. Miner. Eng. 1993, 6, 17–29. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, S.R.; Massicot, J.; McDonagh, A.M. A Straightforward Route to Tetrachloroauric Acid from Gold Metal and Molecular Chlorine for Nanoparticle Synthesis. Metals 2015, 5, 1454-1461. https://doi.org/10.3390/met5031454
King SR, Massicot J, McDonagh AM. A Straightforward Route to Tetrachloroauric Acid from Gold Metal and Molecular Chlorine for Nanoparticle Synthesis. Metals. 2015; 5(3):1454-1461. https://doi.org/10.3390/met5031454
Chicago/Turabian StyleKing, Shirin R., Juliette Massicot, and Andrew M. McDonagh. 2015. "A Straightforward Route to Tetrachloroauric Acid from Gold Metal and Molecular Chlorine for Nanoparticle Synthesis" Metals 5, no. 3: 1454-1461. https://doi.org/10.3390/met5031454